Introduction
Cyanobacteria frequently form water blooms in
eutrophic lakes or reservoirs. Microcystins, as important secondary
metabolites of cyanobacteria, include >90 known structural
variants (1), and have been
demonstrated to contribute to livestock poisoning and higher
incidences of primary human carcinoma, including liver carcinoma
(2), colorectal carcinoma (3) and oral cancer (4) via drinking water and food chains. Among
the variants of microcystins, microcystin-LR (MC-LR) is the most
poisonous and is a tumor initiator in humans (5,6). Recently,
efforts have been made to investigate the molecular mechanisms
underlying MC-LR-induced carcinogenesis (7,8). However,
little is known about the association between MC-LR exposure and
tumor migration and invasion, which is the primary cause of
mortality or poor prognosis in patients with cancer (9).
Cadherins are from a superfamily of >100
transmembrane glycoproteins that mediate intercellular adhesion by
calcium-dependent homophilic interactions (10). Cadherins serve important roles in
cell-cell junctions, tissue architecture and cell polarity in
addition to cell movement and proliferation (11). It has previously been demonstrated
that that CDH1/E-cadherin (12) and
CDH13/H-cadherin (13) are functional
tumor suppressors, while little is known about whether other
cadherins exhibit cancer-associated activity. Cadherin-11 (CDH11),
also known as osteoblast cadherin, is broadly expressed in human
normal mesoderm-derived tissues (14). Previous studies have reported that
CDH11 is associated with tumorigenesis and tumor progression; its
tumor-promoting activity was reported in prostate cancer (15), breast cancer (16) and glioblastoma (17).
Previous studies from our group revealed the active
effect of MC-LR on cancer cell migration and invasion (18–20) and a
previous tumor metastasis PCR array demonstrated that MC-LR
exposure significantly increased the expression of eight genes,
including CDH11 (20). In the present
study, the data demonstrated that MC-LR enhances carcinoma cell
motility and invasiveness by regulating CDH11 expression in human
colorectal cancer HT-29 cells.
Materials and methods
Cell culture and exposure
HT-29 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and maintained in a
monolayer culture in Dulbecco's modified Eagle's medium (DMEM;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), containing
100 µg/ml streptomycin, 100 µg/ml penicillin and 10% fetal bovine
serum (FBS; Hyclone; GE Healthcare Life Sciences). Cells were
cultured in a humidified atmosphere of 5% CO2 at 37°C.
HT-29 cell culture was performed according to the manufacturer's
protocol and as previously described by Miao et al (18). MC-LR (Enzo Life Sciences, Inc.,
Farmingdale, NY, USA) was dissolved in sterile water to make a 0.25
nM stock solution and then added in the medium to reach different
doses (0, 1, 5, 12.5, 25 and 50 nM).
Cellular immunofluorescence
After 48 h treatment with MC-LR of 25 nM, cells
grown on coverslips were washed with PBS, fixed by immersion at
room temperature with 4% polyformaldehyde for 15 min and
permeabilized with 0.1% Triton-X-100 in PBS at 4°C for 15 min.
Slides were then washed with PBS and blocked with blocking buffer
consisting of 4% bovine serum albumin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in PBS for 30 min at room temperature. The
cells were then incubated with a mouse anti-human CDH11 monoclonal
antibody (cat. no. MAB1790; 1 µg/ml; R&D Systems, Inc.,
Minneapolis, MN, USA) in blocking buffer overnight at 4°C, followed
by incubation with a goat anti-mouse phycoerythrin-labeled antibody
(cat. no. sc-3738; dilution, 1:100; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) in blocking buffer at room temperature for 2 h.
Then cells on coverslips were captured and analyzed by fluorescence
microscopy (magnification, ×400; Axio Observer A1; Carl Zeiss AG,
Oberkochen, Germany). Cellular immunofluorescence was performed
according to the manufacturer's protocol and as previously
described by Zhang et al (20).
