Introduction
Osteosarcoma grows quickly and is more likely to
develop pulmonary metastasis during the early phase. Moreover,
patients are prone to multidrug resistance, so there is a certain
degree of difficulty present regarding clinical treatment (1). Although chemotherapy and surgery
techniques have made great progress in recent years, the 5-year
survival rate of osteosarcoma patients has not significantly
improved (2,3). For this reason, it is necessary to
actively explore new potentially effective treatments for
osteosarcoma. Neoadjuvant chemotherapy is a new strategy for tumor
treatment developed in recent years. Phospho-AKT (p-AKT) and heat
shock protein 70 (HSP70) are important proteins which actively
participate in the pathogenesis and development of various diseases
such as liver and gastric cancers. At present, there is no data on
the relationship between p-AKT and HSP70 protein expression levels
in osteosarcoma cells and physical tumor properties after
neoadjuvant chemotherapy. The aim of this study was to investigate
visible changes to osteosarcomas after neoadjuvant chemotherapy and
to determine whether any correlation between these changes and
osteosarcoma cell p-AKT and HSP70 expression exists, in order to
provide a theoretical basis for the clinical treatment of
osteosarcoma.
Materials and methods
General patient information
Thirty patients with osteosarcoma admitted to
Liaocheng People's Hospital between January and October, 2016 were
selected as subjects. Inclusion criteria in the study were as
follows: i) patients with osteosarcoma confirmed by pathological
examination (4); ii) patients who
received the same new auxiliary chemotherapy regimen before
operation; iii) patients and/or guardians who signed informed
consent. Exclusion criteria were as follows: i) patients who
presented osteosarcoma in combination with other cancers; ii)
patients suffering from heart, brain, and other vital organ
diseases; iii) patients who were non-compliant with treatment. The
thirty subjects selected (18 males and 12 females, aged 18–37
years, mean age 23.4±3.5 years) presented 14 cases of osteosarcoma
in the distal femur, 7 cases in the upper tibia, 5 cases in the
proximal femur, 2 cases in the iliac crest, and 2 cases in the
proximal humerus. Treatment regimen was as follows: patients
received two courses of neoadjuvant chemotherapy MMIA (6 weeks)
before operation, rested for 14 days prior to surgery, and then
resumed chemotherapy after operation. The study was approved by the
Ethics Committee of Liaocheng People's Hospital.
Imaging examinations before and after
chemotherapy
All patients received conventional X-ray
examinations before chemotherapy and after 2 courses of
chemotherapy.
Osteosarcoma imaging score
The osteosarcoma imaging was scored as outlined in
Table I, and the imaging features of
the patients before and after chemotherapy were quantified
according to the criteria presented in Table I.
 | Table I.Criteria for image scoring of
osteosarcoma patients. |
Table I.
Criteria for image scoring of
osteosarcoma patients.
| Imaging
characteristic | 0 points | 1 point | 2 points |
|---|
| Bone
calcification | None | Partial | Complete |
| Tumor boundaries | Not clear | Clear | Obviously clear |
| Bone shell
formation | None | Partially formed | Completely
repaired |
| Osteolytic
distruction area | Obvious | Partially
present | None or completely
repaired |
| Pathological
fractures | Clearly present | Callus formation in
broken ending | Healing or no
pathological fracture |
| Periosteal
reaction | Obvious | Less | None |
| Soft tissue mass | Obvious | Less | None |
Pathological examinations and
determination of p-AKT and HSP70 expression
Hematoxylin and eosin (H&E) and
immunohistochemical staining
Needle biopsies were obtained from all patients
before chemotherapy, embedded in wax blocks, and collected. Tumor
specimens were obtained from all patients during the course of the
operation after chemotherapy were also embedded in wax blocks. All
blocks were sectioned and subjected to H&E, p-AKT and HSP70
immunohistochemistry staining. p-AKT, HSP70 and other relevant
immunohistochemical kits were purchased from Shanghai Fusheng
Industrial Co., Ltd. (Shanghai, China). Sections known to present
positive-expression positive controls and phosphate-buffered saline
(PBS) negative controls were generated simultaneously.
Immunohistochemical staining evaluation
criteria
Ten casual fields of view were observed under
microscopy, with yellow or brown particles in the nuclei or
cytoplasm with clear backgrounds being considered as positive
staining. In addition, samples showing positive staining were
divided into strong staining (>95% positive cells), moderate
staining (51–95% positive cells), weak staining (2–50% positive
cells), and absence of expression (<2% positive cells)
categories (5). In this study, weak
staining and absence of expression were interpreted as negative
expression, and strong staining and moderate staining as positive
expression.
