Introduction
Leukemia is the most common form of hematological
malignancy and it has an incidence rate of 3–4/10 million between
1988 and 2002 (1,2) and 5.17/10 million in china between 2003
and 2007 (3). Due to the continuous
optimization of treatment and supportive care, the survival rate
for patients with leukemia has been prolonged significantly over
the last few decades (4,5). As a consequence of these improvements,
the frequency of central nervous system (CNS) involvement in
leukemia has increased (6,7). The diagnosis of CNS leukemia should be
considered in the differential diagnosis of patients with leukemia
and CNS lesions, including those with CNS infection and
neurodegenerative disorders following leukemia treatment. CNS
lesions in leukemia may occur due to the disease itself, or the
treatment (8–13). Disease-associated CNS complications
may consist of leukemic cell involvement of the meninges,
parenchyma, and cerebrovasculature (8), whilst treatment-associated CNS
complications may include leukoencephalopathy, inflammatory
demyelinating polyradiculoneuropathy, infections, vascular
disorders and secondary tumors (9–12).
The clinical presentations of CNS lesions in
leukemia vary. Diagnosing the nature of CNS lesions is often
challenging, as there is a varied set of causes (14). There are no pathognomonic imaging
features for CNS lesions in leukemia, and the histological
verification of a brain biopsy remains the gold standard for
diagnosis (15,16). As numerous CNS lesions in leukemia are
curable, early diagnosis is essential for their proper management
(13). Several studies have reported
the usefulness of magnetic resonance imaging (MRI) and computed
tomography (CT) in the diagnosis of CNS lesions in leukemia
(13,17–20).
However, there are a limited number of reports concerning the
pathological features of CNS lesions in leukemia.
The present study aimed to combine the MRI and
pathological findings observed in 14 patients with leukemia with
CNS lesions, characterize these features and determine their value
in the diagnosis of such patients. Doing so may aid reaching the
correct diagnosis in the future and potentially allow treatment to
be conducted without delay.
Patients and methods
Patients
The clinical data, MRI features and pathology
results of 14 patients (11 male and 3 female), whose ages ranged
from 7 to 60 years old, were retrospectively reviewed at Beijing
Tiantan Hospital (Capital Medical University, Beijing, China)
between April 2003 and May 2015 (Table
I). The patients had various types of leukemia, including 8
cases of acute lymphoblastic leukemia (ALL; 4 B-cell-ALL, 2
T-cell-ALL and 2 unknown type-ALL), 4 cases of acute myelogenous
leukemia (AML; 2 M5, 1 M4 and 1 unknown type), 1 case of acute
promyelogenous leukemia (APL) and 1 case of chronic myelomonocytic
leukemia (CMML).
 | Table I.Medical records of 14 patients with
leukemia with CNS lesions. |
Table I.
Medical records of 14 patients with
leukemia with CNS lesions.
| Case | Age,
years/gender | Leukemia type | Treatments | A/I | Clinical symptoms
and signs | MRI findings | CSF
examination | CNS lesions of
clinical diagnosis | Stereotactic
biopsy/pathology diagnosis | Outcome |
|---|
| 1 | 54/M | APL | Retinoic acid | 53/2 months
following retinoic acid | Headache, limb
numbness, seizure | Bilateral posterior
parietal lobe exhibiting patchy enhancement, leptomeninx ‘gyriform’
meningeal enhancement | CSF pressure 180 mm
H2O; immature cells (+); Pandy test (−); Pr 0.42 g/l;
WBC 210×106/l↑; bacterial/fungal cultures (−). | CNS leukemia | Left parietal lobe
lesion/atypia of white blood cells infiltration | Improved |
| 2 | 7/M | ALL (B cell) | VDCLP | 7/during
chemotherapy | Headache, fever,
dizziness | Cystic lesion in
the right temporal lobe and cerebellum obvious edema. | CSF pressure 160 mm
H2O; immature cells (−); Pandy test (−); Pr 0.42 g/l;
WBC 6×106/l; bacterial/fungal cultures (−) | Brain abscesses of
right temporal occipital lobe | The right temporal
lobe/CNS leukemia (B cell) | Progressed |
| 3 | 25/M | ALL (T cell) | Allo-ASCT, DLI +
immunosuppressive agent | 21/3 years after
DLI + immunosuppressive agent | Headache, tic,
disturbance of consciousness, limb weakness, binocular vision
loss | Mixed signals at
right hemisphere, perilesional mild enhancement, ventricular
expansion | CSF pressure 200 mm
H2O↑; immature cells (+); Pandy test (+); Pr 0.72 g/l↑;
WBC 450×106/l↑; bacterial/fungal cultures (−) | CNS leukemia | The right parietal
and occipital/the brain tissue and blood vessels with lymphocyte
infiltration, in accordance with T lymphoid cell
leukemia/lymphoma | Improved |
| 4 | 60/M | CMML | Allo-ASCT | 57/4 years after
allo-HSCT | Nausea, vomiting,
right limb weakness, disturbance of consciousness | MRI: A 2.5×2
cm2 lesion in left basal ganglia, thalamus, compressive
deformation of left lateral ventricle | CSF pressure 220 mm
H2O↑; immature cells (+); Pandy test (+); Pr 0.56 g/l↑;
WBC 500×106/l↑; bacterial/fungal cultures(−) | CNS leukemia | The left basal
ganglia/mature and immature granulocytes with bleeding, considering
chronic myelomonocytic leukemia intracranial invasion. Granulocyte
immunohistochemical: LC (+++), MPO (++), CD15 (++), Ki-67
>25%. | Succumbed |
| 5 | 26/M | ALL (B cell) | VDCLP | 20/9 months after
last course of chemotherapy | Headache | Crumb placeholder
at left temporal lobe, perilesional with obvious edema and
mass-effect. Enhancement of the crumb neoplasm and meningeal on T1
with Gd | CSF pressure 210 mm
H2O↑; immature cells (+); Pandy test (−); Pr 0.63 g/l↑;
WBC 440×106/l↑; lymphocytes, 90%; bacterial/fungal
cultures (−) | CNS leukemia | The left temporal
lobe/B cell leukemia/lymphoma | Improved |
| 6 | 29/F | AML (M5b) | FLAG +
idarubicin | 27/1 months after
last course of chemotherapy with FLAG + idarubicin | MRI examination