Knockdown of CDH11 using small
interfering RNA in HT-29 cells
The CDH11 and negative control small interfering
RNAs (siRNAs) were purchased from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The sequences of each siRNA
were as follows: CDH11 number 1, 5′-AGGAAGUAGGAAGAGUGAAAGCUAA-3′;
CDH11 number 2, 5′-CAACAUCACUGUCUUUGCAGCAGAA-3′; and CDH11 number
3, 5′-CAUCGUCAUUCUCCUGGUCAUUGUA-3′ (21), these three siRNA sequences were mixed
at a ratio of 1:1:1 prior to the experiment. A random sequence
control was used as the negative control (forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′). Cells were plated at a density of
8×104 cells/well in six-well plates. Following
pre-incubation of the siRNA with Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) in serum-free Opti-MEM
medium (Invitrogen; Thermo Fisher Scientific, Inc.) for 20 min,
cells were transfected with CDH11 or negative control siRNA oligo
duplexes for 6 h, then cells were switched into fresh medium and
incubated at 37°C in a humidified atmosphere of 5% CO2.
Following 24 h, cells were collected and used for migration or
invasion assays, and 48 h later for western blot analysis. All
transfections were performed according to the manufacturer's
protocol and as previously described (18).
Western blotting
To investigate CDH11 expression, a standard western
blotting analysis was conducted. Following 48 h culture with MC-LR,
or being transfected with CDH11 or negative control siRNA
oligoduplexes, the cells were lysed with RIPA cell lysis buffer 1
(Beyotime Institute of Biotechnology, Haimen, China) containing 1
mM phenylmethylsulfonyl fluoride. The total cellular protein (30
µg/lane) was then boiled for 5 min in SDS-sample buffer (Beyotime
Institute of Biotechnology) before being subjected to SDS-PAGE (8%)
and transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Membranes were then blocked for 2 h
with nonfat dry milk in sterile water at room temperature and then
incubated overnight at 4°C with a mouse anti-human CDH11 monoclonal
antibody (cat. no. 32–1700; 2 µg/ml; Invitrogen; Thermo Fisher
Scientific, Inc.). Following four washes in TBS-Tween, membranes
were incubated with a horse anti-mouse secondary antibody (cat. no.
7076; dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA) for 2 h. The blots were visualized by Enhanced
Chemiluminescence (Thermo Fisher Scientific, Inc.) and imaged using
aTanon-5200 imaging system machine (Tanon Science and Technology,
Co., Ltd., Nanjing, China) and the membrane was probed with an
anti-GAPDH antibody (cat. no. 5174; dilution 1:2,000; Cell
Signaling Technology, Inc.) to confirm equal loading. The specific
western blot assay method was performed according to the
manufacturer's protocol and as described by Miao et al
(18), andthe quantitative analysis
was performed usingGel Image System version 4.00 (Tanon Science and
Technology, Co., Ltd., Shanghai, China).
Migration and invasion assay
Transwell assays were conducted to investigate the
motility and invasiveness of HT-29 cells. Cell migration assays
were performed in 24-well Transwell chambers with 8.0-µm pore size
transwell inserts (Costar; Corning Incorporated, Corning, NY,
USA,). Cell invasion assays were investigated using an 8.0-µm
filter coated with 30 µl of 0.5% Matrigel (Sigma-Aldrich; Merck
KGaA). Transwell assays were performed according to the
manufacturer's protocol and as previous described (18). Briefly, 4×104 cells treated
with MC-LR or transfected with siRNA were added to the upper
chamber of DMEM, and DMEM with 10% FBS was added to the lower
chamber. Following incubation for 48 or 72 h (migration assay) and
72 or 96 h (invasion assay) at 37°C in a humidified atmosphere of
5% CO2, cells on the upper surface were removed
completely by gentle wiping with a cotton swab. Cells that
penetrated through pores and adhered to the underside of the
filters were then fixed with 4% paraformaldehyde and stained with
0.02% crystal violet solution containing 20% ethanol. Then cells
were observed and counted under light microscopy (magnification,
×100) (CKX41 microscope; Olympus Corporation, Tokyo, Japan). For
each replicate (n=3), cells in three randomly selected fields per
well were counted and averaged. Data are expressed as a ratio to
the control (no MC-LR treatment but negative control siRNA
treatment). Tumor cell motility and invasiveness was defined as the
mean number of the treated cells.
