Introduction
Concurrent cisplatin-based chemoradiation is
considered the standard treatment for locally advanced cervical
cancer, with the aim of improving local control and overall
survival (OS). However, Lin et al (1) identified that a sizeable proportion of
patients with cervical cancer exhibit para-aortic lymph node (PALN)
involvement, with 14% of International Federation of Gynecology and
Obstetrics stage (2) I, 20% of stage
II and 32% of stage III cancer cases involved with the para-aortic
lymph nodes. Thus, patients treated with standard pelvic field
irradiation possess shortened 5-year disease-free survival (DFS)
times (3). The Radiation Therapy
Oncology Group (RTOG) 79–20 trial demonstrated that prophylactic
para-aortic irradiation using anteroposterior fields may improve OS
and decrease distant metastasis for patients with cervical cancer
with bulky tumors or stage IIB disease (4). However, the cumulative incidence of
grade 4 and 5 toxicities, and the proportion of mortalities as a
result of radiotherapy complications are considerably increased for
EF irradiation compared with pelvic irradiation (4). Therefore, the therapeutic value of
prophylactic irradiation for PALNs remains unclear in conventional
radiotherapy.
Although intensity-modulated radiotherapy (IMRT) is
used to decrease toxicity, treatment of the para-aortic nodal
regions has inherent toxicity. Furthermore, cervical cancer appears
to exhibit an orderly pattern of spread, initially involving the
low pelvic lymph nodes prior to progressing to the high pelvic
lymph nodes and PALNs (3). In
addition, IMRT costs are covered by national insurance in China,
which decreases the financial burden on patients. Therefore, since
January 2011, EF-IMRT has been electively performed for patients
with cervical cancer with common iliac node or PALN involvement,
and pelvic IMRT has been electively performed for patients with or
without low pelvic nodal metastasis. Overall, the aim of the
present study was to evaluate the efficacy and toxicities of
EF-IMRT vs. pelvic IMRT.
Materials and methods
Patients
A total of 181 patients (mean age, 56.3 years;
range, 27–83) with cervical squamous-cell cancer who were treated
with IMRT and high-dose-rate brachytherapy (HDR BT) at the Sun
Yat-Sen University Cancer Center (Guangzhou, China) between March
2011 and May 2013 were retrospectively reviewed. Among them, 74
patients were excluded for meeting any one of the following
criteria: i) Stage IVB disease; ii) distant nodal metastasis in
inguinal, mediastinal or supraclavicular lymphatics; iii) treatment
with salvage, palliative or adjuvant intent; or iv) incomplete
radiotherapy due to patient refusal or poor performance status. The
Institutional Review Board of the Sun Yat-Sen University Cancer
Center approved the present study.
Among the remaining patients, 55 and 52 patients
were treated with EF-IMRT and pelvic IMRT, respectively. All
patients underwent abdominal and pelvic magnetic resonance imaging
(MRI) to assess nodal metastasis and tumor size. As a result of
economic factors, the majority of patients underwent a chest X-ray
and color ultrasound of the supraclavicular region to evaluate the
distant metastasis instead of a positron emission tomography (PET)
and chest computed tomography (CT) scan. The patient
characteristics of the two groups are presented (Table I).
 | Table I.Patient characteristics for
extended-field irradiation and pelvic irradiation. |
Table I.
Patient characteristics for
extended-field irradiation and pelvic irradiation.
| Characteristics | Extended-field
(n=55) | Pelvic (n=52) | P-value |
|---|
| Mean age (range) | 55.1 (27–80) | 59.4 (38–83) | 0.048 |
| FIGO stage, n |
|
| 0.82b |
| I | 4 | 5 |
|
| II | 28 | 28 |
|
| III | 23 | 19 |
|
| Tumor size,
cma | 4.95±1.57 | 4.55±1.80 | 0.21 |
| Vaginal invasion,
n |
|
| 0.59b |
| None | 7 | 7 |
|
| Upper
1/3 | 33 | 34 |
|
| Middle
1/3 | 9 | 9 |
|
| Distal
1/3 | 6 | 2 |
|
| Uterus invasion,
n |
|
| 0.12b |
| No | 19 | 26 |
|
| Yes | 36 | 26 |
|
| Highest level of
involved lymph nodes, n |
|
|
<0.001b |
| None | 0 | 27 |
|
| Lower
pelvic nodes | 0 | 25 |
|
| Common
iliac nodes | 44 | 0 |
|
| PALN | 11 | 0 |
|
| Nadir-Hgb,
g/la | 96.9±20.6 | 100.3±16.1 | 0.36 |
| Cumulative dose of
weekly cisplatin, mg/m2a | 128.8±106.8 | 130.8±120.2 | 0.93 |
| Treatment duration,
daysa | 69.2±22.2 | 63.4±19.9 | 0.16 |
| Total dose of D90
(EQD2, Gy)a | 94.7±7.3 | 95.7±7.1 | 0.44 |
| Tumor response,
n |
|
| 0.89b |
| CR | 45 | 42 |
|
| PR | 10 | 10 |
|
Treatment
All patients were treated with a combination of IMRT
and HDR BT. The IMRT process incorporated CT-based simulation,
performed with a full bladder and an empty rectum, and each patient
underwent a planning CT scan from the upper border of T10 to the
ischialtuberosity, with a 3-mm slice thickness (Philips Medical
Systems, Inc., Bothell, WA, USA). The target volumes and organs at
risk, including the spinal cord, bowel, kidneys, bladder, rectum
and femoral heads, were drawn on each planning CT slice (5). Involved lymph nodes (a short-axis
diameter on CT/MRI of >1 cm) were contoured as gross tumor
volume (GTV-N). The cervical tumor and uterus were contoured as
high-risk CTV (HR-CTV), and the doses were increased in BT, but not
in external-beam radiotherapy.
