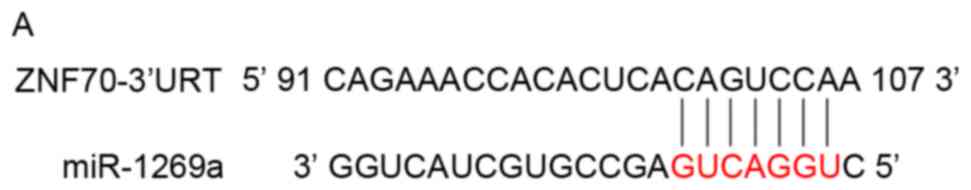Introduction
There are ~738,000 mortalities per year caused by
gastric cancer, which has been recognized as a major cause of
cancer-associated mortality in the world (1). Over 70% of new cases and mortalities are
occurring in developing countries, most of which are in advanced
stage when first diagnosed due to the lack of sensitive and early
diagnosis markers (2). Additionally,
recurrence and metastasis of advanced gastric cancers are commonly
observed following surgery and chemical therapy (3,4).
Therefore, it is urgently required to identify novel and efficient
methods for early diagnosis and offer positive treatments.
microRNAs (miRNAs) are a type of small non-coding
single-strand RNAs that regulate gene expression
post-transcriptionally. It is reported that 30–60% of human genes
are regulated by miRNAs, primarily by binding to target sites on
messenger RNAs (mRNAs) (5–7). miRNAs have a pivotal role in regulating
biological processes in the development of gastric cancer (5). Specifically, miRNAs contribute to
carcinogenesis by altering the expression of oncogenes and tumor
suppressors to affect proliferation, apoptosis motility and
invasion (8). Numerous studies have
revealed that small changes in miRNAs expression may cause
significant changes in target mRNA translation and protein
synthesis and therefore affect the occurrence and development of
gastric cancer (9,10).
Single nucleotide polymorphisms (SNPs) are the third
generation of genetic markers for the study of complex genetic
diseases, pharmacogenomics and human evolution (11,12). SNPs
in microRNA may reconstruct the secondary structure of pre-miRNAs,
which are named pri-miRNAs, or interfere with the maturation or
target selection of miRNA (13),
leading to the alteration of cancer formation. Therefore, miRNA
SNPs may be regarded as biomarkers for cancer diagnosis and
prognosis. However, a limited number of studies have investigated
the molecular mechanisms of microRNA SNPs in gastric cancer.
miR-1269a, which is located at human chromosome 4,
is associated with the occurrence and development of various tumors
including primary hepatocellular carcinoma (14), colorectal cancer (15) and breast cancer (16). miR-1269a has been reported to be
upregulated in hepatocellular carcinoma, as one of the independent
cancer predictors via suppressing Forkhead box protein O1
expression (14). However, little has
been reported in the literature regarding the role of miR-1269a in
gastric cancer or the involvement of its SNP, rs73239138.
In the present study, to the best of our knowledge,
for the first time an association was identified between rs73239138
in microRNA-1269a and the susceptibility to gastric cancer. The
present study further revealed the molecular role of this
variation. The findings of the present study may assist to clarify
the roles of SNPs in miRNAs in the development and occurrence of
gastric cancer and lead to proposals of novel methods for early
prediction, detection, diagnosis, treatment and prognosis.
Materials and methods
Study population
The 373 gastric cancer patients were recruited from
the Huashan Hospital and Huadong Hospital Affiliated to Fudan
University (Shanghai, China), Renji Hospital and Tongren Hospital
Affiliated to Shanghai Jiao Tong University School of Medicine
(Shanghai, China) and PLA 175 Hospital (Xiamen, China), between
January 2011 and December 2014. The mean age was 62.13 years and
the age distribution was 27–90 years. The patients were diagnosed
by pathology or imaging by enhanced computed tomography (CT) scan
or positron emission tomography-CT scan. A total of 402 cancer-free
controls were collected from the Taizhou longitudinal study without
a history of other types of cancer or restriction of age and sex
(17). All subjects were unrelated
Han Chinese. All materials from patients, including peripheral
blood samples and epidemiological investigations, were obtained
with informed consent, and the whole study was approved by the
Ethic Review Committees of Huashan Hospital and School of Life
Science, Fudan University (Shanghai, China).
The prediction of microRNA target
gene
The target genes of the miRNA were obtained
according to the miRNA target gene database (TargetScan 6.2
http://www.targetscan.org/vert_62/).
Gene ontology (GO) analysis
GO analysis was applied in order to organize genes
into hierarchical categories and uncover the target genes of miRNA
on the basis of biological process (18). In detail, two-sided Fisher's exact
test and χ2 test were used to classify the GO category,
and the false discovery rate (FDR) (19) was calculated to correct the P-value.
The present study selected only GOs that had a P-value of <0.01
and a FDR of <0.05.
Pathway analysis
Based on KEGG, pathway analysis was performed using
Fisher's exact test and χ2 test, and the threshold of
significance was defined by P-values and FDRs. The screening
criteria were P<0.05 (20–22).