Evaluation of tumor cell necrosis rate
(TCNR)
TCNR was calculated in accordance with previously
published literature (6) for all
patients with preoperative chemotherapy.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
(IBM, Armonk, NY, USA). The data were expressed as mean ± SD. The
enumeration data were analyzed by paired samples t-tests, and
measurement data were compared using χ2 tests. The
correlation analyses between p-AKT and HSP70 expression levels,
TCNR, and image scoring were performed with χ2 tests. A
P<0.05 was considered as statistically significant.
Results
Changes in osteosarcoma
characteristics before and after chemotherapy
In this study, osteogenic changes were observed in
20 patients, osteoporosis in 6 cases, and a mixture of the two in 4
cases before chemotherapy. All patients exhibited typical
osteosarcoma osteolytic or sclerotic changes, with the tumor border
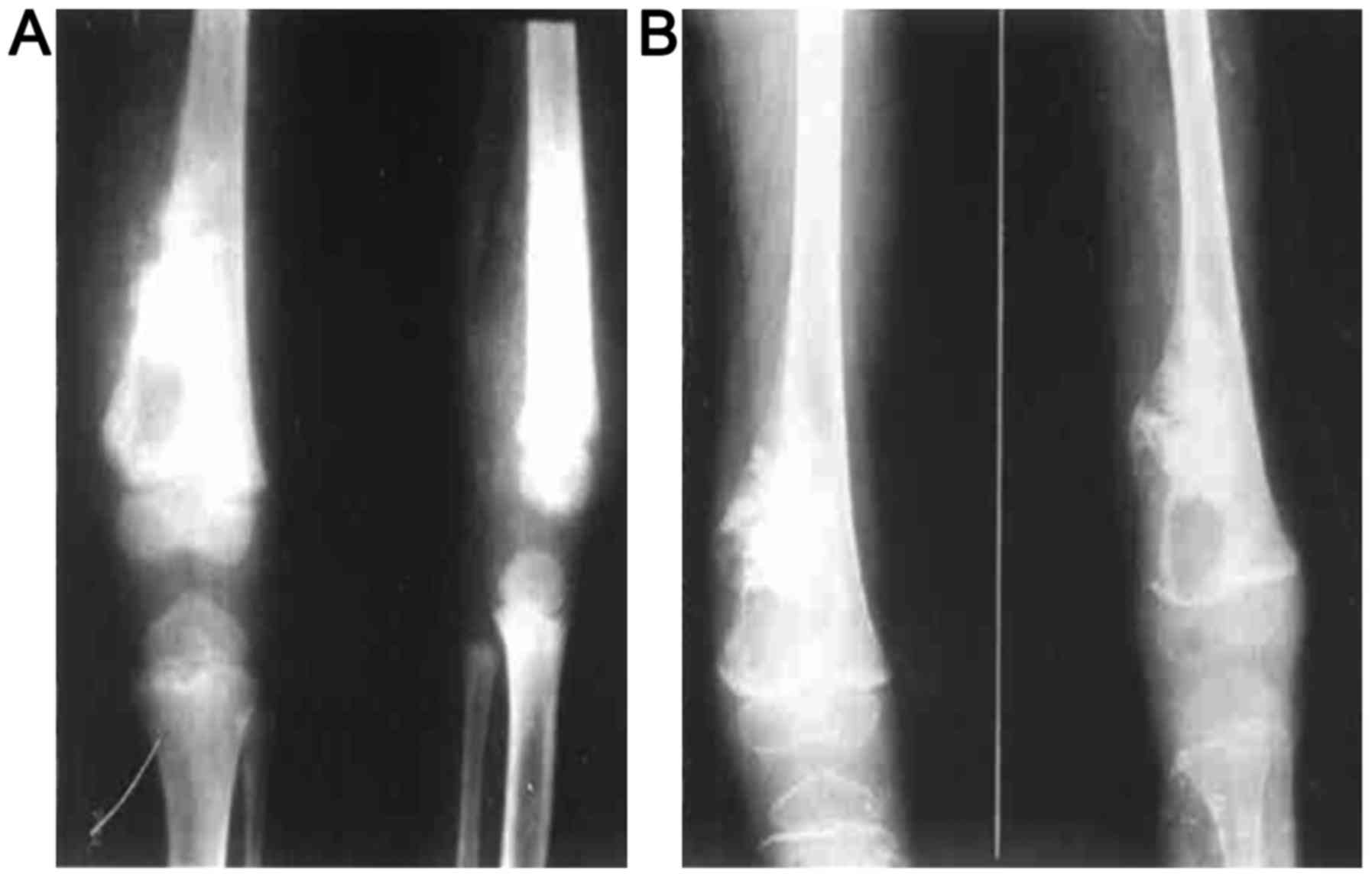
being unclear with cortical bone damage. An example of visible
changes to distal femoral osteosarcoma properties before and after
chemotherapy is shown in Fig. 1. An
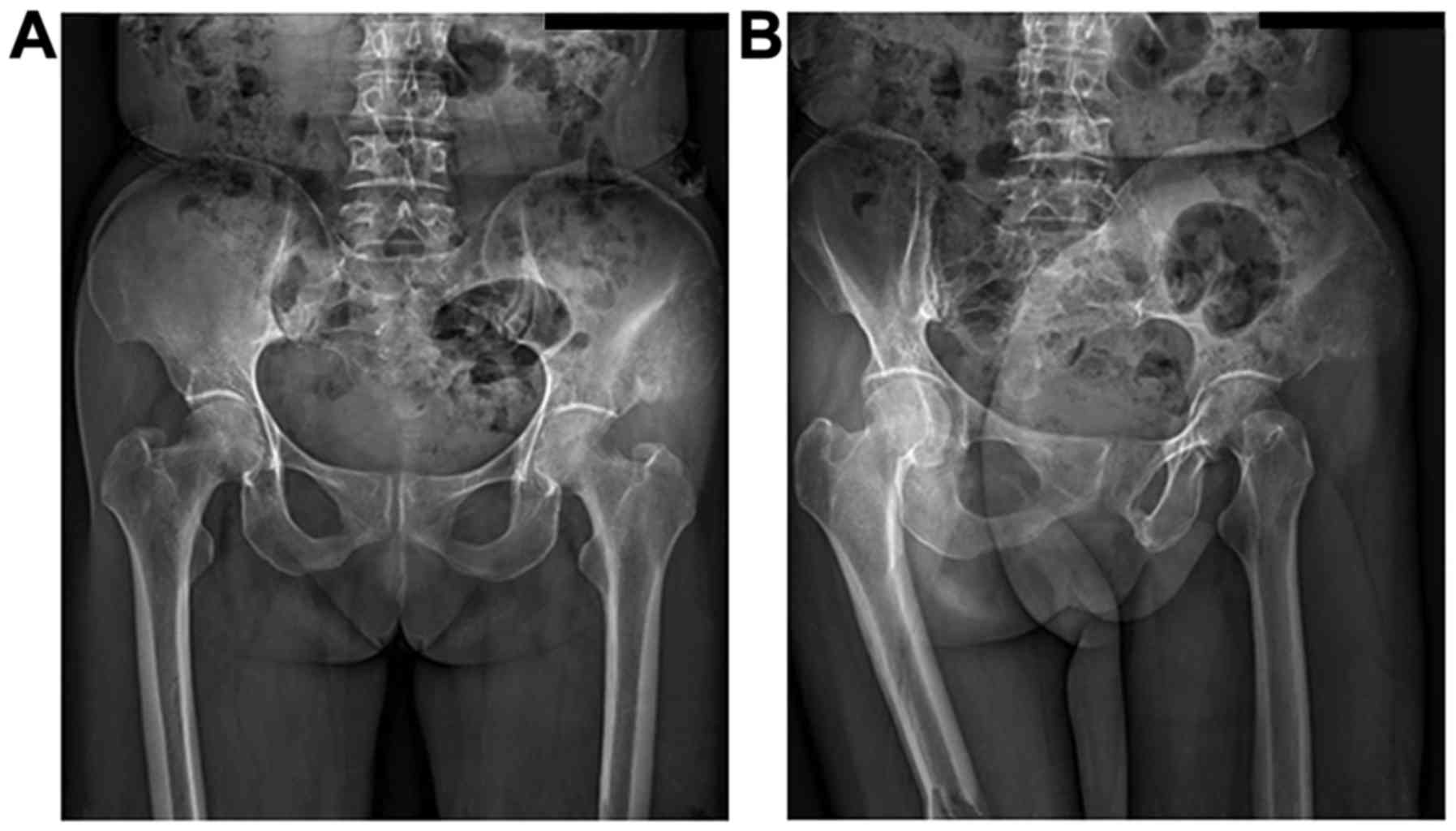
example of visible changes to iliac osteosarcoma properties are
shown in Fig. 2.
Imaging showed a significant increase in
radiographic score after chemotherapy (P<0.05) compared with
before chemotherapy (Table II).
 | Table II.Imaging scores for all 30 patients
before and after chemotherapy. |
Table II.