prior to allo-ASCT found lesions | Bilateral cerebral
hemisphere multiple abnormal signal; bilateral cerebellum, left
occipital lobe abnormal signal enhanced on T1 with Gd. | Not performed | CNS leukemia | The left parietal
occipital brain/hematopoietic malignant tumor, MPO (+++) Ki67,
90%. | Improved |
| 7 | 42/M | ALL | Allo-HSCT | 41/3 months after
allo-HSCT | Dizziness, walking
instability, vomiting | Cerebellar vermis
roof of fourth ventricle, left cerebellar hemisphere visible
nodular enhancement lesions | CSF pressure 170 mm
H2O; immature cells (−); Pandy test (−); Pr 0.37 g/l;
WBC 5×106/l; bacterial/fungal cultures (−) | CNS leukemia | The cerebellar
vermis/atypia of peripheral lymphocytes infiltration | Improved |
| 8 | 16/M | ALL (T cell) | VDCLP | 16/at the diagnosis
of leukemia | Headache, blurred
vision in right eye | Scattered, abnormal
signal of sizes at the hemispheres and cerebellar hemispheres | CSF pressure 180 mm
H2O; immature cells (−); Pandy test (−); Pr 0.35 g/l;
WBC 1×106/l; bacterial/fungal cultures (−) | Leukemia
intracranial invasion with bleeding | Not performed | CNSL recurrence
after 1.4 years of allo-HCST |
| 9 | 15/M | AML (M5) | A modified BU/CY+
decreased ATG+ allo-HSCT; after 3 months of recurrence: FLAG; DLI +
cyclosporin. | 14/3 days after DLI
+ Cyclosporin | Seizure | Left occipital
lobe,right frontal lobe low-density lesions, cystic solid
placeholder with enhancement of capsule wall. | Not performed | CNS infection | The left
occipital/fungal brain abscesses | Improved |
| 10 | 38/M | AML | Allo-HSCT | 34/9 months after
allo-HSCT | Fever, headache,
vomiting | Scattered lesions
at the right frontal lobe and anterior horn of right ventricle,
Short T1 and long T2 signal, the larger lesion at the right
frontal, 20×15 mm2. DWI heterogeneous signal | CSF pressure 220 mm
H2O↑; immature cells (−); Pandy test (+); Pr 0.65 g/l↑;
WBC 1530×106/l↑; bacterial/fungal cultures (−) | CNS infection:
Bacterial meningitis; brain abscess | Not performed | Improved |
| 11 | 20/M | ALL | VDCLP | 20/during second
course of chemotherapy | Headache, fever,
vomiting, limb tic | Bilateral cerebral
hemisphere cortex and subcortical multiple long T1 and T2 signal
nodular lesions, border is not clear, perilesional edema is
apparent. Multiple ‘small capsule lesions’ enhancement on T1 with
Gd. | CSF pressure 165 mm
H2O; immature cells (−); Pandy test (−); Pr 0.33 g/l;
WBC 1×106/l; bacterial/fungal cultures (−) | CNS infection | Right frontal lobe
lesion/broken rotten tissue like dark ‘broken cotton’; pathology
demonstrated fungal brain abscess | Improved |
| 12 | 26/F | AML (M4) | MA | 25/during fourth
course of chemotherapy | Fever, headache,
language is not fluent, tic, right angle deflection, disturbance of
consciousness, nausea, limb weakness | Mixed signals at
the left fronto-temporal top border zone, 4.3×4.5 cm2,
DWI mixed signals, perilesional edema and mass-effect can be seen,
adjacent meningeal reinforcement | CSF Pandy test (+),
CSF protein 0.86 g/l↑, glucose 2.3 mmol/l↓; chlorine 97
mmol/l↓ | CNS infection | Left temporal
lobe/high degree of neutrophil infiltration, brain abscesses | Improved |
| 13 | 49/M | ALL
(Ph+-B-ALL) | CAT | 49/1 months after
CAT | MRI examination
before allo-ASCT found lesions | Enhanced lesions in
left parietal and occipital lobe with surrounding edema; Glial cell
proliferation around lesion at right parietal lobe | CSF cytology
negative | CNS leukemia | The left parietal
and occipital/expansion of blood vessels with vascular
degeneration, surrounding brain tissue degeneration and atrophy,
glial cell hyperplasia with lymphocytes and a small quantity of
neutrophils infiltrating | Improved |
| 14 | 23/F | ALL (B cell) | Allo-ASCT | 23/1 months after
allo-ASCT | Headache | The lateral frontal
of right paracele white matter lesions visible long T1 and long T2
signal, the boundary is not clear, edema is not obvious, no
enhancement of lesions on T1 with Gd. | CSF pressure 160 mm
H2O; immature cells (−); Pandy test (−); Pr 0.40 g/l;
WBC 0.5×106/l; bacterial/fungal cultures (−) | Degenerative
disease | Right frontal lobe
lesions/Nerve cell degeneration, glial cells mild hyperplasia. No
abnormal lymphocytes. Some loss of myelin staining, considering
brain white matter reaction of chemotherapy | Improved |
The majority of patients presented with nonspecific
symptoms, including seizure, headache, nausea, vomiting and a
change in mental status. Few patients had focal neurological
deficiencies, including limb weakness and vision loss. Systemic
symptoms, including fever, night sweat and weight loss, were
common.
Patient medical records were reviewed with
particular attention to the type of treatment given, time of onset
of neurological symptoms, the interval between the onset of
neurological symptoms and final treatment for leukemia,
cerebrospinal fluid (CSF) results (pressure, Pandy test,
quantitative protein levels, cell count and bacterial/fungal
cultures), outcome of the CNS lesions, MRI findings in 14 patients,
pathological features in 12 patients and the consistency of the
clinical diagnosis and pathological diagnosis (Table I). The study protocol was approved by
the Ethics Committee of Beijing Tiantan Hospital (Beijing, China).
All of the patients provided written informed consent.
Imaging
Two experienced neuroradiologists (Beijing Tiantan
Hospital, Capital Medical University, Beijing, China)
retrospectively and independently evaluated the images. All scans
were reviewed, noting the brain lesion locations, size, margin and
signal characteristics, as well as the presence of perilesional
edema, mass-effect, hemorrhage, necrosis and meningeal
enhancement.
All of the patients were imaged using T1- and
T2-weighted and post-contrast T1-weighted scans (0.1–0.15 mmol
gadolinium-diethylenetriaminepenta-acetate/kg body weight).