Pelvic irradiation
The clinical target volume (CTV) included all areas
of gross and potentially microscopic diseases. The pelvic CTV
consisted of a 0.7 to 2-cm margin around the vessels, cervix,
uterus, parametria, presacral space and vagina (6). The inguinal region was drawn as part of
the CTV when the distal vagina or inguinal lymph nodes was
considered to be involved, according to imaging and clinical
examination.
EF irradiation
Patients with evidence for positive involvement of
para-aortic or high common iliac nodes were treated with EFR to the
superior border of the first lumbar vertebra. The CTV in the
para-aortic region was contiguous with the pelvic lymph node
stations and encompassed the aorta and inferior vena cava with a 1-
to 1.5-cm minimum margin (7).
The GTV-N was expanded by 0.5 cm to create the
planning target volume of the GTV-N (PTVGTV-N) and the CTV was
expanded by 0.6–1 cm to create the PTVCTV, accounting for patient
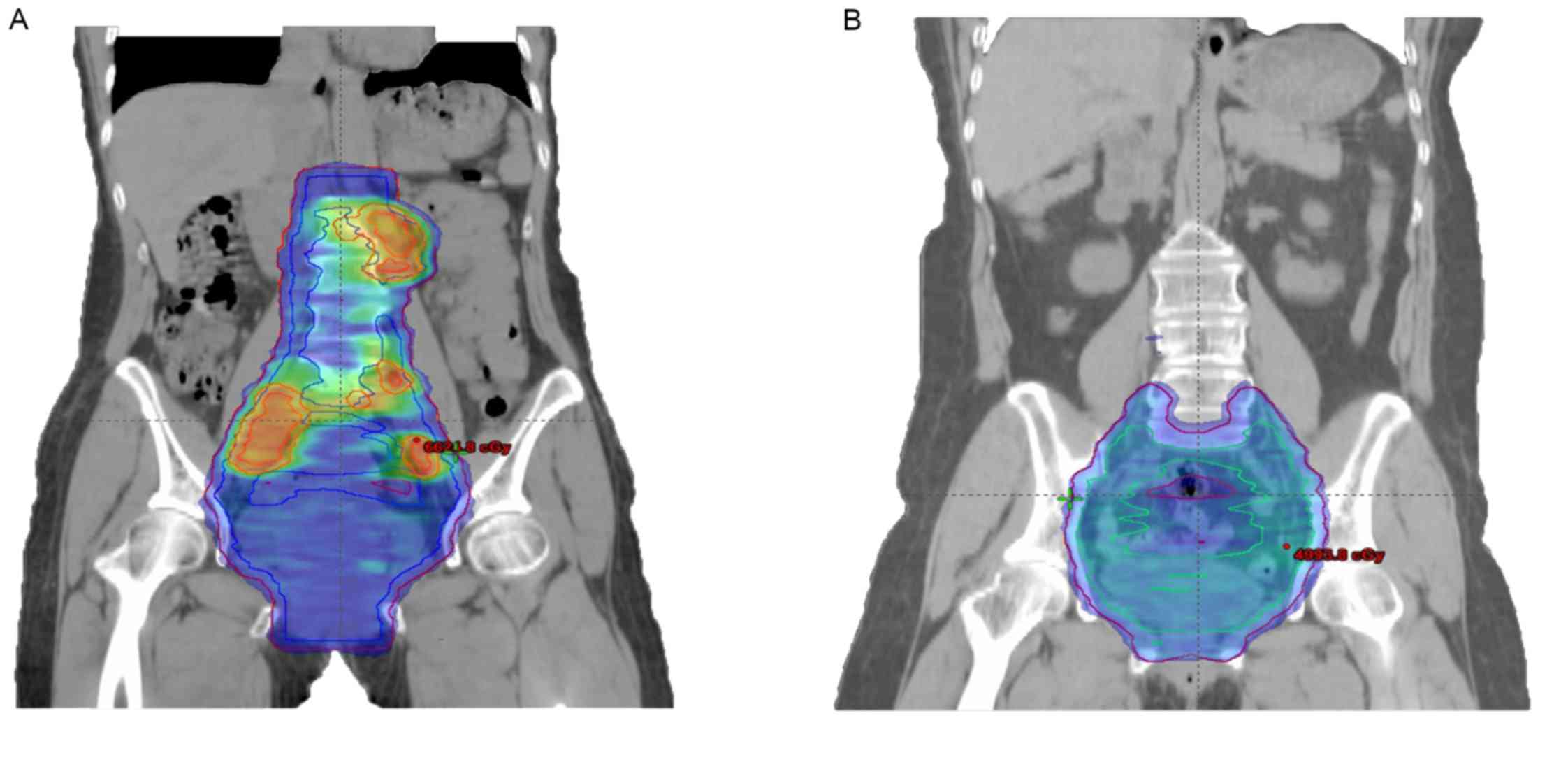
motion and set-up uncertainty. The CTV dose was 45 Gy in 25
fractions, with a concomitant boost of GTV-N to a dose of 60 Gy in
25 fractions (Fig. 1A and B).