Imaging scores for all 30 patients
before and after chemotherapy.
|
|
Patient
number |
|---|
|
|
|
|---|
| Time-points | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
|---|
| Before
chemotherapy | 4 | 4 | 7 | 5 | 6 | 8 | 7 | 6 | 7 | 6 | 4 | 5 | 5 | 6 | 7 | 8 | 5 | 7 | 8 | 7 | 6 | 6 | 6 | 4 | 4 | 5 | 5 | 7 | 8 | 7 |
| After
chemotherapy | 8 | 7 | 9 | 10 | 11 | 12 | 10 | 9 | 11 | 10 | 8 | 8 | 7 | 12 | 10 | 11 | 8 | 11 | 12 | 10 | 9 | 9 | 10 | 8 | 8 | 9 | 8 | 10 | 12 | 11 |
The relationship between imaging score
changes and TCNR after chemotherapy
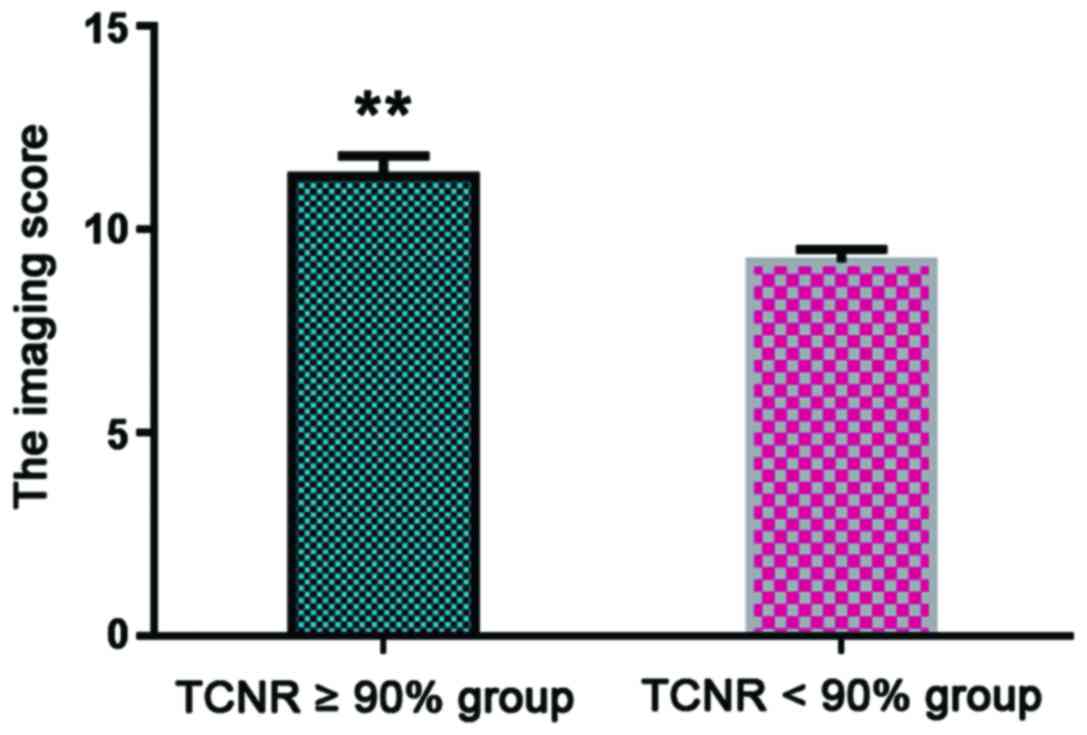
A TCNR ≥90% indicated good patient prognosis
(7). Patients were divided into TCNR
≥90% (n=21) and TCNR <90% groups (n=9), with statistical
analysis showing that the average imaging score of the TCNR ≥90%
group was 11.3±0.5 points, which was significantly higher than that
of the TCNR <90% group (8.7±0.3, P<0.05) (Fig. 3).