Statistical analysis
All statistical analyses were carried out by
two-tailed Student's t-test or a one-way analysis of variance. Data
are presented as the mean ± standard deviation from three different
experiments. P<0.05 was considered to indicate a statistically
significant difference. Computer-based calculations were conducted
using Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA,
USA).
Results
Activation of cell migration and
invasion in microcystin-LR-treated HT-29 cells
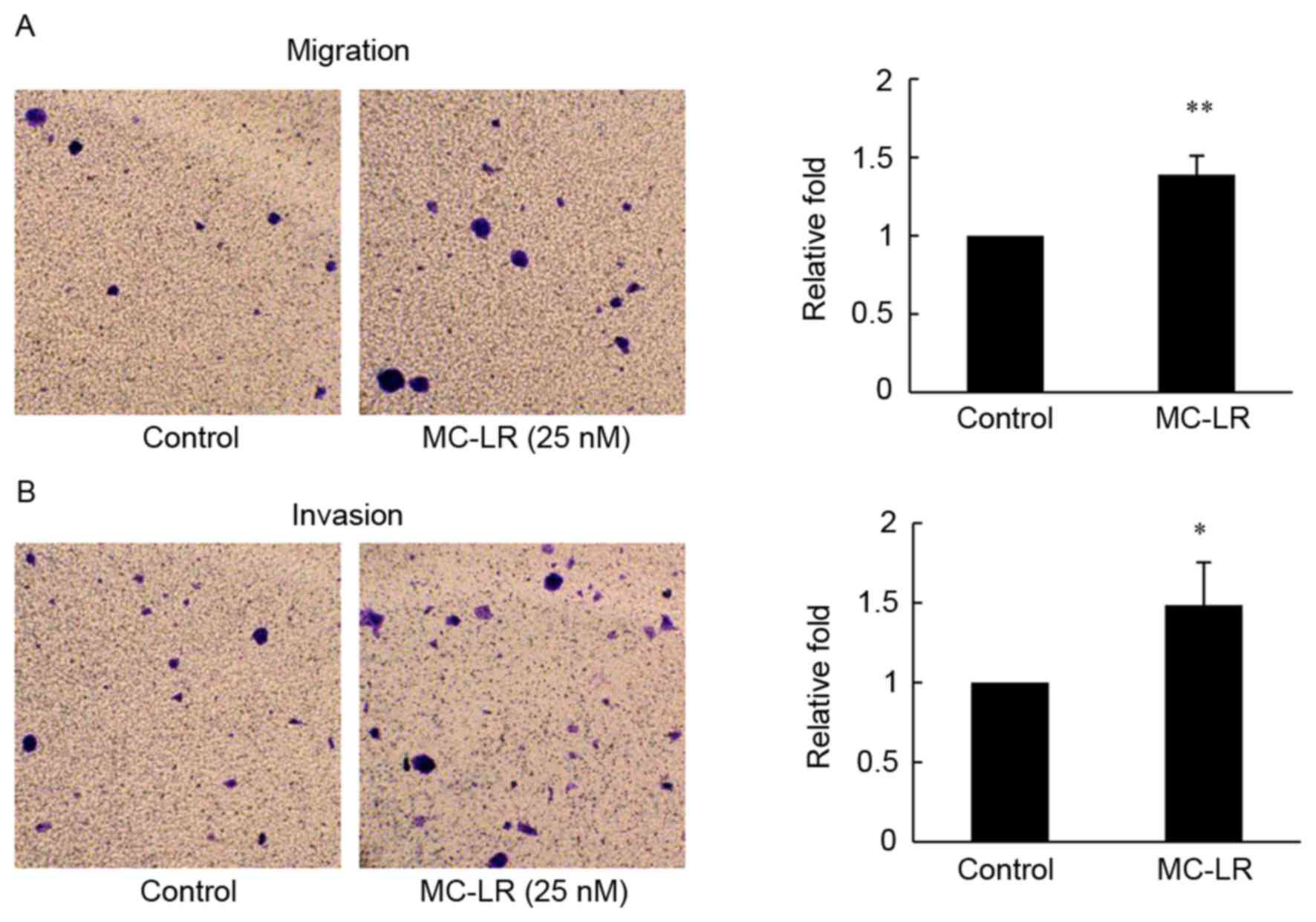
To investigate the motility and invasiveness of the
HT-29 cells following MC-LR treatment, Transwell chamber assays
were performed. In the present study, MC-LR at 25 nM promoted
migration and invasion of HT-29 cells and significantly increased
the number of translocated cells compared with the control group
(Fig. 1). MC-LR treatment resulted in
a 1.4-fold increase in cell migration (P<0.01; Fig. 1A), suggesting that MC-LR induces
migration of HT-29 cells. The results from the invasion assay in
HT-29 cells demonstrated that MC-LR exposure led to a 1.5-fold
increase in cell number compared with the control group (P<0.05;
Fig. 1B).