HDR BT
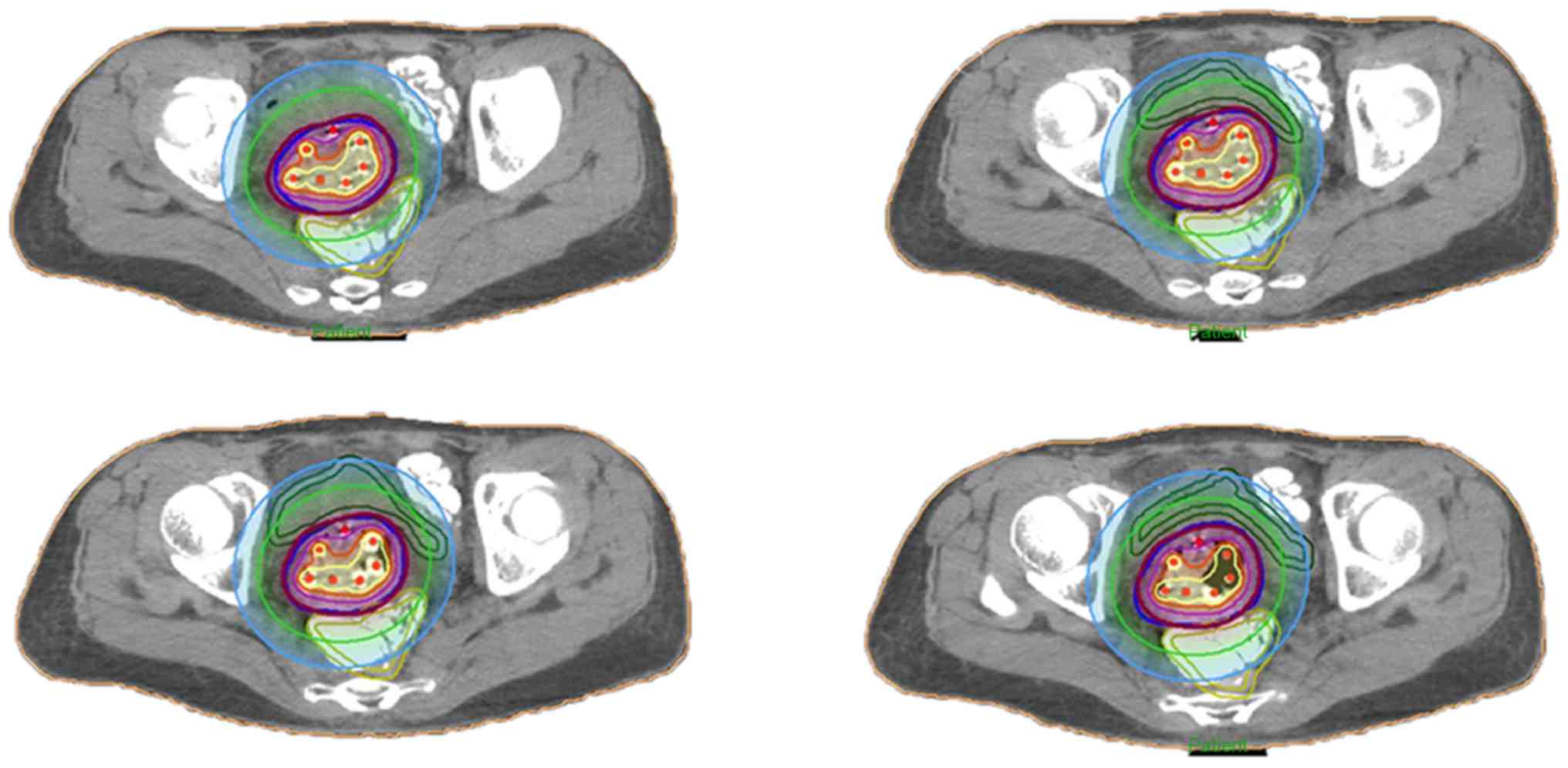
The median BT dose was 36 Gy in 6 fractions to the
periphery of the HR-CTV, with a combined intracavitary/interstitial
technique. The prescribed dose to the periphery of the HRCTV was 6
Gy. The radiation source (192Ir) dwell time was adjusted
using graphic optimization until the dose-volume constraints were
optimally matched (Fig. 2). The
planning aimed to deliver a minimum of 85 Gy to 90% of the HR-CTV
in 2 Gy equivalent (EqD2), adding BT and external beam radiotherapy
doses, and applying the linear quadratic model with an α/β ratio of
10 Gy.
Chemotherapy
It was planned that the patients would receive
cisplatin (40 mg/m2) via intravenous infusion every week
for 6 weeks during the course of external beam radiotherapy, and
that patients with the following conditions would not receive
concurrent chemotherapy: i) Elderly age (>70 years); ii)
Performance Status score >2 (8);
or iii) rejection of chemotherapy. Complete blood count tests were
performed weekly and chemotherapy was withheld until resolution to
at least grade 1 if patients presented with grade 3 or 4
hematological or gastrointestinal toxicity.
Follow-up
All patients were examined at 1 month
post-radiotherapy, every 3 months during the first and second
years, and every 6 months in the third year of follow-up. Follow-up
investigations included clinical examination, Papanicolau smears,
serum tumor marker (squamous cell carcinoma antigen) analysis and
cross-sectional imaging (pelvic MRI and abdominal CT). Chest CT
examination, supraclavicular lymph node ultrasound examination and
bone scintigraphy were performed once a year to evaluate distant
recurrence (lung, supraclavicular nodes and bone).