The relationship between the
expression of p-AKT and HSP70 in tumor cells and TCNR after
chemotherapy
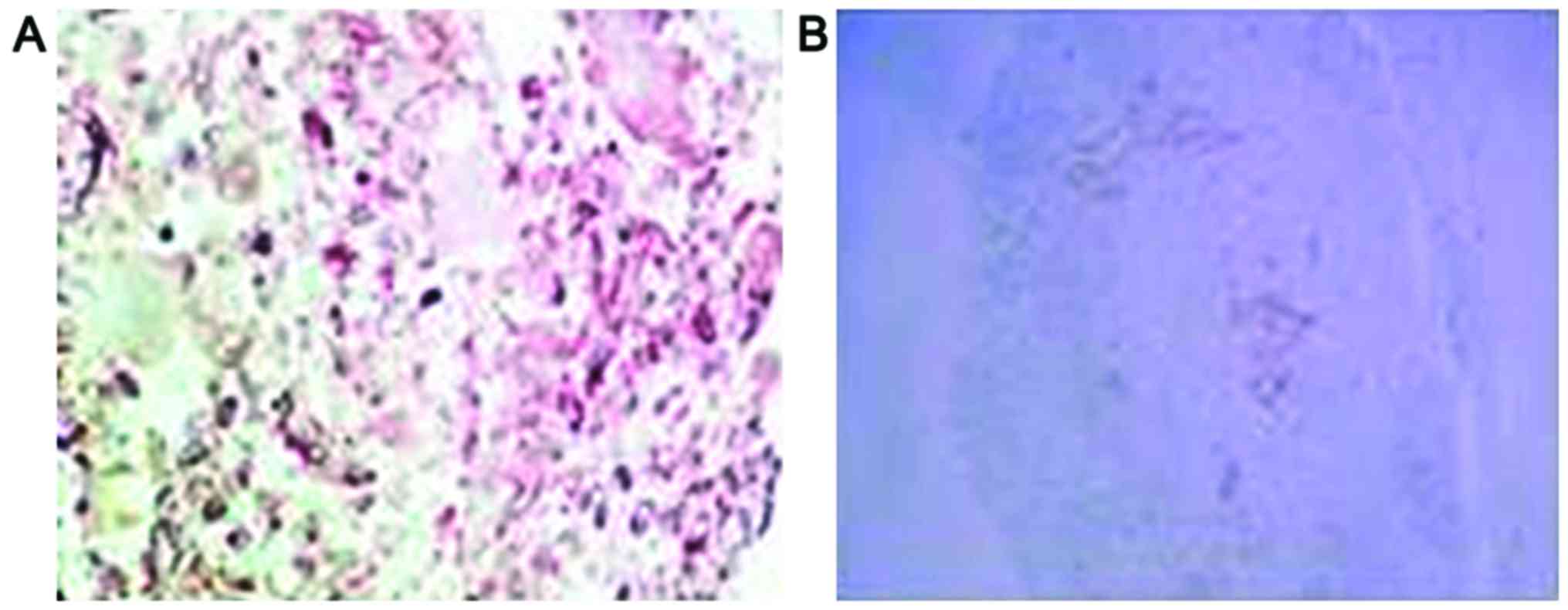
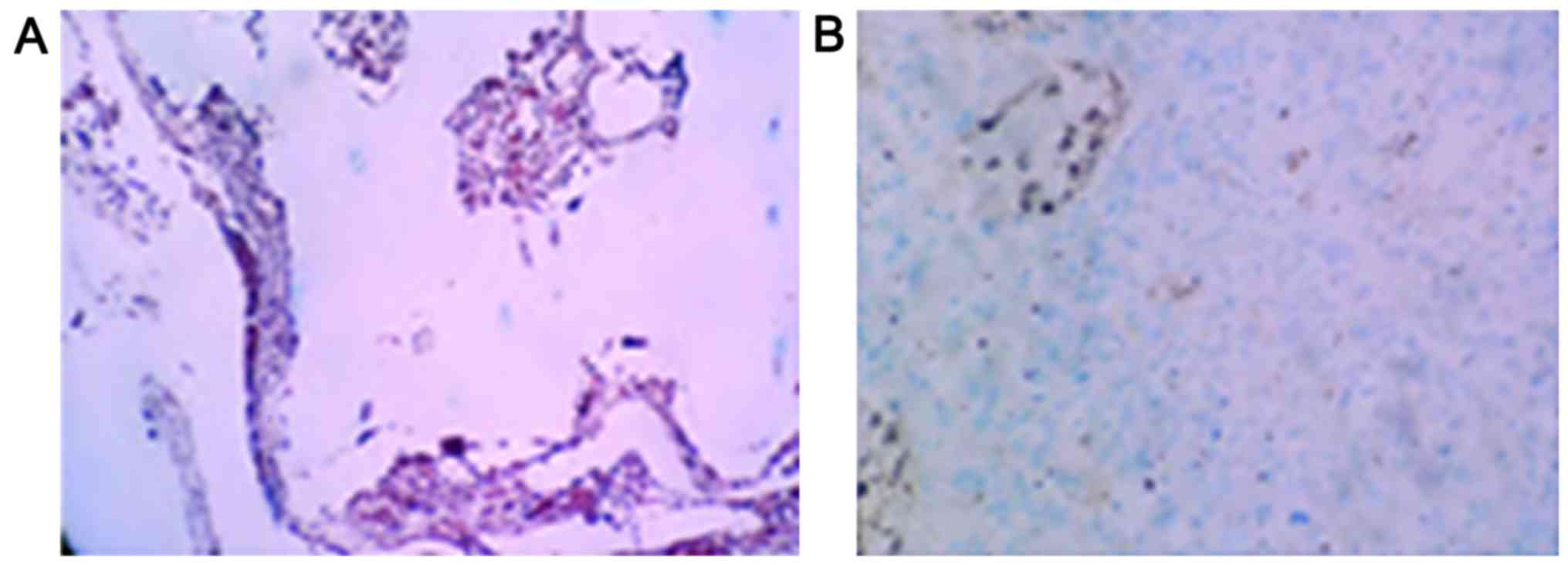
Positive staining for p-AKT and HSP70 proteins
manifested as purple particles in the cytoplasm (Figs. 4 and 5).