DNA extraction and genotyping
Genomic (g)DNA of each subject was isolated by using
the AxyPrep™ Blood Genomic DNA Miniprep kit (Axygen Biosciences;
Corning Incorporated, Corning, NY, USA). Concentration of gDNA was
determined using NanoDrop (C723 ND-1000 UV/Vis; NanoDrop
Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
DNA samples were detected by agarose gel electrophoresis to ensure
the DNA quality and a concentration >20 ng/µl were diluted into
a working dilution of 10 ng/µl and added to 96-well plates for
subsequent genotyping.
The SNPs were genotyped using matrix-assisted laser
desorption/ionization-time of flight-based assay (MALDI-TOF)
(23). The primers were designed
using MassARRAY Assay Design software (Table I) and synthetized by Shanghai Generay
Biotech Co., Ltd (Shanghai, China). The primer sequences are listed
in Table I. Genotyping was performed
using the MassARRAY Compact system (Sequenom Inc., CA, USA), and
the data were analyzed using TyperAnalyzer software version 4.0
(Sequenom Inc.).
 | Table I.Primer sequences for miR-1269a
variant rs73239138. |
Table I.
Primer sequences for miR-1269a
variant rs73239138.
|
|
|
|
|
| Primer sequences
(5′-3′) |
|---|
|
|
|
|
|
|
|
|---|
| SNP IDa | Substitution | SNP location | miRNA | Location | Amplification
primer 1 | Amplification
primer 2 | Extension
primer |
|---|
| rs73239138 | G/A | 67142620 | hsa-mir-1269a | Mat | ACGTTGGATGA | ACGTTGGATGA | CAGGGAAGC |
|
|
|
|
|
| AGTCTCATGAT | CCTGAGGAATG | CAGTAGCA |
|
|
|
|
|
| AGGCCATC | CCTGGAC |
|
Cell lines and cell culture
A total of two human gastric cell lines (HGC-27 and
MGC-803) used in the present study, and the cell lines were
purchased from Cell Bank of the Chinese Academy of Sciences
(Shanghai, China), which were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc,) with 10% FBS
(Biological Industries Israel, Kibbutz Beit Haemek, Israel). The
cells were all maintained in a humidified incubator containing 5%
CO2 at 37°C.
Reverse transcription-quantitative PCR
analysis
miRNA was quantified using the miRcute miRNA qPCR
Detection kit (SYBR green; Tiangen Biotech Co., Ltd., Shanghai,
China) and analyzed using the 7500 Fast Real-Time Sequence
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The primers used for stem-loop reverse-transcription PCR for
miR-1269a and 5S were purchased from Tiangen Biotech Co., Ltd. The
relative expression levels of the miRNA were normalized to the
expression of small nuclear RNA 5S and calculated using 2−[(Cq
of miRNA) - (Cq of 5S)] (24).
Cell transfection
The wild-type and variant miR-1269a expression
plasmids were generated by cloning the wild-type and variant
pre-miR-1269a sequence into the retroviral transfer plasmid
CMV-MCS-SV40-neomycin (Shanghai GeneChem Co., Ltd., Shanghai,
China), respectively. The concentration of DNA plasmids transfected
was 1 µg/ml in DMEM with Penicillin-Streptomycin (Gibco; Thermo
Fisher Scientific, Inc.). The miRNA-1269a mimics and miRNA-1269a
inhibitor of the wild-type and variant type as well as the
miR-negative control (NC) were purchased from GenePharma (Shanghai
GenePharma Co., Ltd., Shanghai, China). Zinc-finger protein 70
(ZNF70) small interfering (si)RNA and the siRNA NC were purchased
from Biotend (Shanghai, China). Transfections were performed using
Lipofectamine 2000 (Invitrogen) according to the manufacturer's
protocol.
Cell apoptosis assay
HGC-27 and MGC-803 cell lines were seeded in 6-well
plate at a suitable density (60–70%) and after 24, 48 and 72 h of
transfection. The cells were washed in PBS, harvested by trypsin,
and subsequently treated with 100 µl Muse™ Annexin V Dead Cell
reagent (EMD Millipore; Billerica, MA, USA) at room temperature in
the dark for 20 min. This was followed by detection of apoptosis
using Muse™ Cell Analyzer (EMD Millipore).
Cell proliferation assay
Proliferation was measured using the cell-counting
kit-8 (CCK-8 kit; Dojindo Molecular Technologies, Inc., Shanghai,
China). According to the manufacturer's instructions,
2×103 cells were seeded into 96-well culture plates and
allowed to adhere for 24 h at 37°C. The cells were subsequently
transfected with 50 nM negative control or siRNA and incubated at
37°C for 24, 48, 72 or 96 h. At the endpoint, 20 µl CCK-8 (5 g/l)
was added for a further 4 h at 37°C. A Multiscan GO
spectrophotometer (Thermo Fisher Scientific, Inc.) was used to
measure the absorbance at 450 nm.