Follow-up abdominal CT and pelvic MRI were performed
between 1 and 2 months after the completion of radiotherapy to
evaluate the response to therapy. Complete response was defined as
images and clinical examination resultsidentifying no evidence of
local or regional nodal disease. Partial response was defined as
any persistence of tumor at the site of local or regional nodes on
the axial scan within 3 to 6 months after completion of
radiotherapy. Local failure was defined as the recurrence or
residual disease at the cervix, uterus or adjacent pelvic organs,
e.g., parametria, bladder and vagina. Regional nodal relapse was
defined as residual or recurrent, cancer in the pelvic or
para-aortic lymph nodes (if the distal vagina was involved,
inguinal lymph nodes were also considered regional relapse;
otherwise, the nodes were considered distant metastases). Distant
failure was defined as recurrence in non-regional lymph nodes
(mediastinal and supraclavicular region) or hematogenous metastasis
(including in the bones, liver and lungs). Failure was recorded on
the basis of clinical examination and follow-up imaging (MRI/CT or
PET/CT), and the majority of patients received biopsy
confirmation.
Acute toxicity associated with radiotherapy was
assessed in accordance with the RTOG acute radiation morbidity
scoring criteria (9) and was defined
as toxicity occurring between the initiation of treatment and 90
days after completion. Acute toxicity was assessed weekly during
the course of radiotherapy, at the completion of RT and after 1
month; late effects were evaluated at each clinical visit. Adverse
events for >90 days after the completion of treatment were
graded in accordance with the RTOG late radiation morbidity scoring
system (10).
Statistical analysis
Survival time and time to recurrence were measured
from the date of initial radiation treatment. An unpaired t-test or
χ2 test was used to analyze the associations between
patient characteristics, recurrence patterns and toxicities. The
Kaplan-Meier estimator method was used to derive estimates of
survival. Differences in OS and DFS were assessed using the
log-rank test. OS time was calculated from the date of RT start to
the date of mortality from any cause or last follow-up. The DFS
time was calculated from the date of RT start to the date of
disease progression, relapse or initiation of any new, unplanned,
anticancer therapies associated with the disease.
Disease-associated significant variables on univariate analysis
were utilized for Cox's regression analysis to control the
confounding factors for OS and DFS. P<0.05 was considered to
indicate a statistically significant difference for all study
outcomes. All statistical analyses were performed using SPSS
(version 17.0; SPSS, Inc., Chicago, IL, USA). Results are presented
as the mean ± standard deviation.
Results
Survival
The total length of time for the two treatments
ranged from 49 to 104 days (median, 61 days). The median duration
of follow-up was 29.5 months (range, 7.4 to 50.6 months). A total
of 19 out of the 107 patients included in the present study
succumbed, including 5 of whom succumbed to concomitant disease (1
cerebral infarction, 1 heart failure, 1 myocardial infarction and 2
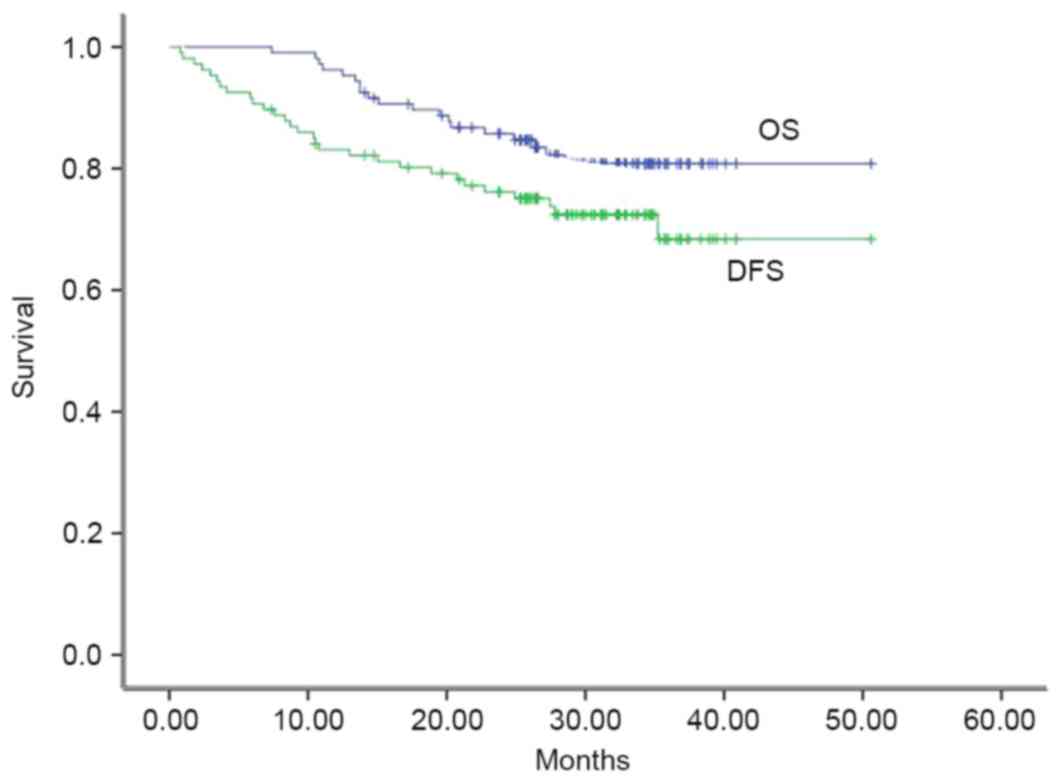
severe pulmonary infections). The mean survival time was 46.1
months, and the 2- and 3-year OS rates were 87.7 and 80.7%,
respectively. A total of 29/107 patients (27.1%) exhibited failure
at either local, regional node or distant sites. The mean DFS time
was 41.2 months, and the 2- and 3-year DFS rates were 77.1 and
72.4%, respectively. The OS and DFS rates are presented (Fig. 3).