The positive expression rate of p-AKT in osteosarcoma cells was
13.3% (4 of 30 cases), which was significantly lower than the 73.3%
found before chemotherapy (22 of 30 cases, p<0.05). After
chemotherapy, the positive expression rate of HSP70 in osteosarcoma
cells was 6.7% (2 of 30 cases), which was significantly lower than
the 83.3% found before chemotherapy (25 of 30 cases, P<0.05)
(Table III).
 | Table III.Expression of p-AKT and HSP70 in
osteosarcoma before and after chemotherapy. |
Table III.
Expression of p-AKT and HSP70 in
osteosarcoma before and after chemotherapy.
|
|
| p-AKT | HSP70 |
|---|
|
|
|
|
|
|---|
| Time-points | n (cases) | Positive | Negative | Positive | Negative |
|---|
| Before
chemotherapy | 30 | 22 | 8 | 25 | 5 |
| After
chemotherapy | 30 | 4 | 26 | 2 | 28 |
| χ2 |
| 8.425 | 9.107 |
|
|
| P-value |
| <0.05 | <0.05 |
|
|
The expression of p-AKT and HSP70 was inversely
correlated with TCNR after chemotherapy (P<0.05). The lower the
expression of p-AKT and HSP70, the higher the patient TCNR value
(Table IV).
 | Table IV.The relationship between expression
of p-AKT and HSP70 and TCNR after chemotherapy. |
Table IV.
The relationship between expression
of p-AKT and HSP70 and TCNR after chemotherapy.
|
| p-AKT
expression | HSP70
expression |
|---|
|
|
|
|
|---|
| TCNR | Positive | Negative | Positive | Negative |
|---|
| ≥90% | 2 | 19 | 1 | 18 |
| <90% | 2 | 7 | 1 | 10 |
| χ2 | 6.128 |
| 7.603 |
|
| P-value | <0.05 |
| <0.05 |
|
Relationship between imaging score and
p-AKT and HSP70 expression after chemotherapy
Based on previous comprehensive clinical practice, a
postoperative radiographic score ≥9 points was considered as
effective chemotherapy, on the contrary, a score of <9 points
was considered ineffective (8).
Imaging score was inversely correlated with the expression of p-AKT
and HSP70 in tumor cells after chemotherapy. The higher the imaging
score, the lower the expression rates of p-AKT and HSP70 (Table V).
 | Table V.Patient imaging score and expression
of p-AKT and HSP70 after chemotherapy. |
Table V.
Patient imaging score and expression
of p-AKT and HSP70 after chemotherapy.
|
| p-AKT
expression | HSP70
expression |
|---|
|
|
|
|
|---|
| Score | Positive | Negative | Positive | Negative |
|---|
| ≥9 points | 2 | 19 | 1 | 18 |
| <9 points | 2 | 7 | 1 | 10 |
| χ2 | 6.128 |
| 7.603 |
|
| P-value | <0.05 |
| <0.05 |
|
Discussion
Neoadjuvant chemotherapy improves 5-year survival
rate for osteosarcoma (9,10). After patients with osteosarcoma
receive chemotherapy, tumor cells undergo necrosis, tumor tissue is
directly absorbed by the body, and the gaps left are replaced by
new bone (11–13), leading to increased complexity with
regards to imaging results after osteosarcoma chemotherapy. The
occurrence and development of osteosarcoma is closely related to
cellular molecular expression levels. Moreover, changes at the
molecular level are related to morphological changes which can be
detected by imaging (14–17).
This study showed that typical osteosarcoma
osteolytic or sclerotic changes combined with cortical bone damage
occurred in all 30 patients after chemotherapy, which was
consistent with previous findings (18). These changes may be related to
alterations in osteosarcoma pathogenesis. After scoring patient
images, we found that the imaging scores of all 30 patients
significantly increased after chemotherapy (P<0.05). The
radiographic score of the TCNR ≥90% group was significantly higher
than that of the TCNR <90% group (P<0.05), suggesting that
imaging score was positively correlated with TCNR, and accordingly,
with improved patient prognosis. Therefore, imaging-detected
characteristic changes can be used to predict patient chemotherapy
effectiveness, thus providing a theoretical basis for clinical
treatment (19).