Western blot analysis
Treated cells were washed twice with ice-cold PBS
and then treated with RIPA lysis buffer supplemented with 1 mM
phenylmethylsulfonyl fluoride (both from Beyotime Institute of
Biotechnology, Haimen, China). Following centrifugation at 10,000 ×
g for 15 min at 4°C, concentration of the proteins was measured
using BCA protein assay kit (Beyotime Institute of Biotechnology).
Cell protein lysates were separated on 12% SDS denatured
polyacrylamide gel and electro-transferred onto polyvinylidene
fluoride membranes. The membranes were blocked with 5% non-fat milk
and were incubated with primary antibodies against ZNF70
(zinc-finger protein 70; cat. no. ab49339; 5:1,000; Abcam,
Cambridge, UK), GAPDH (cat. no. 5174S, Cell Signaling Technology
Inc., Danvers, MA, USA), Bik (cat. no. 4592S, Cell Signaling
Technology Inc., Danvers, MA, USA), Bim (cat. no. 2933S, Cell
Signaling Technology Inc., Danvers, MA, USA) and Bak (cat. no.
12105S, Cell Signaling Technology Inc., Danvers, MA, USA) at 4°C
overnight, which were all diluted to 1:1,000. The membranes were
then washed 3 times with TBST (Bioscience Shanghai, Inc., China)
and incubated with anti-rabbit immunoglobulin G at room temperature
~1 h, horseradish peroxidase-linked antibody (cat. no. 7074;
1:2,000; Cell Signaling Technology Inc.), according to the
manufacturer's instructions. Finally, the images were captured
using a gel imaging analysis system (Tanon 4100; Tanon Science and
Technology Co., Ltd., Shanghai, China).
Statistical analysis
All statistical tests were performed using the
software SPSS (version 19. 0; IBM Corp., Armonk, NY, USA) and Excel
(Microsoft Corporation, Redmond, WA, USA). Using Pearson's
chi-squared test of goodness of fit, genotype frequencies were
evaluated for Hardy-Weinberg equilibrium. Binary logistic
regression was applied to analyze the association between the
genotypes of miRNA SNP and the susceptibility to gastric cancer
following adjustments for age, sex, smoking status and drinking
status. Odds ratios and 95% confidence intervals (CI) were also
calculated. Pearson's chi-squared test of independence was then
used to investigate the association between the SNPs and
qualitative clinical indexes, including age, sex, smoking and
alcohol consumption status, and tumor size in gastric cancer
patients. A two-sided P<0.05 was considered to indicate a
statistically significant difference.
The data from cellular experiments are expressed as
the mean ± standard deviation for three independent experiments.
Subsequently, ANOVA and multiple comparisons test were used to
evaluate the significant difference between three groups of data,
and the student's t-test was used to evaluate the significant
difference between two groups of data. Statistics was performed
with the use of GraphPad Prism (version 6.0; GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-1269a rs73239138 decreases the
risk of gastric cancer
The present study included 373 patients with gastric
cancer and 402 healthy subjects, and the demographic
characteristics were summarized in Table
II. It was indicated that there were significant differences
between the patients with gastric cancer and the healthy controls
in terms of age, sex and smoking status. However, there were no
differences between the two groups for alcohol consumption.
 | Table II.General characteristics of gastric
cancer patients and unaffected controlsa. |
Table II.
General characteristics of gastric
cancer patients and unaffected controlsa.
| Characteristic | Cases (n=373) | Controls
(n=402) | P-value |
|---|
| Age | 62.13±12.10 | 62.37±8.76 |
<0.001b |
| Sex, n (%) |
|
|
<0.001b |
| Total | 367 | 402 |
|
|
Male | 267 (72.8) | 175 (43.5) |
|
|
Female | 100 (27.2) | 227 (56.5) |
|
| Smoking, n (%) |
|
| 0.012b |
|
Total | 357 | 402 |
|
|
Neverc | 273 (76.5) | 274 (68.2) |
|
|
Everc | 84 (23.5) | 128 (31.8) |
|
| Consumption of
alcohol, n (%) |
|
| 0.099 |
|
Total | 357 | 402 |
|
|
Neverc | 315 (88.2) | 338 (84.1) |
|
|
Everc | 42 (11.8) | 64 (15.9) |
|
| Size of tumor foci
(n=209), mean ± SD | 4.28±2.70 |
|
|
| Tumor sites, n
(%) |
|
|
|
|
Total | 303 |
|
|
|
Non-cardia cancer | 230 (75.9) |
|
|
| Cardia
cancer | 73 (24.1) |
|
|
| Organ metastasis, n
(%) | 321 |
|
|
|
Negative, M0 | 253 (78.8) |
|
|
|
Positive, M1 | 68
(21.2) |
|
|
| Lymph-node
metastasis, n (%) |
|
|
|
|
Total | 340 |
|
|
|
Negative (N0) | 158 (46.5) |
|
|
|
Positive (N1-N3) | 182 (53.5) |
|
|
As shown in Table
III, compared with the wild-type GG, the AA genotype was
associated with a significant decreased risk of gastric cancer
(P=0.045; OR, 0.610; 95% CI, 0.376–0.990). However, there was no
statistically significant association between the GA genotype and
gastric cancer risk (P=0.567; OR, 1.106; 95% CI, 1.106
(0.783–1.564). A similar association was identified in the
recessive model (AA vs. GG + AG; P=0.014; OR, 0.576; 95% CI,
0.371–0.896). Collectively, the findings indicated that miR-1269a
rs73239138 may have a role in decreasing the risk of gastric
cancer.