A total of 11 out of the 55 patients treated with
EF-IMRT succumbed. The mean survival time was 35.0±1.40 months, and
the 2- and 3-year OS rates were 81.5 and 79.4%, respectively. A
total of 16 patients developed recurrence and the mean DFS was
31.2±1.93 months. The 2- and 3-year DFS rates were 73.7 and 61.0%,
respectively.
A total of 8 patients treated with pelvic IMRT
succumbed during the follow-up period. The mean survival time for
pelvic irradiation was 45.4±1.67 months, and the 2- and 3-year OS
rates were 90.2 and 82.3%, respectively. A total of 13 patients
experienced failure and the mean DFS time was 40.7±2.42 months. The
2- and 3-year DFS rates for patients treated with pelvic IMRT were
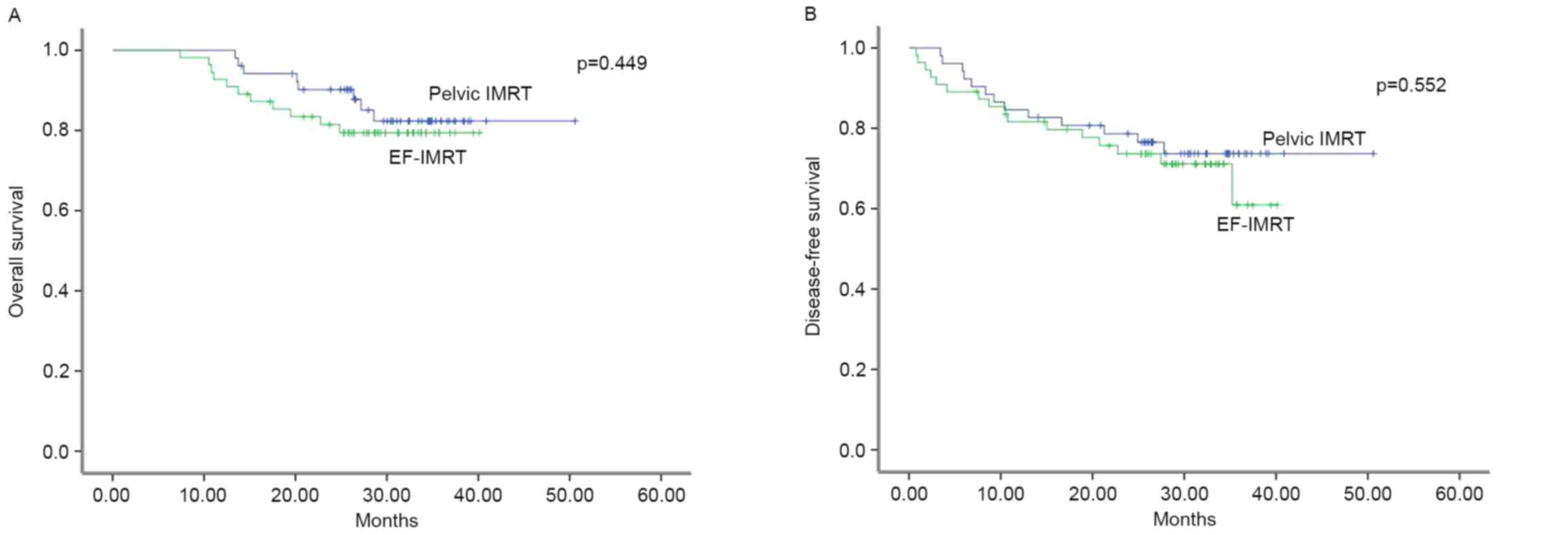
78.3 and 73.7%, respectively. The differences in OS and DFS between
EF-IMRT and pelvic IMRT patients were assessed using the log-rank
test and no statistically significant differences were identified
(Fig. 4A and B).
The univariate analysis revealed that patients with
bulky tumors, a decreased nadir for hemoglobin concentration,
increased treatment duration or increased level of LN metastasis
were associated with diminished survival rates (Table II). The irradiation fields (EFor
pelvic field) were entered and significant variables on univariate
analysis were utilized for Cox's regression analysis to evaluate
the effect of irradiation field on survival (Table III). It was revealed that patients
treated with EF irradiation exhibited an improved prognosis
compared with those treated with pelvic field when confounding
factors, including the nadir-hemoglobin level, the most distant
level of lymph nodes involved, and treatment duration, were
controlled.
 | Table II.Univariate analysis for OS and
DFS. |
Table II.
Univariate analysis for OS and
DFS.
|
| 3-year OS | 3-year DFS |
|---|
|
|
|
|
|---|
|
Characteristics | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Tumor size | 1.323 | 1.021–1.713 | 0.034 | 1.340 | 1.078–1.666 | 0.008 |
| Nadir-Hgb | 0.976 | 0.954–0.999 | 0.039 | 0.975 | 0.956–0.993 | 0.008 |
| Treatment
duration | 1.024 | 1.009–1.041 | 0.003 | 1.016 | 1.001–1.030 | 0.031 |
| Level of LN
involved | 1.322 | 1.150–1.520 | 0.042 | 1.240 | 1.114–1.381 | 0.045 |
| Irradiation field
(extended vs. pelvic) | 1.419 | 0.571–3.529 | 0.451 | 1.248 | 0.600–2.597 | 0.553 |
 | Table III.Cox's regression for 3-year OS and
DFS. |
Table III.