Upregulation of CDH11 in
microcystin-LR-treated HT-29 cells
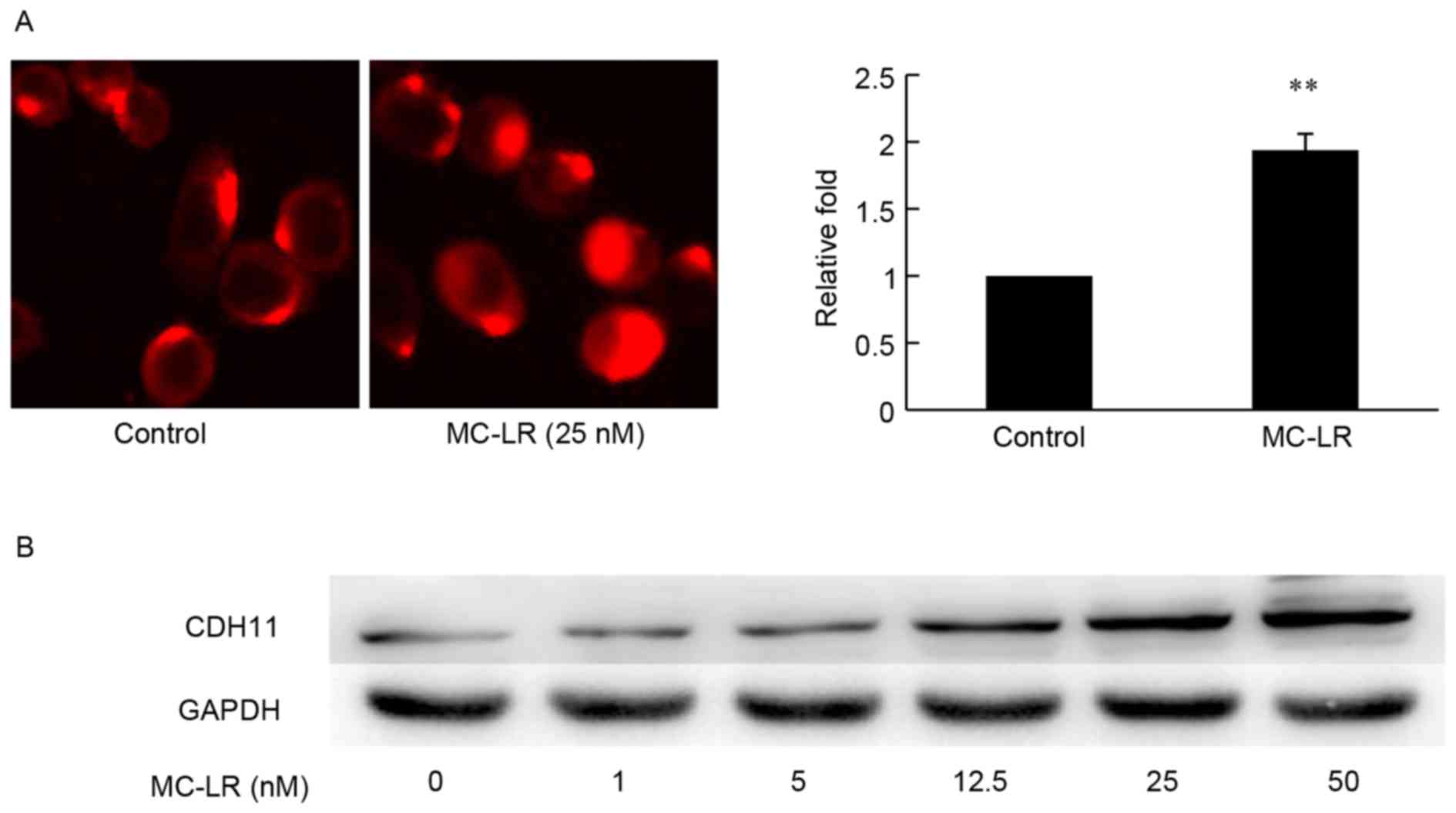
To confirm the effect of MC-LR treatment on CDH11
expression, the protein levels of CDH11 in HT-29cancer cells
treated with different concentrations of MC-LR were investigated.