Proton-magnetic resonance-spectroscopy (1H-MRS) was
performed in 3 patients. MRI was performed on a 1.5-T Siemens
MAGNETOM® Avanto machine (Siemens AG, Munich, Germany).
On this machine, T1 images were fast spin-echo sequences with
repetition time (TR), 500 ms and echo time (TE), 7.8 ms. T2 images
were fast spin-echo sequences with TR, 3,630 ms and TE, 93 ms. The
pre- and post-contrast T1 sequences were obtained along the three
orthogonal planes. The T2 sequences were obtained axially. The pre-
and post-contrast T1 sequences were isotropic, ultrafast spoiled
gradient echo sequences (TR, 8.3 ms and TE, 3.8 ms). The T2
sequences were 5-mm axial fast spin echo sequences with TR, 4,000
ms and TE, 110 ms. 1H-MRS was obtained with a long echo
time (135 ms) as a multivoxel 2D exam encompassing the lesion and
normal white matter.
Stereotactic biopsy
Among the 14 patients with leukemia, 12 were
pathological, as confirmed by stereotactic biopsy with a framework
of the stereotactic surgery planning system (AeroTech) and
robot-assisted planning of frameless stereotactic surgery (Computer
Stereotactic Assistant, type R; CSA-R type), in order to place the
stereotactic frame on the head or post four marker points on the
head and then used MRI to locate the lesion. Through the local area