Cox's regression for 3-year OS and
DFS.
|
| 3-year OS | 3-year DFS |
|---|
|
|
|
|
|---|
|
Characteristics | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Tumor size | 1.623 | 1.238–2.127 | 0.024 | 1.276 | 1.017–1.601 | 0.036 |
| The nadir Hgb | 0.983 | 0.970–0.0996 | 0.016 | 0.977 | 0.958–0.997 | 0.025 |
| The level of LN
metastasis | 1.795 | 1.554–2.073 | 0.039 | 1.510 | 1.353–1.685 | 0.005 |
| Irradiation
fielda (extended vs.
pelvic) | 0.767 | 0.590–0.996 | 0.011 | 0.874 | 0.783–0.976 | 0.043 |
| Treatment
duration | 1.024 | 1.008–1.041 | 0.003 | 1.006 | 0.984–1.028 | 0.094 |
Recurrent patterns
Of the 16 patients treated with EF-IMRT and who
developed recurrence, 7 patients presented with more than one site
of failure. A total of 7 of the 55 EF-IMRT patients (12.7%)
developed local failure, 6 (10.9%) patients developed regional
relapse (3 developed in para-aortic nodes) and 9 (16.4%) patients
exhibited distant metastasis. Among the patients treated with
pelvic IMRT, 13 patients relapsed and 4 patients presented more
than one site of failure. A total of 6 of the 52 pelvic IMRT
patients (11.5%) developed local failure, 3 (5.8%) developed
regional failures (2 developed in para-aortic nodes) and 6 (11.5%)
exhibited distant metastasis. No significant differences in the
failure rate or proportion of recurrent sites between the two
groups were identified in the patients (P=0.67 and P=0.88,
respectively).
Toxicities
Acute toxicities are presented in Table IV. The two treatments were
well-tolerated, with 19 (34.5%) and 10 (19.2%) patients
experiencing grade 3 or greater acute toxicities for EF-IMRT and
pelvic IMRT, respectively (P=0.048, Fisher's exact test). For
EF-IMRT, 2 patients experienced severe nausea or vomiting, and 3
patients experienced severe diarrhea, which required
pharmacological intervention. Of the 14 patients with grade 3 or
greater hematological toxicities, 4 patients developed anemia and
required a blood transfusion, and 10 patients with grade 3 or 4
leukopenia also presented with neutropenia. Of the patients treated
with pelvic IMRT, 2 patients exhibited severely altered bowel
habits, 2 possessed nadir-hemoglobin <70 g/l (normal range,
110–130 g/l) and 5 patients developed grade 3 leukopenia
accompanied with neutropenia.
 | Table IV.Acute toxicities according to the
Radiation Therapy Oncology Group acute radiation morbidity scoring
criteria. |
Table IV.
Acute toxicities according to the
Radiation Therapy Oncology Group acute radiation morbidity scoring
criteria.
|
| EF-IMRT, n (%) | Pelvic IMRT, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Toxicity | Grade 3 | Grade 4 | Grade 3 | Grade 4 | P-value |
|---|
|
Gastrointestinal |
|
|
|
| 0.44a |
| Upper
GI | 2 (3.6) | 0 | 0 | 0 | 0.50a |
| Lower
GI | 3 (5.5) | 0 | 2 (3.8) | 0 | 0.439a |
| Hematological |
|
|
|
| 0.18 |
|
Hgb/Hct | 4 (7.3) | 0 | 2 (3.8) | 0 | 0.68a |
|
WBC | 8 (14.5) | 2 (3.6) | 5 (9.6) | 0 (0.0) | 0.27 |
| Neutrophils | 7 (12.7) | 2 (3.6) | 4 (7.7) | 1 (1.9) | 0.58 |
|
Platelets | 0 | 0 | 0 | 0 |
|
|
Genitourinary | 0 | 0 | 0 | 0 |
|
As for late toxicities, 5 patients treated with
EF-IMRT and 4 treated with pelvic IMRT experienced grade 3 rectal
bleeding requiring transfusion or surgery, and 1 patient treated
with pelvic IMRT was diagnosed with grade 4 rectovaginal fistula as
a result of local recurrence.
Discussion
Salama et al (10) reported that IMRT planning improves
dosimetry, decreases the volume of normal tissue irradiated, and
exhibits favorable local control and decreased acute
gastrointestinal, genitourinary and bone marrow toxicities in
comparison with conventional treatment. EFRT is conventionally
indicated for patients with cervical cancer with grossly detected
common iliac or PALN metastasis on the basis of orderly spread
patterns and decreased rates of skip metastasis (11). Therefore, patients with cervical
cancer in the Sun Yat-Sen University Cancer Center received EF-IMRT
when common iliac nodes or PALN were involved, and pelvic IMRT when
negative nodes or lower pelvic nodes were involved.