MC-LR treatment resulted in a significant increase in CDH11
expression in HT-29cancer cells as measured by cellular immune
fluorescence. Compared with the control, cell treatment with 25 nM
of MC-LR produced a 1.9-fold raise in fluorescence intensity of
CDH11 (P<0.01; Fig. 2A). Similar
to the results of immunofluorescence assays, western blot analysis
demonstrated a marked increase in protein levels with MC-LR
treatment, particularly at 12.5, 25, 50 nM compared with GAPDH
expression (Fig. 2B). The results
from cellular immunofluorescence and western blotting revealed that
the protein level of CDH11 is upregulated by MC-LR in HT-29 cells,
which may mediate the effects of MC-LR on cell migration and
invasion.
Inhibition of migration and invasion
following knockdown of CDH11 in HT-29 cells
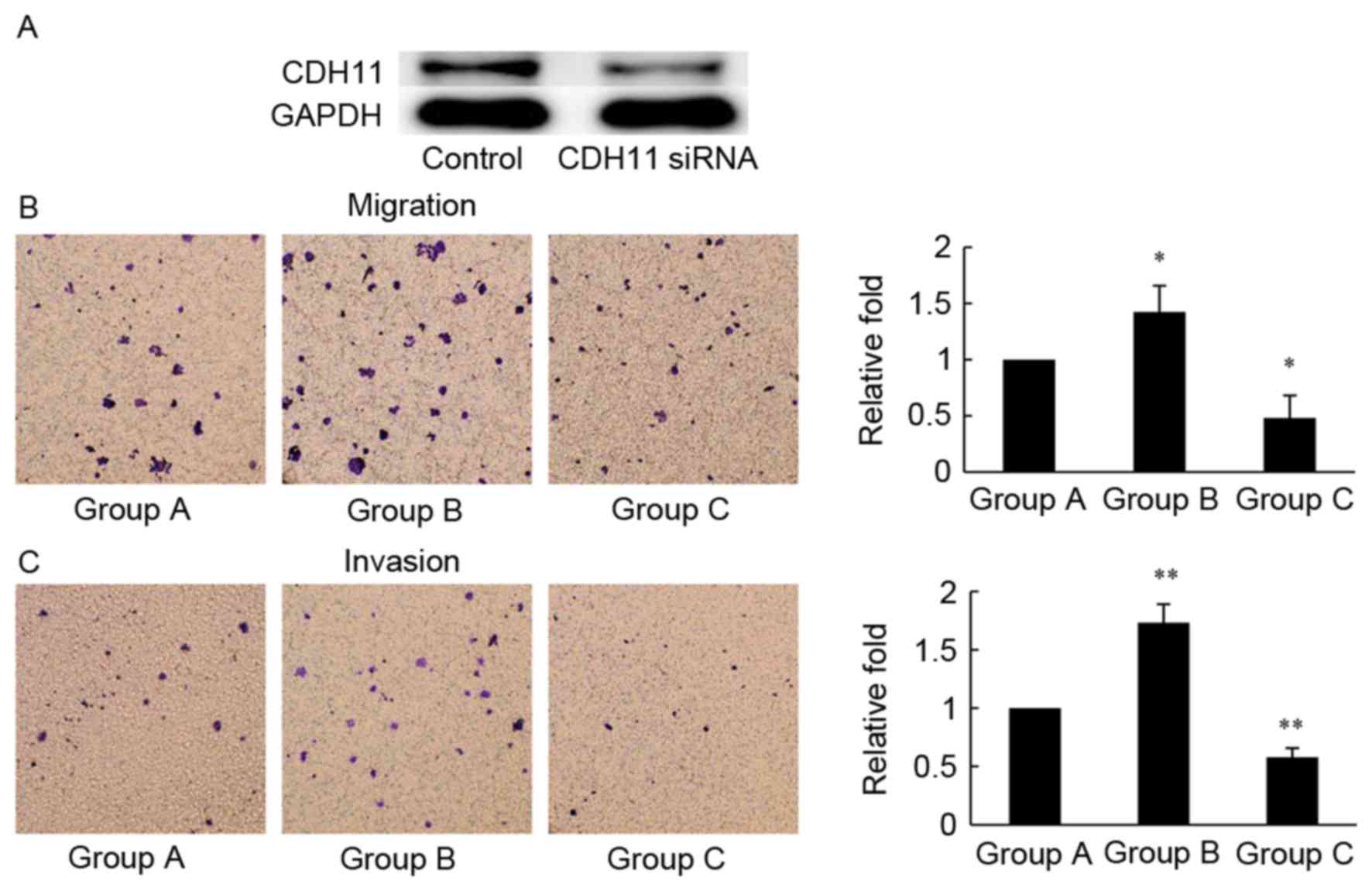
To further evaluate the association between
MC-LR-induced CDH11 overexpression, and tumor migration and
invasion, an RNA interference assay was performed. Western blot
analysis demonstrated that CDH11-siRNA markedly reduced the
expression of CDH11 in HT-29 cells (Fig.
3A). Following treatment with CDH11-siRNA, cells exposed to
MC-LR (25 nM; Group C) exhibited significantly decreased motility
(0.52-fold; P<0.05) and invasiveness (0.42-fold; P<0.01)
compared with the control group (Group A, negative control siRNA),
whereas treatment with MC-LR in the presence of the negative
control siRNA (Group B) significantly increased cell migration
(0.43-fold; P<0.05) and invasion (0.73-fold; P<0.01) compared
with the control group (Group A), and in the presence of MC-LR, the
knockdown of CDH11 reduced cell migration and invasion ability by
approximately0.67-fold (Fig. 3B and
C). These data revealed that MC-LR-induced migration and
invasion decreases following CDH11 knockdown, suggesting that CDH11