 | Table III.Association between genotypes/alleles
of miR-1269a rs73239138 and the risk of gastric cancer. |
Table III.
Association between genotypes/alleles
of miR-1269a rs73239138 and the risk of gastric cancer.
|
| Gastric cancer
patients |
|---|
|
|
|
|---|
| Genotype | Control, n (%) | Number, n (%) | OR (95%
CI)a |
P-valueb |
|---|
| rs7329138 |
|
|
|
|
| GG | 144 (36.1) | 131 (35.1) |
|
|
| GA | 180 (45.1) | 193 (51.7) | 1.106
(0.783–1.564) | 0.567 |
| AA | 75 (18.8) | 49 (13.1) | 0.610
(0.376–0.990) | 0.045b |
| Dominant model |
|
|
|
|
| AA + GA
vs. GG |
|
| 0.956
(0.689–1.326) | 0.788 |
| Recessive
model |
|
|
|
|
| AA vs.
GG + GA |
|
| 0.576
(0.371–0.896) | 0.014b |
| G | 468 (58.6) | 455 (61.0) |
|
|
| A | 330 (41.4) | 291 (39.0) | 0.841
(0.669–1.056) | 0.135 |
Decreased expression of miR-1269a
variant in human gastric cancer cell lines
Subsequently, the expression levels of miR-1269a in
HGC-27 and MGC-803 gastric cancer cell lines transfected with
pre-miR1269a and per-miR1269a variant plasmid were analyzed by
RT-qPCR. As shown in Fig. 1, the
expression level of miR-1269a was significantly downregulated in
cells that were transfected with pre-miR1269a-variant compared with
pre-miR1269a wild-type-transfected cells (P<0.0001), indicating
that the decreased expression of miR-1269a by the SNP may have
contributed to the development of gastric cancer.
Increased apoptosis in cells
transfected with miR-1269a variant compared with cells transfected
with wild-type miR-1269a
To determine the functional alteration of miR-1269a
variant in gastric cancer progression, the authors of the present
study examined the rate of apoptosis of MGC-803 and HGC-27 cells
transfected with wild-type miR-1269a and mimics of miR-1269a
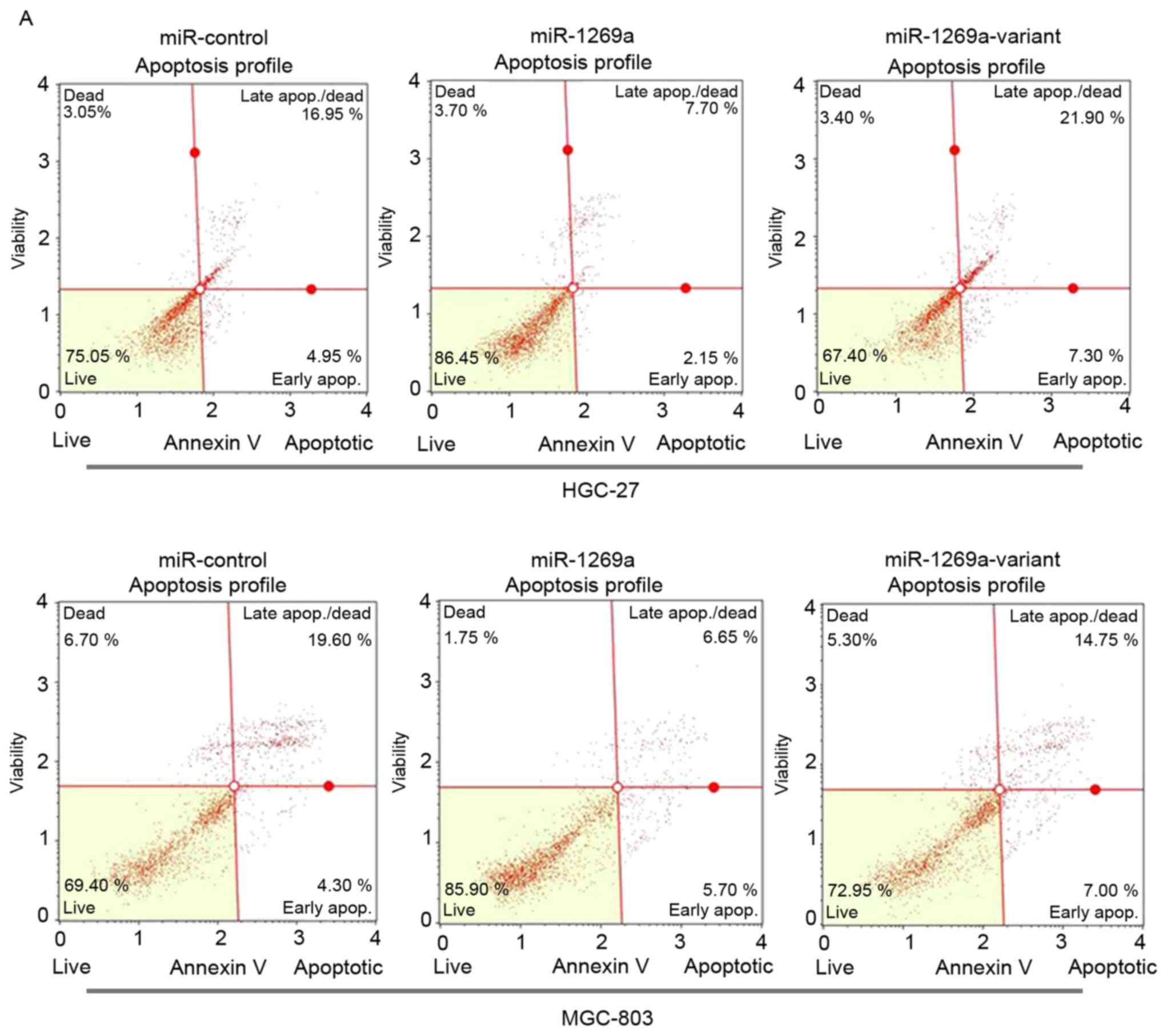
variant by flow cytometric (FCM) analysis. As shown in Fig. 2A and B, the proportion of apoptotic
cells was significantly decreased in MGC-803 or HGC-27 cells
transfected with wild-type miR-1269a mimics after 72 h compared
with control miR mimics. However, the apoptotic rate of cells
transfected with miR-1269a variant type was significantly increased
compared with cells transfected with miR-1269a wild-type (miR-NC
vs. miR-1269a vs. miR-1269a variant, 19.10 vs. 11.62 vs. 22.95% in
HGC-27; 21.17 vs. 13.97 vs. 20.73% in MGC-803). These results