AKT belongs to a class of important proteins in the
PI3K/AKT signaling pathway, and AKT activation is dependent on the
activation of upstream PI3K. p-AKT is the activated form of AKT
(20). Previous data showed (21) that abnormal expression of p-AKT was
found in laryngeal squamous cell carcinoma, gastric cancer, and
other cancers. Heat shock proteins are stress proteins which
regulate the activity and physiological function of a wide variety
of proteins. HSP70 is an important member of the heat shock protein
family (22). A previous study
suggested (23) that HSP70 was
abnormally expressed in cervical cancer, nasopharyngeal carcinoma,
colorectal, and other cancers, and involved in the occurrence and
development of cancers.
This study showed that the positive expression rate
of p-AKT in osteosarcoma cells was 13.3% (4 of 30 cases) after
chemotherapy, which was significantly lower than the 73.3% found
before chemotherapy (22 of 30 cases, P<0.05). The positive
expression rate of HSP70 in osteosarcoma cells was 6.7% (2 of 30
cases) after chemotherapy, which was significantly lower than the
83.3% found before chemotherapy (25 of 30 cases, P<0.05),
suggesting that neoadjuvant chemotherapy drugs could effectively
inhibit the proliferation of osteosarcoma cells. We also found that
the expressions of p-AKT and HSP70 were correlated with TCNR after
chemotherapy (P<0.05). The lower the expression rates of p-AKT
and HSP70 were, the higher the TCNR was, suggesting that the
expression of p-AKT and HSP70 in osteosarcoma cells after
neoadjuvant chemotherapy could be used to predict the efficacy of
neoadjuvant chemotherapy. In addition, imaging scores were
significantly correlated with the expression rates of p-AKT and
HSP70 in tumor cells. The higher the imaging score was, the lower
the expression rates of p-AKT and HSP70 were, which implied that
image analysis after neoadjuvant chemotherapy could be used to
posit the expressions of p-AKT and HSP70 in patient tumor cells,
and thus could be used to predict and evaluate the efficacy of
neoadjuvant chemotherapy.
In conclusion, osteosarcoma characteristics, as
detected by imaging, after neoadjuvant chemotherapy were closely
related to the expressions of p-AKT and HSP70 in osteosarcoma
cells. The effect of chemotherapy can be evaluated by observing the
above examination results.
References
|
1
|
Laux CJ, Berzaczy G, Weber M, Lang S,
Dominkus M, Windhager R, Nöbauer-Huhmann IM and Funovics PT: Tumour
response of osteosarcoma to neoadjuvant chemotherapy evaluated by
magnetic resonance imaging as prognostic factor for outcome. Int
Orthop. 39:97–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kubo T, Furuta T, Johan MP, Adachi N and
Ochi M: Percent slope analysis of dynamic magnetic resonance
imaging for assessment of chemotherapy response of osteosarcoma or
Ewing sarcoma: Systematic review and meta-analysis. Skeletal
Radiol. 45:1235–1242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li H, Li XY, Bu J and Xiao T: How to
explain the role of magnetic resonance imaging on evaluating tumour
response of osteosarcoma to neoadjuvant chemotherapy? Int Orthop.
39:1031–1032. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ji WP and He NB: Investigation on the DNA
repaired gene polymorphisms and response to chemotherapy and
overall survival of osteosarcoma. Int J Clin Exp Pathol. 8:894–899.
2015.PubMed/NCBI
|
|
5
|
Hagleitner MM, Coenen MJ, Gelderblom H,
Makkinje RR, Vos HI, de Bont ES, van der Graaf WT, Schreuder HW,
Flucke U, van Leeuwen FN, et al: A first step towards personalized
medicine in osteosarcoma: Pharmacogenetics as predictive marker of
outcome after chemotherapy based treatment. Clin Cancer Res.
21:3436–3441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarman H, Bayram R and Benek SB:
Anticancer drugs with chemotherapeutic interactions with
thymoquinone in osteosarcoma cells. Eur Rev Med Pharmacol Sci.