serves an important role in MC-LR-induced migration and invasion of
HT-29 cells.
Discussion
Colorectal carcinoma is one of the most frequent
forms of cancer in humans and is among the leading causes of
cancer-associated mortality, which is primarily caused by cancer
cell metastasis (9). Previous studies
have demonstrated that MC-LR serves a role as a tumor initiator
(2–4,6); however,
the molecular mechanisms underlying this effect remain unclear. A
previous study by our group demonstrated that MC-LR promotes
migration and invasion of a number of colorectal cell lines,
including HT-29 cells (18). A
previous tumor metastasis PCR array demonstrated that the
expression of CDH11geneincreased significantly following MC-LR
exposure (20). The results from the
present study suggested that MC-LR upregulates CDH11, and enhances
migration and invasion, as migration and invasion induced by MC-LR
was suppressed by silencing of CDH11 in HT-29 cells. These results
suggest that CDH11 is a tumor promoter that serves an important
role in MC-LR-induced migration and invasion.
A previous study reported MC-LR induces
reorganization or collapse of the cytoskeleton (22), which may be responsible for increased
migration and invasion induced by MC-LR. CDH11 contains a
cytoplasmic tail that binds to p120 catenin and β-catenin like
other cadherin family members (23).
p120 catenin mediates small GTPases, including Rho and Rac
(24), and attaches cadherin to
microtubules (25), while β-catenin
interacts with α-catenin, which regulates the actin cytoskeleton
(24). In addition, Li et al
(26) has also reported that CDH11
recruits Rac-specific GEFTrio to the plasma membrane, which
elevates Rac activity and promotes the formation of membrane
ruffles (27) in breast tumorigenesis
(26,28). Therefore, CDH11 may serve a role in
cytoskeleton reorganization and cell motility induced by MC-LR.