suggested that wild-type miR-1269a may have promoted tumor genesis
in gastric cancer through prohibiting the apoptosis of gastric
cancer cells.
The authors further detected the expression levels
of proteins involved in apoptosis (Bik, Bim and Bak) between cells
transfected with wild-type and miR-1269a variant by western
blotting. As expected, compared with cells transfected with
miR-1269a variant, the expression of Bik, Bim and Bak was
downregulated in gastric cancer cells transfected with wild-type
miR-1269a. However, the expression of these proteins was
upregulated in cells transfected with the wild-type inhibitor
compared with cells transfected with inhibitor of miR-1269a variant
(Fig. 2C). Collectively, these
results indicated that wild-type miR-1269a may have a role in the
proliferation of gastric cancer cells by inhibiting apoptosis.
However, expression of miR-1269a variant increased the apoptosis of
gastric cancer cells and reduced the tumor-promoting effect of
miR-1269a.
ZNF70 is a candidate target gene of
miR-1269a and serves as a tumor suppressor gene in gastric cancer
cells
It is important to investigate potential mRNAs,
which are regulated by miR-1269a, which may further evaluate the
function of miR-1269a. The potential target genes were obtained
using the miRNA target gene database (TargetScan 6.2). Then, the
authors used gene ontology (GO) analysis and pathway analysis to
exclude any unlikely targets. The authors obtained a total of 23
predicted target genes of hsa-miR-1269a (Table IV), and excluded those by
overexpressing miR-1269a in order to investigate which target did
not respond (data not shown). As presented in Fig. 3A, a binding site in ZNF70 mRNA
3′-untranslated region (UTR) was identified. Using western
blotting, it was demonstrated that ZNF70 expression was
downregulated in MGC-803 and HGC-27 cells overexpressing miR-1269a
compared with cells transfected with miR-control. However, this
effect was reversed in cells overexpressing miR-1269a variant.
Similarly, ZNF70 expression was upregulated in cells transfected
with miR-1269a inhibitor and reversed in cells transfected with
miR-1269a variant inhibitor compared with cells transfected with
miR-inhibitor control (Fig. 3B).
Therefore, these results suggest that miR-1269a targets and
inhibits ZNF70 in gastric cancer cells.
 | Table IV.The predicted target genes of
miRNA-1269a. |
Table IV.