network, images were uploaded into the workstation to formulate the
surgical plan, determine the biopsy site, avoid the important
functional areas and select the appropriate cranial puncture point
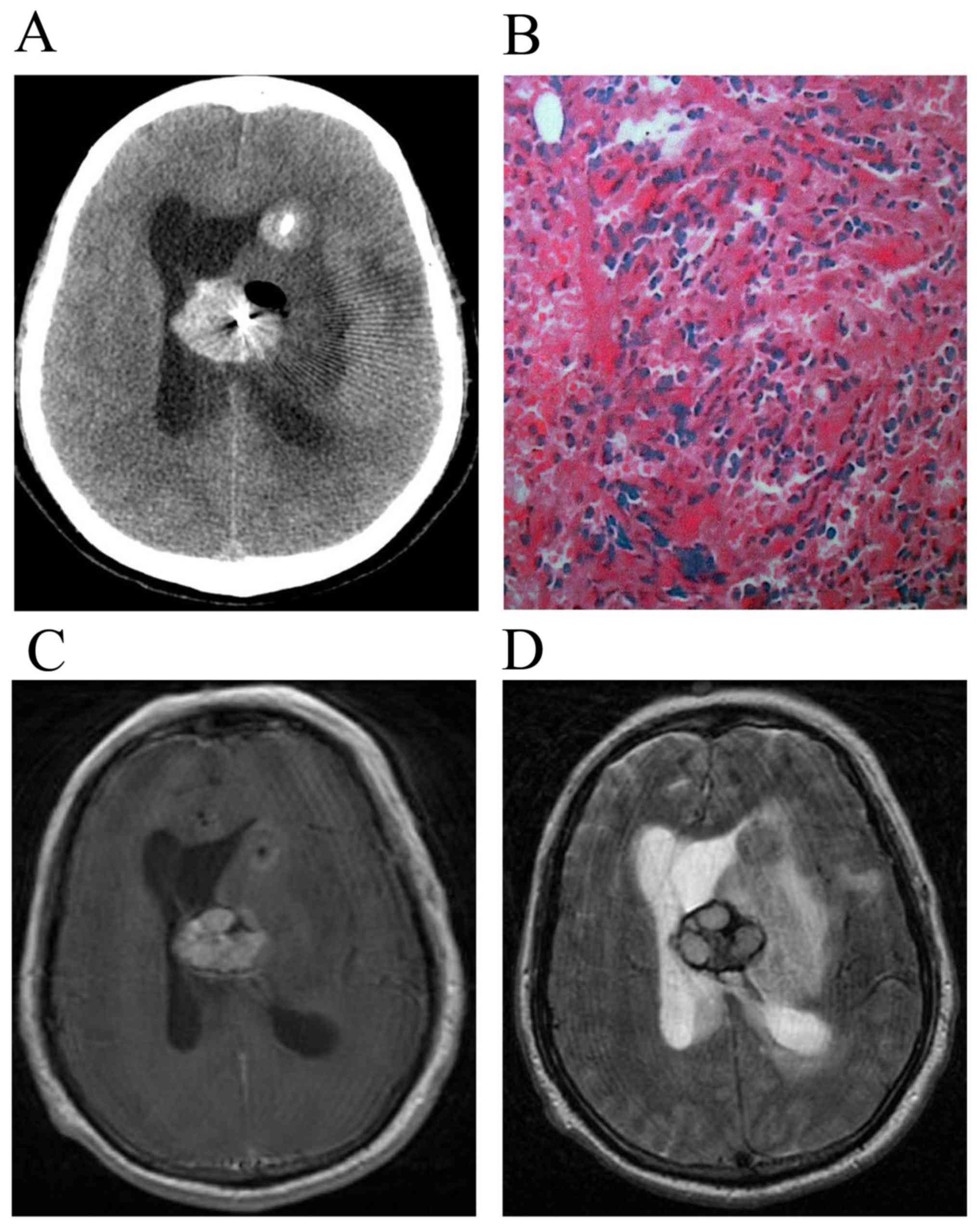
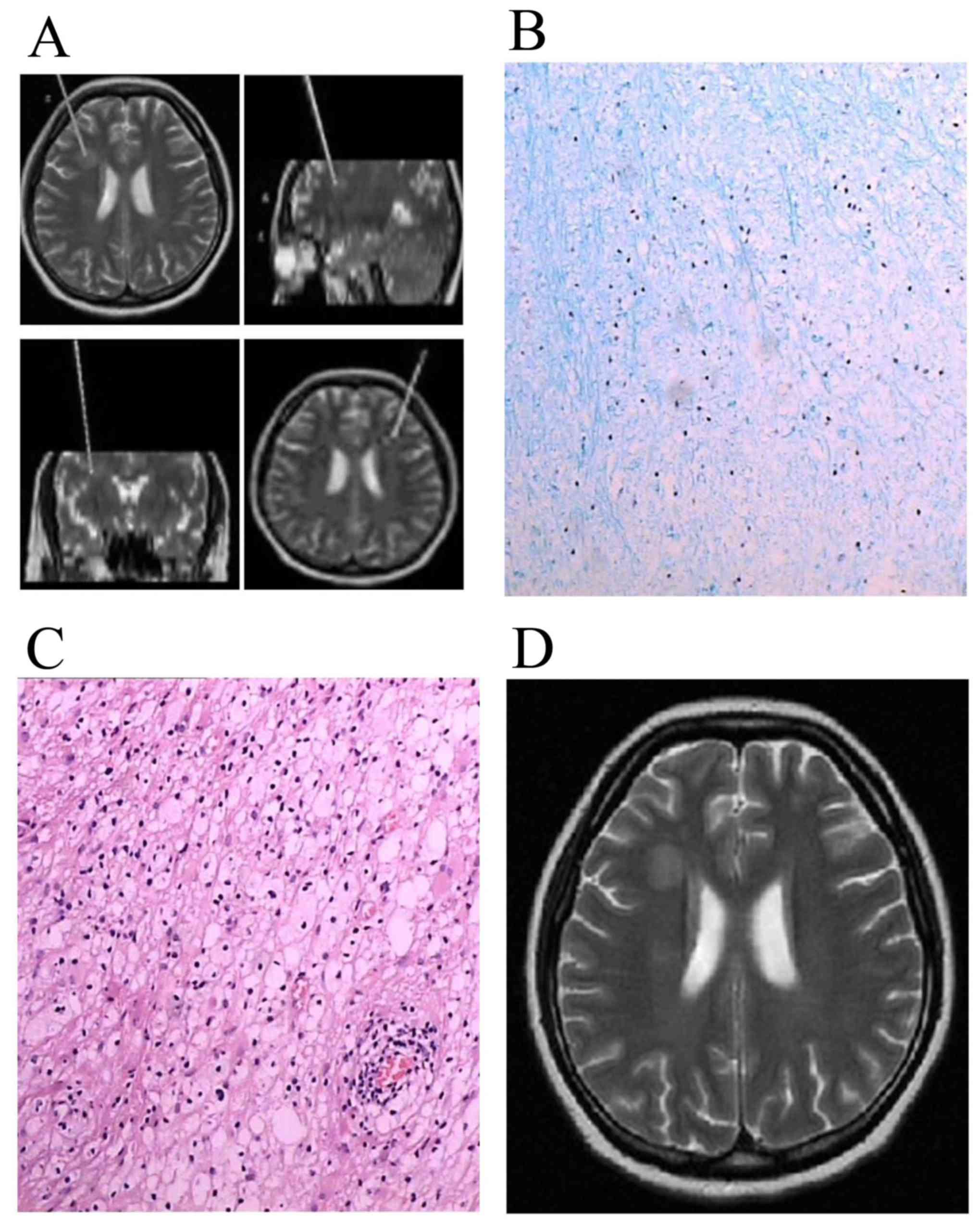
and best surgical puncture path (Figs.
1A and 2A). A biopsy needle was
inserted following a hole being drilled with a 3-mm diameter drill.
Four or five pieces of tissues measuring 1.0×0.3×0.3 cm were
removed from the lesion area. An intraoperative snap-frozen tissue
section used liquid nitrogen and a conventional paraffin-embedded
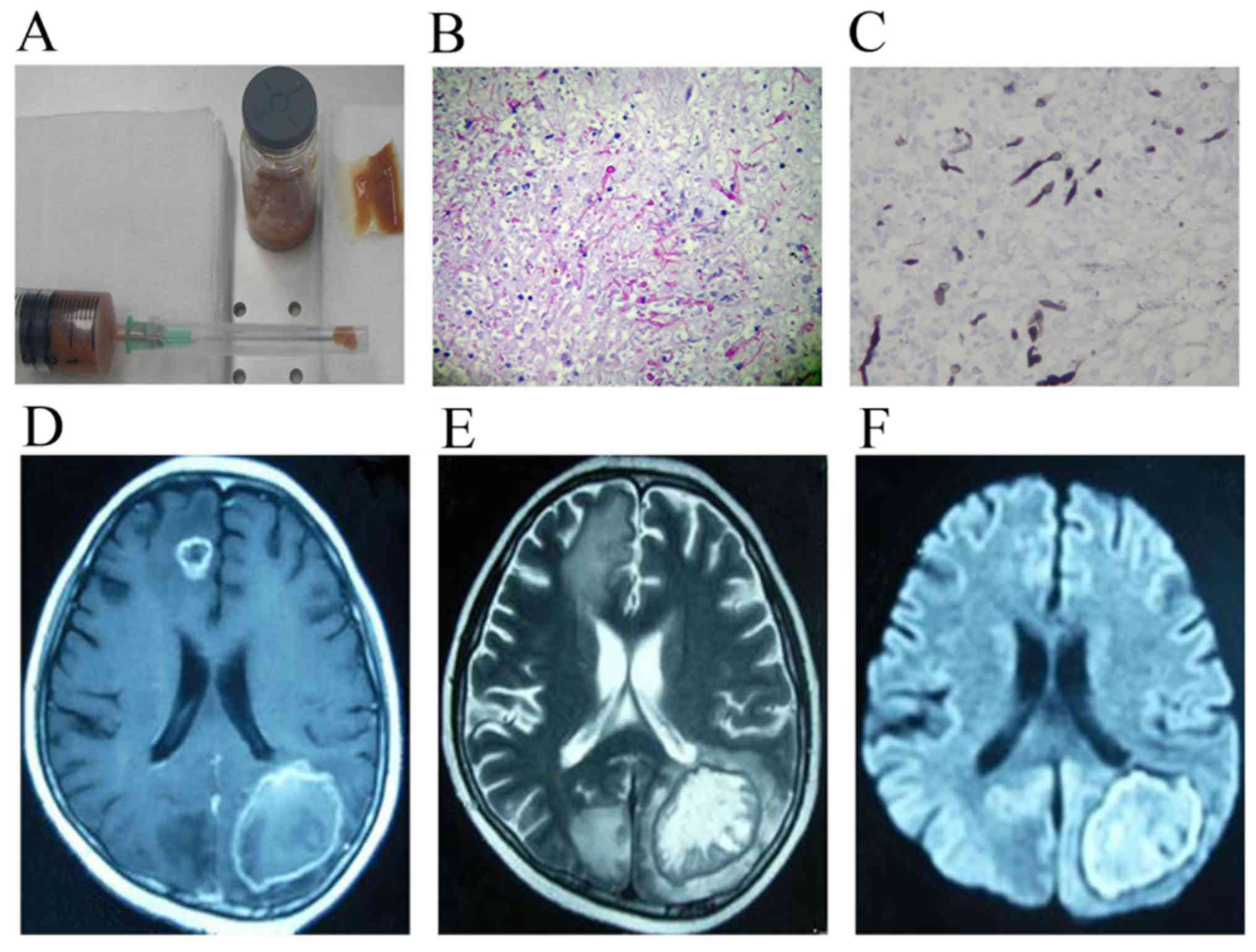
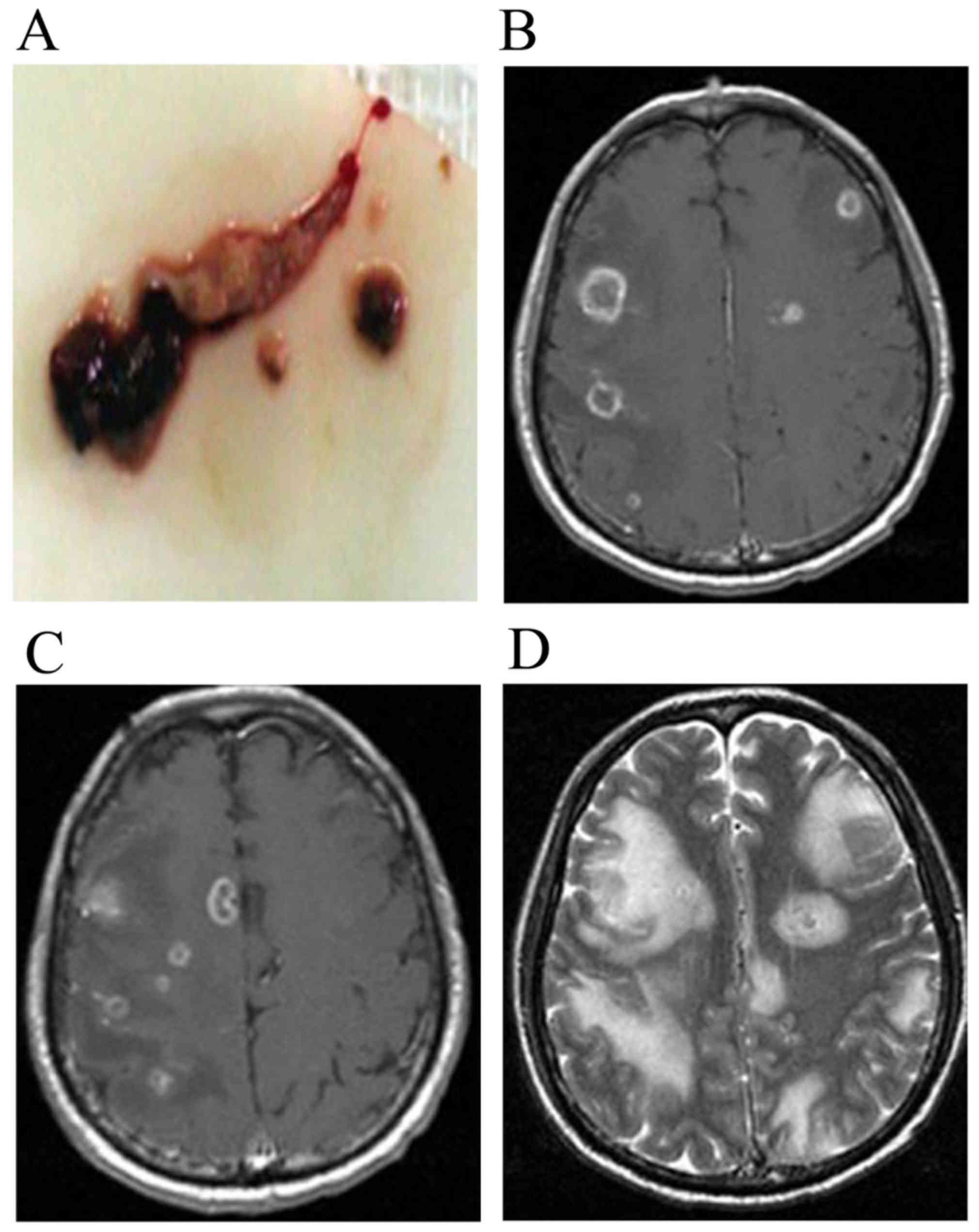
tissue section was sent for evaluation (Figs. 3A and 4A). Following the biopsy, the equipment was
withdrawn and the wound was sutured.
Results
Among the 14 patients, the causes of CNS lesions
were divided into three groups. The first group had CNS leukemia
(n=8; Fig. 1B), the second group had
CNS infection (n=4; Fig. 3B and C)
and the third group had a neurodegenerative disorder (n=2, 1
leukoencephalopathy and 1 glial cell hyperplasia 1; Fig. 2B and C). CNS leukemia included 1 APL
case, 3 ALL (B cell) cases, 2 ALL (T cell) cases, 1 CMML case and 1