Wu et al (12)
retrospectively analyzed the data of 55 patients with PALN-positive
cervical cancer treated with (27 patients) or without (28 patients)
EFRT, and revealed that the 3-year OS rates were ~50 and 23%,
respectively (12). The results from
another study on 39 patients with grossly involved common iliac
nodes or PALN treated with EFRT demonstrated that the 3-year OS and
DFS rates were 45 and 23% (13),
which is inferior to the patients in the current study treated with
EFRT (79.4 and 61.1%). In the aforementioned studies, EBRT was
delivered to the pelvis and para-aortic regions using four-field or
antero-posterior/postero-anterior (AP/PA) field techniques
(12,13). The inferior survival rate was
attributed to the limitation of conventional radiotherapy in dose
escalation to positive periaortic nodes due to concerns of toxicity
to adjacent critical structures (14). According to a previous dosimetric
publication, grossly involved PALNs may be treated with a
simultaneous integrated boost (SIB) to 60 Gy while limiting dose to
the small bowel, bone marrow and kidney using IMRT (15). Use of a SIB results in a decrease in
overall treatment time, limiting tumor repopulation, with delivery
of an increased dose per fraction resulting in an increased
biologically equivalent dose and potentially resulting in a higher
rate of local control (16).
As presented in Table
I, pretreatment characteristics were well-balanced across the
two treatment groups, with the exception of age and the extent of
lymph node involvement (common iliac nodes or PALN vs. negative or
lower pelvic nodes). Lymph node status in patients with cervical
cancer is an important determinant of prognosis. Furthermore, the
most distant level of lymph node involvement influenced the
cervical cancer survival outcome (17). As such, an increased level of lymph
node involvement predicts poorer survival, which support the
univariate analysis results of the present study. Therefore,
patients in the EF-IMRT group of the present study were
hypothesized to present with poorer survival rates than patients in
the pelvic IMRT group if the same irradiation volume of external
beam radiotherapy was delivered. However, these OS and DFS rates
were not significantly different between the two groups
(P>0.05), and Cox's regression analysis indicated that
EFirradiation was a protective prognostic factor for OS and DFS
time, demonstrating that EF-IMRT is an efficient treatment for
patients with uterine cervical cancer with involved common iliac
nodes or PALNs to eradicate lymphatic micrometastasis and cure
grossly involved PALNs.
Locoregional failure is known to be the predominant
site of failure in patients treated with curative intent using
radiotherapy alone (18), and a
markedly increased rate of distant failure was previously
documented in patients treated with concurrent chemoradiotherapy
(11). However, in the present study,
the locoregional failure rate was increased compared with the
distant failure rate, potentially due to the fact that: i) Only 72
patients received concurrent chemotherapy, which may sensitize
tumor cells to radiation, further enhance shrinkage of the primary
tumor and achieve favorable locoregional control; ii) the present
study had a shorter duration of follow-up; iii) para-aortic node
relapse was defined as distant failure in previous studies and
defined as regional failure in the present study; and iv) the size
criterion for detecting LN of >1 cm was employed, which may fail
to deliver a boosted dose to the involved nodes with a short
diameter of <1 cm, and lead to increased regional failure
rates.
Kidd et al (3)
reviewed the clinical data of 560 patients with cervical cancer who
underwent pretreatment PET/CT staging and concluded that the risk
of recurrence increased incrementally on the basis of the most
distant level of nodal involvement, with a hazard ratio of 2.40 for
pelvic nodes and 5.88 for para-aortic nodes. However, the failure
rate and the proportions of failure sites did not indicate a
difference between the two groups in the present study, further
illustrating that EF-IMRT may decrease the risk of recurrence for
patients with an increased level of lymphatic involvement.
With conventional radiotherapy techniques, generous
portions of the small bowel and vertebrae are included in the
treatment field, resulting in markedly increased gastrointestinal
and hematological toxicities, as well as treatment interruption and
severe late toxicities. According to Yoon et al (11), grade 3 or 4 acute toxicities were
observed in 42% of 90 patients treated with AP/PA EF irradiation.
Furthermore, 38% of patients treated with four-field EFRT, in a
study conducted by Rajasooriyar et al (13), experienced overall grade 3 or 4 acute
toxicities. In comparison, grade 3 acute toxicity was experienced
by 34.5% of the patients treated with EF-IMRT in the current study;
a superior result to those demonstrated in previous studies. IMRT
use assisted in the conformity of dose distribution, confined the
high-dose portions of radiation fields, and decreased the absorbed
dose and volume in critical organs, resulting in decreased overall
toxicity. Gerszten et al (19)
identified a significant decrease in critical organ irradiation
following EF-IMRT treatment and subsequently suggested that this
treatment may decrease acute and late treatment-associated side
effects.
The target volume and the irradiated volume of
vertebrae and small bowel tend to be considerably increased for EF
irradiation than that for pelvic irradiation. Thus, the rate and
severity of acute and late toxicities are expected to increase for
EFRT. Although IMRT, as a means to decrease toxicities, was applied
in the present study, the rate of acute toxicities increased within
a tolerated range for EF-IMRT. Beriwal et al (20) reported that 1/3 of the patients
treated with EF-IMRT exhibited grade 3 or higher acute toxicities.
These results are comparable with the results of the present study.
The use of IMRT assisted in the conformation of the radiation dose
and thus decreased the exposure of the small bowel and marrow,
resulting in decreased toxicity.
In conclusion, para-aortic irradiation with IMRT may
improve survival and alter the recurrence patterns of patients with
involved common iliac nodes or PALNs who tended to experience
poorer survival and an increased risk of recurrence, but were
similar to those results of patients with or without lower pelvic
nodal metastasis. Furthermore, with regard to EF-IMRT, the rate of
acute toxicities increased, but remained within the acceptable
range in comparison with that of pelvic IMRT. Overall, EF
irradiation with IMRT should be electively performed on patients
with cervical cancer with common iliac or periaortic nodal
involvement to improve survival.