The predicted target genes of
miRNA-1269a.
| No. | Gene symbol | Transcript ID | Gene full name | Total
context+score |
|---|
| 1 | DACT1 | NM_001079520 | Dapper, antagonist
of β-catenin, homolog 1 (Xenopus laevis) | −0.47 |
| 2 | INTS6 | NM_001039938 | Integrator complex
subunit 6 | −0.47 |
| 3 | RBMS3 | NM_001003792 | RNA binding motif,
single stranded interacting protein 3 | −0.41 |
| 4 | ZNF70 | NM_021916 | Zinc finger protein
70 | −0.37 |
| 5 | DAZ2 | NM_001005785 | Deleted in
azoospermia 2 | −0.35 |
| 6 | VPS13B | NM_017890 | Vacuolar protein
sorting 13 homolog B (yeast) | −0.33 |
| 7 | DDX5 | NM_004396 | DEAD
(Asp-Glu-Ala-Asp) box polypeptide 5 | −0.27 |
| 8 | AFAP1 | NM_001134647 | Actin filament
associated protein 1 | −0.23 |
| 9 | APPBP2 | NM_006380 | Amyloid β precursor
protein (cytoplasmic tail) binding protein 2 | −0.22 |
| 10 | NLN | NM_020726 | Neurolysin
(metallopeptidase M3 family) | −0.21 |
| 11 | ONECUT1 | NM_004498 | One cut homeobox
1 | −0.19 |
| 12 | NFX1 | NM_002504 | Nuclear
transcription factor, X-box binding 1 | −0.19 |
| 13 | USP9Y | NM_004654 | Ubiquitin specific
peptidase 9, Y-linked | −0.18 |
| 14 | RAB3GAP2 | NM_012414 | RAB3 GTPase
activating protein subunit 2 (non-catalytic) | −0.18 |
| 15 | CACNA1E | NM_000721 | Calcium channel,
voltage-dependent, R type, α 1E subunit | −0.16 |
| 16 | WNT10A | NM_025216 | Wingless-type MMTV
integration site family, member 10A | −0.12 |
| 17 | CUX2 | NM_015267 | Cut-like homeobox
2 | −0.11 |
| 18 | SPTB | NM_001024858 | Spectrin, β,
erythrocytic | −0.1 |
| 19 | KLF3 | NM_016531 | Kruppel-like factor
3 (basic) | −0.1 |
| 20 | FAM55C | NM_001134456 | Family with
sequence similarity 55, member C | −0.07 |
| 21 | SLC24A2 | NM_001193288 | Solute carrier
family 24 (sodium/potassium/calcium exchanger), member 2 | −0.04 |
| 22 | MTDH | NM_178812 | Metadherin | −0.04 |
| 23 | LRP6 | NM_002336 | Low density
lipoprotein receptor-related protein 6 | >-0.02 |
To further confirm ZNF70, as a target of miR-1269a,
is involved in the pathology of gastric cancer, the authors of the
present study have used siRNA to inhibit the endogenous ZNF70, and
the siRNA interference efficiency is presented as Fig. 3C. As shown in Fig. 3D, the CCK-8 analysis revealed that
when ZNF70 was suppressed, the growth rates of gastric cancer cells
were increased significantly compared with the negative control.
This result suggested that ZNF70 may be a tumor suppressor gene and
the suppression of ZNF70 may have an important role in
miR-1269a-mediated gastric cancer progression.
Discussion
Numerous studies have reported that miRNAs are
upregulated or downregulated in gastric cancer, indicating that
overexpressed oncogenic miRNAs (oncomiRs) or downregulated tumor
suppressor miRNAs may affect apoptosis and proliferation in gastric
cancer by inhibiting target genes (10). For example, it was reported that the
overexpression of miR-181a, an oncomiR, in a gastric cancer cell
line led to increased proliferation and inhibition of apoptosis via
repression of tumor suppressor kruppel like factor 6 (25). Furthermore, a number of recent studies
have focused on SNPs in miRNAs and have identified a number of SNPs
that contribute to gastric cancer risk, including rs3746444 (in
miR-499), rs2296616 (in miR-107) and rs2910164 (in miR-146a)
(26–28). However, the specific functions of SNPs
in miRNA remain unknown.
Apoptosis is an important cell death process that
occurs in physiological and pathological conditions. An imbalance
of the delicate relationship between physiological and pathological
conditions can lead to the development of cancer. Previously,
little is known about the effect of miR-1269a on apoptosis in
cancer. To the best of our knowledge, the present study describes
the first report of the observation that overexpression of
miR-1269a may significantly inhibit the apoptosis of gastric cancer
cells in vitro.
Additionally, it was indicated that miR-1269a
inhibited the expression of apoptosis proteins, including Bik, Bim
and Bak. Meanwhile, the low expression of miR-1269a variant had a
reverse effect of wild-type, which suggested that miR-1269a variant
suppressed the gastric cancer development by promoting the
apoptosis of gastric cancer cells.