20:1263–1270. 2016.PubMed/NCBI
|
|
7
|
Li S, Sun W, Wang H, Zuo D, Hua Y and Cai
Z: Research progress on the multidrug resistance mechanisms of
osteosarcoma chemotherapy and reversal. Tumour Biol. 36:1329–1338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Zheng H, Shou T, Tang C, Miao K
and Wang P: Effectiveness of multi-drug regimen chemotherapy
treatment in osteosarcoma patients: A network meta-analysis of
randomized controlled trials. J Orthop Surg Res. 12:522017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu XZ and Mei J: Effect and mechanism
analysis of siRNA in inhibiting VEGF and its anti-angiogenesis
effects in human osteosarcoma bearing rats. Eur Rev Med Pharmacol
Sci. 19:4362–4370. 2015.PubMed/NCBI
|
|
10
|
Ahn JH, Cho WH, Lee JA, Kim DH, Seo JH and
Lim JS: Bone mineral density change during adjuvant chemotherapy in
pediatric osteosarcoma. Ann Pediatr Endocrinol Metab. 20:150–154.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Byun BH, Kim SH, Lim SM, Lim I, Kong CB,
Song WS, Cho WH, Jeon DG, Lee SY, Koh JS, et al: Prediction of
response to neoadjuvant chemotherapy in osteosarcoma using
dual-phase (18)F-FDG PET/CT. Eur Radiol. 25:2015–2024. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
1Chang KJ, Kong CB, Cho WH, Jeon DG, Lee
SY, Lim I and Lim SM: Usefulness of increased 18F-FDG uptake for
detecting local recurrence in patients with extremity osteosarcoma
treated with surgical resection and endoprosthetic replacement.
Skeletal Radiol. 44:529–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Avril P, Le Nail LR, Brennan MÁ, Rosset P,
De Pinieux G, Layrolle P, Heymann D, Perrot P and Trichet V:
Mesenchymal stem cells increase proliferation but do not change
quiescent state of osteosarcoma cells: Potential implications
according to the tumor resection status. J Bone Oncol. 5:5–14.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai S, Zhang T, Zhang D, Qiu G and Liu Y:
Volume-sensitive chloride channels are involved in cisplatin
treatment of osteosarcoma. Mol Med Rep. 11:2465–2470. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo J, Glass JO, McCarville MB, Shulkin
BL, Daryani VM, Stewart CF, Wu J, Mao S, Dwek JR, Fayad LM, et al:
Assessing vascular effects of adding bevacizumab to neoadjuvant
chemotherapy in osteosarcoma using DCE-MRI. Br J Cancer.
113:1282–1288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao X, Wang W, Zhang H, Gao P, Fan B,
Huang C, Fu J, Chen G, Shi L, Zhu H, et al: Individualized
chemotherapy for osteosarcoma and identification of gene mutations
in osteosarcoma. Tumour Biol. 36:2427–2435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JA, Jeon DG, Cho WH, Song WS, Yoon HS,
Park HJ, Park BK, Choi HS, Ahn HS, Lee JW, et al: Higher
gemcitabine dose was associated with better outcome of osteosarcoma
patients receiving gemcitabine-docetaxel chemotherapy. Pediatr
Blood Cancer. 63:1552–1556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katagiri H, Sugiyama H, Takahashi M,
Murata H, Wasa J, Hosaka S and Miyagi M: Osteosarcoma of the pelvis
treated successfully with repetitive intra-arterial chemotherapy
and radiation therapy: A report of a case with a 21-year follow-up.
J Orthop Sci. 20:568–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kleinerman E: Maximum benefit of
chemotherapy for osteosarcoma achieved-what are the next steps?
Lancet Oncol. 17:1340–1342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang X, Jiang Y, Feng L, Chen H, Zhen C,
Ding M and Wang X: Blockade of PI3K/AKT pathway enhances
sensitivity of Raji cells to chemotherapy through down-regulation
of HSP70. Cancer Cell Int. 13:482013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chatterjee M, Andrulis M, Stühmer T,
Müller E, Hofmann C, Steinbrunn T, Heimberger T, Schraud H,
Kressmann S, Einsele H, et al: The PI3K/Akt signaling pathway
regulates the expression of Hsp70, which critically contributes to
Hsp90-chaperone function and tumor cell survival in multiple
myeloma. Haematologica. 98:1132–1141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boon E, van der Graaf WT, Gelderblom H,
Tesselaar ME, van Es RJ, Oosting SF, de Bree R, van Meerten E,
Hoeben A, Smeele LE, et al: Impact of chemotherapy on the outcome
of osteosarcoma of the head and neck in adults. Head Neck.
39:140–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan T, Li X, Xie W, Jankovic J and Le W:
Valproic acid-mediated Hsp70 induction and anti-apoptotic
neuroprotection in SH-SY5Y cells. FEBS Lett. 579:6716–6720. 2005.
View Article : Google Scholar : PubMed/NCBI
|