References
|
1
|
Lin MY, Jobling TW and Narayan K: Surgical
staging of para-aortic LN in patients with locally advanced cervix
cancer and no evidence of metastases in preoperative PET/CT
imaging. J Gynecol Oncol. 26:352–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
FIGO Committee on Gynecologic Oncology:
FIGO staging for carcinoma of the vulva, cervix, and corpus uteri.
Int J Gynaecol Obstet. 125:97–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kidd EA, Siegel BA, Dehdashti F, Rader JS,
Mutch DG, Powell MA and Grigsby PW: Lymph node staging by positron
emission tomography in cervical cancer: Relationship to prognosis.
J Clin Oncol. 28:2108–2113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rotman M, Pajak TF, Choi K, Clery M,
Marcial V, Grigsby PW, Cooper J and John M: Prophylactic
extended-field irradiation of para-aortic lymph nodes in stages IIB
and bulky IB and IIA cervical carcinomas. Ten-year treatment
results of RTOG 79–20. JAMA. 274:387–393. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gay HA, Barthold HJ, O'Meara E, Bosch WR,
El Naqa I, Al-Lozi R, Rosenthal SA, Lawton C, Lee WR, Sandler H, et
al: Pelvic normal tissue contouring guidelines for radiation
therapy: A radiation therapy oncology group consensus panel atlas.
Int J Radiat Oncol Biol Phys. 83:e353–e362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim K, Small W Jr, Portelance L,
Creutzberg C, Jürgenliemk-Schulz IM, Mundt A, Mell LK, Mayr N,
Viswanathan A, Jhingran A, et al: Consensus guidelines for
delineation of clinical target volume for intensity-modulated
pelvic radiotherapy for the definitive treatment of cervix cancer.
Int J Radiat Oncol Biol Phys. 79:348–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kabolizadeh P, Fulay S and Beriwal S: Are
radiation therapy oncology group para-aortic contouring guidelines
for pancreatic neoplasm applicable to other malignancies-assessment
of nodal distribution in gynecological malignancies. Int J Radiat
Oncol Biol Phys. 87:106–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the radiation therapy oncology group (RTOG) and the
European organization for research and treatment of cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salama JK, Mundt AJ, Roeske J and Mehta N:
Preliminary outcome and toxicity report of extended-field,
intensity-modulated radiation therapy for gynecologic malignancies.
Int J Radiat Oncol Biol Phys. 65:1170–1176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoon HI, Cha J, Keum KC, Lee HY, Nam EJ,
Kim SW, Kim S, Kim YT, Kim GE and Kim YB: Treatment outcomes of
extended-field radiation therapy and the effect of concurrent
chemotherapy on uterine cervical cancer with para-aortic lymph node
metastasis. Radiat Oncol. 10:182015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu SY, Huang EY, Chanchien CC, Lin H, Wang
CJ, Sun LM, Chen HC, Fang FM, Hsu HC and Huang YJ: Prognostic
factors associated with radiotherapy for cervical cancer with
computed tomography-detected para-aortic lymph node metastasis. J
Radiat Res. 55:129–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajasooriyar C, Van Dyk S, Bernshaw D,
Kondalsamy-Chennakesavan S, Barkati M and Narayan K: Patterns of
failure and treatment-related toxicity in advanced cervical cancer
patients treated using extended field radiotherapy with curative
intent. Int J Radiat Oncol Biol Phys. 80:422–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coulombe G, Thiessen B, Balkwill S and
Aquino-Parsons C: Polyradiculopathy post-concomitant chemoradiation
for carcinoma of the uterine cervix treated with pelvic and
para-aortic fields. Gynecol Oncol. 99:774–777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmed RS, Kim RY, Duan J, Meleth S, De Los
Santos JF and Fiveash JB: IMRT dose escalation for positive
para-aortic lymph nodes in patients with locally advanced cervical
cancer while reducing dose to bone marrow and other organs at risk.
Int J Radiat Oncol Biol Phys. 60:505–512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boyle J, Craciunescu O, Steffey B, Cai J
and Chino J: Methods, safety and early clinical outcomes of dose
escalation using simultaneous integrated and sequential boosts in
patients with locally advanced gynecologic malignancies. Gynecol
Oncol. 135:239–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grigsby PW, Siegel BA and Dehdashti F:
Lymph node staging by positron emission tomography in patients with
carcinoma of the cervix. J Clin Oncol. 19:3745–3749. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakurai H, Mitsuhashi N, Takahashi M,
Akimoto T, Muramatsu H, Ishikawa H, Imai R, Yamakawa M, Hasegawa M
and Niibe H: Analysis of recurrence of squamous cell carcinoma of
the uterine cervix after definitive radiation therapy alone:
Patterns of recurrence, latent periods, and prognosis. Int J Radiat
Oncol Biol Phys. 50:1136–1144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerszten K, Colonello K, Heron DE, Lalonde
RJ, Fitian ID, Comerci JT, Selvaraj RN and Varlotto JM: Feasibility
of concurrent cisplatin and extended field radiation therapy (EFRT)
using intensity-modulated radiotherapy (IMRT) for carcinoma of the
cervix. Gynecol Oncol. 102:182–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beriwal S, Gan GN, Heron DE, Selvaraj RN,
Kim H, Lalonde R, Kelley JL and Edwards RP: Early clinical outcome
with concurrent chemotherapy and extended-field,
intensity-modulated radiotherapy for cervical cancer. Int J Radiat
Oncol Biol Phys. 68:166–171. 2007. View Article : Google Scholar : PubMed/NCBI
|