In the present study, three databases, TargetScan
6.2, gene ontology (GO) analysis and pathway analysis, were used to
determine the target genes of miR-1269a. A total of 23 potential
target genes were obtained, and the expression levels of the target
genes were analyzed by RT-qPCR following transfection with
miR-1269a mimics in HGC-27 and MGC-803 cells (data not shown). It
was observed that there was a decreased level of ZNF70 expression
in cells transfected with miR-1269a mimics in HGC-27 and MGC-803
cells compared with cells transfected with miR-control (Fig. 3B). Additionally, the effect on ZNF70
expression was reversed in gastric cells overexpressing miR-1269a
variant mimics. Therefore, the findings indicated that ZNF70 is a
target gene of miR-1269a and ZNF70 is involved in mediating the
inhibitory effect of miR-1269a variant on gastric cancer cells.
Zinc finger proteins are a type of abundant proteins
present in eukaryotic genomes. The functions of zinc finger
proteins are extraordinarily diverse, including DNA recognition,
RNA packaging, transcriptional activation, regulation of apoptosis,
protein folding and assembly and lipid binding (29). For example, ZNF217 is an oncogenic
protein that is involved in various types of human cancer and has a
role in regulating transcription, and inducing positive
transcriptional regulation of target gene (30). ZNF70, located at human chromosome 22,
was reported to be a part of the transcription factor complex,
which determines cell fate and participates in gene regulation,
tissue differentiation and mammalian evolution (31). However, the specific function of ZNF70
in cancer remains unknown. In the present study, the findings
indicated that ZNF70 is a target gene of miR-1269a. Furthermore,
the results suggest that ZNF70 may serve as a tumor-suppressor gene
in gastric cancer. Therefore, these results indicate that ZNF70 is
involved in epigenetic regulation via miRNAs. The regulation of
ZNF70 by miR-1269a is important in tumor development and
progression and its downstream pathway would be of interest for
future studies.
In conclusion, microRNA-1269a rs73239138 has a
protective role in the susceptibility of gastric cancer. The
primary mechanism may be that a decreased expression of miR-1269a
variant increases the expression of the target tumor suppressor
gene ZNF70 and decreases the risk of gastric cancer by upregulating
apoptosis. To the best of our knowledge, the present study has, for
the first time, revealed an important association between a
miR-1269a SNP and gastric cancer progression. A potential molecular
mechanism was elucidated, and ZNF70 was identified as a target gene
of target gene of miR-1269a, which is also a tumor suppressor gene.
The present study indicates an important role in cancer development
for miRNAs and SNPs that are present in miRNAs. Furthermore, the
present study indicates that rs73239138 is a potential predictive
marker and therapeutic target for gastric cancer.
Acknowledgements
The present study was supported by grants from the
Shanghai Municipal Commission of Health and Family Planning (grant
no. 20134132), the Ministry of Science and Technology of China
(grant no. 2013CB945401), the National Natural Science Foundation
of China (grant no. 81300327) and the Ministry of Education of
China (grant no. 20130071110048), the Key Discipline Construction
Project of Pudong Health Bureau of Shanghai (grant no. PWZx2014-07)
and the Shanghai Municipal Science and Technology Commission (grant
no. 13ZR1437200).
Glossary
Abbreviations
Abbreviations:
|
miRNA
|
microRNA
|
|
FCM
|
flow cytometry
|
|
mRNA
|
messenger RNA
|
|
SNP
|
single nucleotide polymorphism
|
|
oncomiR
|
oncogenic miRNA
|
|
GO
|
gene ontology
|
|
ZNF217
|
zinc-finger protein 217
|
|
ZNF70
|
zinc-finger protein 70
|
|
NC
|
negative control
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
|
RT-qPCR
|
quantitative reverse transcription
polymerase chain reaction
|
|
siRNA
|
small-interfering RNA
|
References
|
1
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
PDQ Screening and Prevention Editorial
Board: Stomach (Gastric) Cancer Prevention (PDQ®): Health
Professional Version, . PDQ Cancer Information Summaries. National
Cancer Institute (US); Bethesda (MD): 2002
|
|
3
|
Li J, Zhang S, Liu J, Shao M and Chen L:
Review of clinical investigation on recurrence of gastric cancer
following curative resection. Chin Med J. 125:1479–1495.
2012.PubMed/NCBI
|
|
4
|
Roukos DH and Kappas AM: Limitations in
controlling risk for recurrence after curative surgery for advanced
gastric cancer are now well-explained by molecular-based
mechanisms. Ann Surg Oncol. 8:620–621. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang R and Su B: Small but influential:
The role of microRNAs on gene regulatory network and 3′UTR
evolution. J Genet Genomics. 36:1–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song JH and Meltzer SJ: MicroRNAs in
pathogenesis, diagnosis, and treatment of gastroesophageal cancers.