AML (M5b) case. CNS infection included 3 AML cases and 1 ALL case
(T cell), whereas neurodegenerative disorders included 2 ALL cases
(B cell).
All the patients received chemotherapy, and 6
received hematopoietic stem cell allotransplantation (allo-HSCT).
In group 1, CNS leukemia occurred prior to chemotherapy (n=1, case
8), during chemotherapy (n=1, case 2), following chemotherapy
patients obtained complete remission (n=3, case 1, 5, 6 and case 5
with systemic relapse) and following allo-HSCT (n=3, case 3, 4, 7
and case 4 with systemic relapse).
In the second group, 2 patients with CNS infection
following allo-HSCT with graft vs. host disease (GVHD; 1 GVHD of
the intestinal tract and 1 GVHD of the liver; cases 9 and 10,
respectively) were given immunosuppressive therapy, including
cyclosporin and/or antithymocyte globulin against GVHD. Another 2
patients had CNS infection during the second and fourth courses of
chemotherapy for anti-leukemic treatment (cases 11 and 12,
respectively).
In group 3, 1 patient had leukoencephalopathy
following 3 courses of systemic chemotherapy and 5 intrathecal
injections (case 13). Another patient had glial cell hyperplasia 1
month following high-dose chemotherapy and allo-HSCT (case 14).