Gastroenterology. 143(35–47): e22012.
|
|
9
|
Mizuno K, Seki N, Mataki H, Matsushita R,
Kamikawaji K, Kumamoto T, Takagi K, Goto Y, Nishikawa R, Kato M, et
al: Tumor-suppressive microRNA-29 family inhibits cancer cell
migration and invasion directly targeting LOXL2 in lung squamous
cell carcinoma. Int J Oncol. 48:450–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song S and Ajani JA: The role of microRNAs
in cancers of the upper gastrointestinal tract. Nat Rev
Gastroenterol Hepatol. 10:109–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chung CC and Chanock SJ: Current status of
genome-wide association studies in cancer. Hum Genet. 130:59–78.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng H, Song F, Zhang L, Yang D, Ji P,
Wang Y, Almeida M, Calin GA, Hao X, Wei Q, et al: Genetic variants
at the miR-124 binding site on the cytoskeleton-organizing IQGAP1
gene confer differential predisposition to breast cancer. Int J
Oncol. 38:1153–1161. 2011.PubMed/NCBI
|
|
13
|
Saunders MA, Liang H and Li WH: Human
polymorphism at microRNAs and microRNA target sites. Proc Natl Acad
Sci USA. 104:pp. 3300–3305. 2007, View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang XW, Shen GZ, Cao LQ, Jiang XF, Peng
HP, Shen G, Chen D and Xue P: MicroRNA-1269 promotes proliferation
in human hepatocellular carcinoma via downregulation of FOXO1. BMC
Cancer. 14:9092014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bu P, Wang L, Chen KY, Rakhilin N, Sun J,
Closa A, Tung KL, King S, Kristine Varanko A, Xu Y, et al: miR-1269
promotes metastasis and forms a positive feedback loop with TGF-β.
Nat Commun. 6:68792015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Persson H, Kvist A, Rego N, Staaf J,
Vallon-Christersson J, Luts L, Loman N, Jonsson G, Naya H, Hoglund
M, et al: Identification of new microRNAs in paired normal and
tumor breast tissue suggests a dual role for the ERBB2/Her2 gene.
Cancer Res. 71:78–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma Y, Wang R, Zhang J, Li W, Gao C, Liu J
and Wang J: Identification of miR-423 and miR-499 polymorphisms on
affecting the risk of hepatocellular carcinoma in a large-scale
population. Genet Test Mol Biomarkers. 18:516–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dupuy D, Bertin N, Hidalgo CA, Venkatesan
K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, et al:
Genome-scale analysis of in vivo spatiotemporal promoter activity
in Caenorhabditis elegans. Nat Biotechnol. 25:663–668. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanehisa M, Goto S, Kawashima S, Okuno Y
and Hattori M: The KEGG resource for deciphering the genome.
Nucleic Acids Res. 32:D277–D280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi M, Horton JD, Cohen JC, Hobbs HH and
Stephens RM: WholePathwayScope: A comprehensive pathway-based
analysis tool for high-throughput data. BMC Bioinformatics.
7:302006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Draghici S, Khatri P, Tarca AL, Amin K,
Done A, Voichita C, Georgescu C and Romero R: A systems biology
approach for pathway level analysis. Genome Res. 17:1537–1545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie L, Shen Y, Franke D, Sebastian V,
Bawendi MG and Jensen KF: Characterization of indium phosphide
quantum dot growth intermediates using MALDI-TOF mass spectrometry.
J Am Chem Soc. 2016. View Article : Google Scholar
|
|
24
|
Wang Y, Tang N, Hui T, Wang S, Zeng X, Li
H and Ma J: Identification of endogenous reference genes for
RT-qPCR analysis of plasma microRNAs levels in rats with
acetaminophen-induced hepatotoxicity. J Appl Toxicol. 33:1330–1336.
2013.PubMed/NCBI
|
|
25
|
Zhang X, Nie Y, Du Y, Cao J, Shen B and Li
Y: MicroRNA-181a promotes gastric cancer by negatively regulating
tumor suppressor KLF6. Tumour Biol. 33:1589–1597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang S, Lv C, Jin H, Xu M, Kang M, Chu H,
Tong N, Wu D, Zhu H, Gong W, et al: A common genetic variation in
the promoter of miR-107 is associated with gastric adenocarcinoma
susceptibility and survival. Mutat Res. 769:35–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu B, Song P, Lu M, Wang B and Zhao Q: The
association between miR-146a gene rs2910164 polymorphism and
gastric cancer risk: A meta-analysis. Biomed Pharmacother.
68:923–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai M, Zhang Y, Ma Y, Li W, Min P, Qiu J,
Xu W, Zhang M, Li M, Li L, et al: Association between microRNA-499
polymorphism and gastric cancer risk in Chinese population. Bull
Cancer. 102:973–978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Laity JH, Lee BM and Wright PE: Zinc
finger proteins: New insights into structural and functional
diversity. Curr Opin Struct Biol. 11:39–46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cohen PA, Donini CF, Nguyen NT, Lincet H
and Vendrell JA: The dark side of ZNF217, a key regulator of
tumorigenesis with powerful biomarker value. Oncotarget.
6:41566–41581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ravasi T, Suzuki H, Cannistraci CV,
Katayama S, Bajic VB, Tan K, Akalin A, Schmeier S,
Kanamori-Katayama M, Bertin N, et al: An atlas of combinatorial
transcriptional regulation in mouse and man. Cell. 140:744–752.
2010. View Article : Google Scholar : PubMed/NCBI
|