The MRI features in patients with CNS leukemia
indicated multiple, scattered, round solid nodules of lesions in
the brain parenchyma, a slightly long or equal T1 and long T2
signal and apparent perilesional edema as well as an enhanced mass
effect (Fig. 1C and D).
1H-MRS was performed in 3 patients with CNS leukemia
(case 1, 2 and 3). 1H-MRS revealed a marked increase in
choline (Cho), and a marked reduction in N-acetylaspartate (NAA)
and creatinine (Cr) peaks in the center of the lesion. However, in
perilesional brain tissue, a slight decrease in NAA and no increase
in Cho peaks were observed. MRI findings in patients with CNS
infection exhibited scattered, small capsule lesions, a short/long
T1 and long T2 signal, an unclear border and apparent perilesional
edema (Figs. 3D-F, 4B-D). MRI findings in patients with
neurodegenerative disorders revealed glial cell proliferation
around the lesion and white matter lesions that were visible on a
long T1 and long T2 signal, whilst edema was not apparent (Fig. 2D).
Except for cases 8 and 10, 12 patients obtained a
pathological diagnosis through stereotactic biopsy. The clinical
diagnosis was not consistent with the pathological diagnosis in 2
patients (cases 2 and 13). One patient's clinical diagnosis was CNS
infection, although the pathological diagnosis was CNS leukemia
(case 2). Another patient's clinical diagnosis and pathological
diagnosis was CNS leukemia and glial cell hyperplasia, respectively
(case 13). Among the 14 patients with leukemia, the outcomes of 12
patients improved, 1 disease progression (case 2) and 1 succumbed
(case 4).
Discussion
Leukemia with CNS involvement is not uncommon in
clinical practice (13). In the
present study, 14 patients with leukemia and CNS lesions were
evaluated. It was observed that MRI aided the characterization of
CNS lesions caused by the leukemic involvement of CNS structures,
treatment-associated CNS complications and CNS infections due to
immunocompromised states. However, two clinical diagnoses were not
consistent with the pathological diagnoses, and pathological
verification of brain biopsy tissues remains the gold standard for
diagnosis (21–23).
CNS leukemia is more common in childhood, with
hyperleukocytic acute leukemia, ALL and M5 often occurring
following complete remission (4,6,7). Over the last two decades, clinical
trials have significantly improved the response rates in patients
with leukemia. Adults with ALL have a 60–90% chance of reaching a
first complete remission following combination chemotherapy
(24–26). Advancements in the understanding of
disease biology, adaptations to anti-leukemic treatment and better
supportive care have all contributed to these improvements
(6,7).
However, CNS involvement is still a primary cause of mortality and
has become a major limitation to long-term survival (27). CNS-directed treatment is a significant
contributing factor in improving the survival rate of patients with
ALL. This essential treatment decreases the rate of CNS relapse, in
addition to reducing the incidence of bone marrow recurrence. While
<5% of patients with ALL present with overt CNS leukemia,
>50% will develop CNS disease in the absence of prophylactic
CNS-directed treatment (28). In the
present study, 8 patients had CNS leukemia due to the disease
itself. Cases 3, 4 and 7 developed CNS leukemia following
allo-HSCT. CNS relapse subsequent to allo-HSCT indicates a poor
prognosis in patients with leukemia (29,30).
Preventing CNS relapse following allo-HSCT remains a therapeutic
challenge, and the criteria for post-HSCT CNS prophylaxis have yet
to be addressed (27).
As chemotherapy drugs for the treatment of leukemia
(anthracyclines, vinca alkaloids, cyclophosphamide) exhibit poor
penetration of the blood brain barrier (BBB), the CNS becomes a
‘shelter’ for leukemia cells. CNS involvement is associated with a
poor prognosis. However, methotrexate and cytarabine display a
moderate capability to cross the BBB at high doses sufficient to
obtain therapeutic concentrations within the CNS. However, as well
as CNS radiation therapy (31), this
contributes to neurotoxicities. Examples of neurotoxicity include
leukoencephalopathy and spontaneous intracranial hemorrhage
(11,32–34).
Infection may be caused by leukemia itself, as well as the bone
marrow suppression observed with intense chemotherapy (13). In the present study, two patients
developed a treatment-associated neurodegenerative disorder due to
systemic high-dose chemotherapy and/or intrathecal injections. In a
total of 4 patients, CNS infections occurred in the bone marrow
suppression period during intense anti-leukemia chemotherapy, or
anti-GVHD immunosuppressive therapy following allo-HSCT.
Among the eight patients with CNS leukemia, seven
were pathologically confirmed by stereotactic biopsy. The pathology
of CNS leukemia was variable, which led to complex and changeable
presentations during MRI. The pathological presentations of five
patients were of tumor cells with nodular infiltration in the brain
parenchyma. MRI scans exhibited a scattered, round and solid nodule
of lesions in the brain parenchyma, with a slightly long or equal
T1 and long T2 signal. Perilesional edema was marked, as was an
enhanced mass-effect. One patient's pathology results indicated
that tumor cells had infiltrated the meninges and meningeal
vessels. The ‘lace’ strengthening of the meninges and the
thickening of local meninges were observed using MRI. Tumor cells
infiltrated and blocked vessels of the parenchyma in one patient
and this patient's MRI scans revealed a partial small infarct,
infarction, hemorrhagic infarction, or venous stasis of brain edema
or cerebral hemorrhage.
When patients with CNS infections or
neurodegenerative disorders demonstrated nodular infiltration
lesions, these lesions tended to be confused with CNS leukemia on
MRI scans. MRI of case 13 exhibited enhanced lesions in the left
parietal and occipital lobe with surrounding edema, which was
misdiagnosed as CNS leukemia. MRI of case 2 revealed a cystic
lesion in the right temporal lobe and cerebellum, as well as marked
edema, which were misdiagnosed as a CNS infection.
MRS allows for the noninvasive acquisition of
biochemical information from biological tissues. Within a defined
volume of interest, signals are detected from chemical nuclei, with
protons (hydrogen ions) being most frequently used (35). During the present study, in patients
with CNS leukemia MRS consistently demonstrated an increase in Cho,
and a decrease in or absence of NAA. The Cr peak was consistent
with previous data from the literature (36,37). This
indicates the ‘exogenous’ features of the tumor, which possibly aid
the differentiation of CNS leukemia from other lesions.
Due to the diversity in pathological changes and
imaging findings within CNS leukemia, it is challenging to identify
the precise nature of CNS lesions in patients with leukemia, which
is used to determine the type of treatment (38,39).
Various studies have demonstrated that the misdiagnosis rate of CNS
leukemia may be ≤75%, with misdiagnoses including intracranial
hemorrhage, cerebral infarction, meningitis, infection,
demyelinating multiple sclerosis, spinal cord compression syndrome
and Guillain-Barre syndrome (38,39). It is
possible to obtain pathological confirmation safely through
stereotactic biopsy with minimal trauma (21,22).
Clinical misdiagnosis and administering experimental treatments
delays the correct treatment, which is an important factor for
tumor recurrence (13). Therefore,
the medical history, hematology and bone marrow test, CSF and
biochemical examination, in combination with the pathology of CNS
lesions following stereotactic biopsy, may improve the rate of
correct diagnosis (23), avoiding
unnecessary treatment and associated morbidity. At the same time,
attention must be given to the function of blood coagulation prior
to biopsy as numerous patients with leukemia have blood coagulation
dysfunctions (32). In the present
study, 12 patients with leukemia with CNS lesions underwent
stereotactic biopsy without surgical complications for the
pathological confirmation of diagnosis. Stereotactic biopsy has the
advantages of convenience, as well as minimal invasion (21,22).
The incidence of CNS lesions in leukemia has
increased due to advances in treatment and prolonged survival time
(6,7).
CNS leukemia typically presents as a scattered, round solid nodule
of lesions in the brain parenchyma that reflects tumor cell nodular
infiltration. These lesions typically exhibit slightly long or
equal T1 and long T2 signal, with marked perilesional edema,
mass-effect and contrast enhancement (13,20).
Differential diagnoses of CNS leukemia on MRI scans include CNS
infection in immunocompromised patients and neurodegenerative
disorders caused by anti-leukemia treatment (14,20).
The novel imaging technique 1H-MRS is
important in the diagnosis of CNS leukemia, and in differentiating
it from other brain lesions in patients with leukemia. This is
particularly vital when the characteristic imaging findings that
usually appear on traditional images are absent (37–41).
To conclude, the present study demonstrated that the
clinical diagnosis was not consistent with the pathological
diagnosis in 2/14 patients. Numerous CNS lesions in patients with
leukemia are potentially curable; therefore, correct diagnosis is
crucial. Pathological confirmation remains the gold standard for
diagnosing the nature of CNS lesions. In addition, the present
study demonstrated that stereotactic biopsy is useful in diagnosing
and differentiating CNS lesions in patients with leukemia. This
technique may aid early recognition of the nature of CNS lesions
and potentially allow for timely therapeutic intervention.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China General Program (grant no.
81272842) and the Beijing Natural Science Foundation (grant no.
7172071).
References
|
1
|
Parkin DM, Ferlay J, Curado MP, Bray F,
Edwards B, Shin HR and Forman D: Fifty years of cancer incidence:
C15 I–IX. Int J Cancer. 127:2918–2927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lei T, Mao WM, Yang HJ, Chen XZ, Lei TH,
Wang XH, Ying Q, Chen WQ and Zhang SW: Study on cancer incidence
through the cancer registry program in 11 cities and counties,
China. Zhonghua Liu Xing Bing Xue Za Zhi. 30:1165–1170. 2009.(In
Chinese). PubMed/NCBI
|
|
3
|
Chen WQ, Shan BE, Zheng RS, Lin GZ, Chen
JZ, Chen JG and HE YT: Analysis of incidence and mortality of
leukemia in registration areas of China from 2003 to 2007. Tumor.
32:251–255. 2012.
|
|
4
|
Rodriguez-Abreu D, Bordoni A and Zucca E:
Epidemiology of hematological malignancies. Ann Oncol. 18:3–8.
2007. View Article : Google Scholar
|
|
5
|
Allemani C, Weir HK, Carreira H, Harewood
R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A,
et al: Global surveillance of cancer survival 1995–2009: Analysis
of individual data for 25,676,887 patients from 279
population-based registries in 67 countries (CONCORD-2). Lancet.
385:977–1010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evans AE, Gilbert ES and Zandstra R: The
increasing incidence of central nervous leukemia in children
(Children's Cancer Study Group A). Cancer. 26:404–409. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niemeyer CM, Hitchcock-Bryan S and Sallan
SE: Comparative analysis of treatment programs for childhood acute
lymphoblastic leukemia. Semin Oncol. 12:122–130. 1985.PubMed/NCBI
|
|
8
|
Walker RW: Neurologic complications of
leukemia. Neurol Clin. 9:989–999. 1991.PubMed/NCBI
|
|
9
|
Feinberg WM and Swenson MR:
Cerebrovascular complications of L-asparaginase therapy. Neurol.
38:127–133. 1988. View Article : Google Scholar
|
|
10
|
Flament-Durand J, Ketelbant-Balasse P,
Maurus R, Regnier R and Spehl M: Intracerebral calcifications
appearing during the course of acute lymphocytic leukemia treated
with methotrexate and X rays. Cancer. 35:319–325. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rubinstein LJ, Herman MM, Long TF and
Wilbur JR: Disseminated necrotizing leukoencephalopathy: A
complication of treated central nervous system leukemia and
lymphoma. Cancer. 35:291–305. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Biti GP, Magrini SM, Villari N, Caramella
D, Guazzelli G, Rosi A and Lippi A: Brain damage after treatment
for acute lymphoblastic leukemia. A report on 34 patients with
special regard to MRI findings. Acta Oncol. 28:253–256. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CY, Zimmerman RA, Faro S, Bilaniuk
LT, Chou TY and Molloy PT: Childhood leukemia: Central nervous
system abnormalities during and after treatment. AJNR Am J
Neuroradiol. 17:295–310. 1996.PubMed/NCBI
|
|
14
|
Faraci M, Lanino E, Dini G, Fondelli MP,
Morreale G, Dallorso S, Manzitti C, Calevo MG, Gaggero R,
Castagnola E and Haupt R: Severe neurologic complications after
hematopoietic stem cell transplantation in children. Neurology.
59:1895–1904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pui CH and Thiel E: Central nervous system
disease in hematologic malignancies: Historical perspective and
practical applications. Semin Oncol. 36 4 Suppl 2:S2–S16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phillips ME, Ryals TJ, Kambhu SA and Yuh
WT: Neoplastic vs inflammatory meningeal enhancement with Gd-DTPA.
J Comput Assist Tomogr. 14:536–541. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ball WS Jr, Prenger EC and Ballard ET:
Neurotoxicity of radio/chemotherapy in children: Pathologic and MR
correlation. AJNR Am J Neuroradiol. 13:761–776. 1992.PubMed/NCBI
|
|
18
|
Asato R, Akiyama Y, Ito M, Kubota M,
Okumura R, Miki Y, Konishi J and Mikawa H: Nuclear magnetic
resonance abnormalities of the cerebral white matter in children
with acute lymphoblastic leukemia and malignant lymphoma during and
after central nervous system prophylactic treatment with
intrathecal methotrexate. Cancer. 70:1997–2004. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsuruda JS, Kortman KE, Bradley WG,
Wheeler DC, Van Dalsem W and Bradley TP: Radiation effects on
cerebral white matter: MR evaluation. AJR Am J Roentgenol.
149:165–171. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Packer RJ, Zimmerman RA and Bilaniuk LT:
Magnetic resonance imaging in the evaluation of treatment-related
central nervous system damage. Cancer. 58:635–640. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ligima K, Hirato M, Miyagishima T,
Horiguchi K, Sugawara K, Hirato J, Yokoo H and Yoshimoto Y:
Microrecording and image-guided stereotactic biopsy of deep-seated
brain tumors. J Neurosurg. 123:978–988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malikova H, Liscak R, Latnerova I,
Guseynova K, Syrucek M and Pytlik R: Complications of MRI-guided
stereotactic biopsy of brain lymphoma. Neuro Endocrinol Lett.
35:613–618. 2014.PubMed/NCBI
|
|
23
|
Göçmen S, Kutlay M, Erikçi A, Atabey C,
Sayan O and Haholu A: Central nervous system involvement of T-cell
prolymphocytic leukemia diagnosed with stereotactic brain biopsy:
Case report. Turk J Haematol. 31:75–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas X, Boiron JM, Huguet F, Dombret H,
Bradstock K, Vey N, Kovacsovics T, Delannoy A, Fegueux N, Fenaux P,
et al: Outcome of treatment in adults with acute lymphoblastic
leukemia: Analysis of LALA-94 trial. J Clin Oncol. 22:4075–4086.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kantarjian HM, O'Brien S, Smith TL, Cortes
J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C, et al:
Results of treatment with hyper-CVAD, a dose-intensive regimen, in
adult acute lymphocytic leukemia. J Clin Oncol. 18:547–561. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thomas X and Le QH: Central nervous system
involvement in adult acute lymphoblastic leukemia. Hematology.
13:293–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sung SH and Jang IS: Isolated central
nervous system relapse of acute lymphoblastic leukemia. Brain Tumor
Res Treat. 2:114–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barredo J and Ritchey AK: Controversies in
the management of central nervous system leukemia. Pediatr Hematol
Oncol. 27:329–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Robertson KA: Pediatric bone marrow
transplantation. Curr Opin Pediatr. 5:103–109. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pui CH and Howard SC: Current management
and challenges of malignant disease in the CNS in paediatric
leukemia. Lancet Oncol. 9:257–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valk PE and Dillon WP: Radiation injury of
the brain. AJNR Am J Neuroradiol. 12:45–62. 1991.PubMed/NCBI
|
|
32
|
Priest JR, Ramsay NK, Steinherz PG,
Tubergen DG, Cairo MS, Sitarz AL, Bishop AJ, White L, Trigg ME,
Levitt CJ, et al: A syndrome of thrombosis and hemorrhage
complicating L-asparaginase therapy for childhood acute
lymphoblastic leukemia. J Pediatr. 100:984–989. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Price RA and Jamieson PA: The central
nervous system in childhood leukemia. II. Subacute
leukoencephalopathy. Cancer. 35:306–318. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Törnebohm E, Lockner D and Paul C: A
retrospective analysis of bleeding complications in 438 patients
with acute leukemia during the years 1972–1991. Eur J Haematol.
50:160–167. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mansour A, Qandeel M, Abdel-Razeq H and
Ali Abu HA: MR imaging features of intracranial primary CNS
lymphoma in immune competent patients. Cancer Imaging.
14:222014.PubMed/NCBI
|
|
36
|
Chan YL, Roebuck DJ, Yuen MP, Yeung KW,
Lau KY, Li CK and Chik KW: Long-term cerebral metabolite changes on
proton magnetic resonance spectroscopy in patients cured of acute
lymphoblastic leukemia with previous intrathecal methotrexate and
cranial irradiation prophylaxis. Int J Radiat Oncol Biol Phys.
50:759–763. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ficek K, Blamek S, Syguła D, Miszczyk L,
Sońta-Jakimczyk D and Tarnawski R: Evaluation of the late effects
of CNS prophylactic treatment in childhood acute lymphoblastic
leukemia (ALL) using magnetic resonance spectroscopy. Acta
Neurochir Suppl. 106:195–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumar R, Nijalingappa S, Grainger J and
Ismayl O: Acute disseminated encephalomyelitis mimicking late CNS
relapse of acute lymphoblastic leukaemia: Case report. J Med Case
Rep. 1:42007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferrés GM, Bidart HT and Zubieta AM:
Important of images and etiological diagnosis of central nervous
system involvement in immunocompromised patients. Rev Chilena
Infectol. 27:541–543. 2010.(In Spanish). PubMed/NCBI
|
|
40
|
Brandt MD, Brandt K, Werner A, Schönfeld
R, Loewenbrück K, Donix M, Schaich M, Bornhäuser M, von Kummer R,
Leplow B and Storch A: Preventive brain radio-chemotherapy alters
plasticity associated metabolite profile in the hippocampus but
seems to not affect spatial memory in young leukemia patients.
Brain Behav. 5:e003682015. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chan YL, Roebuck DJ, Yuen MP, Yeung KW,
Lau KY, Li CK and Chik KW: Long-term cerebral metabolite changes on
proton magnetic resonance spectroscopy in patients cured of acute
lymphoblastic leukemia with previous intrathecal methotrexate and
cranial irradiation prophylaxis. Int J Radiat Oncol Biol Phys.
50:759–763. 2001. View Article : Google Scholar : PubMed/NCBI
|