Another study demonstrated that human angiomotin (Amot)-p80 is a
distinct component of the CDH11 protein complex and deletion of the
Amot binding motif in the CDH11 cytoplasmic domain significantly
reduces migration of human prostate cancer cells (29), which is in line with the results of
the present study, which demonstrated that knockdown of CDH11 leads
to inhibition of cell migration. In addition, it was reported that
CDH11 engagement increases the expression of matrix
metalloproteinases (MMPs) through a mitogen-activated protein
kinase (MAPK) and nuclear factor-κB (NF-κB)-dependent signaling
pathway in rheumatoid arthritis synovial fibroblasts (30). MMP overexpression promotes cell
invasion through degradation of the basement membrane (31); this may be responsible for the
reduction of cell invasion and inhibition following CDH11
knockdown. A previous study reported that MC-LR exposure increases
MMP-2/−9 expression and promotes melanoma cell invasion by
activating phosphatidylinositol 3-kinase/RAC-alpha
serine/threonine-protein kinase (19)
and NF-κB signaling (20). MMPs may
serve an important role in MC-LR and CDH11-induced cell invasion.
MC-LR may upregulate CDH11 through a MC-LR-NF-κB signaling axis,
which requires further study. In addition, it has been reported
that tumor necrosis factor-α (TNF-α) induces CDH11 expression in
vascular smooth muscle cell and inhibition of CDH11 attenuates
smooth muscle cell migration (32).
TNF-α is a inflammation and tumor promoter (33) that may be induced by activation of the
NF-κB signaling pathway (34). A
previous study revealed that MC-LR induced TNF-α expression in the
cells of target tissues and that TNF-α is an endogenous tumor
promoter (35). Therefore, TNF-α may
serve a role in MC-LR-induced CDH11 upregulation.
The present study suggests that silencing of CDH11
expression may be a potential novel and promising therapeutic
strategy for treating and improving the survival rate of patients
with colorectal cancer induced by MC-LR or other CDH11-dependent
malignancies. Future work may include prolonged exposure of
sublethal concentrations of MC-LR in nude mice models, and the
elucidation of possible molecular mechanisms mediating
MC-LR-induced CDH11 activation and CDH11-promoted migration and
invasion.
Acknowledgements
The present study was financially supported by the
National Natural Science Foundation of China (grant nos. 21177062
and 21577066), the Priority Academic Program Development of Jiangsu
Higher Education Institutions (grant no. JX10131801049) and the
Innovation and Entrepreneurship Training Program for College
Students in Jiangsu Province (grant no. 201410312044Y).
Glossary
Abbreviations
Abbreviations:
|
MC-LR
|
microcystin-LR
|
|
CDH11
|
cadherin-11
|
|
siRNA
|
small interfering RNA
|
|
amot
|
angiomotin
|
|
MMPs
|
matrix metalloproteinases
|
|
MAPKs
|
mitogen-activated protein kinases
|
|
NF-κB
|
nuclear factor-κB
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Chakrabarti SK, Bai C and Subramanian KS:
DNA-Protein crosslinks induced by nickel compounds in isolated rat
renal cortical cells and its antagonism by specific amino acids and
magnesium ion. Toxicol Appl Pharmacol. 154:245–255. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ueno Y, Nagata S, Tsutsumi T, Hasegawa A,
Watanabe MF, Park HD, Chen GC, Chen G and Yu SZ: Detection of
microcystins, a blue-green algal hepatotoxin, in drinking water
sampled in Haimen and Fusui, endemic areas of primary liver cancer
in China, by highly sensitive immunoassay. Carcinogenesis.
17:1317–1321. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou L, Yu H and Chen K: Relationship
between microcystin in drinking water and colorectal cancer. Biomed
Environ Sci. 15:166–171. 2002.PubMed/NCBI
|
|
4
|
Choi P, Jordan CD, Mendez E, Houck J, Yueh
B, Farwell DG, Futran N and Chen C: Examination of oral cancer
biomarkers by tissue microarray analysis. Arch Otolaryngol Head
Neck Surg. 134:539–546. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Cai C, Fang W, Wang J, Zhang Y,
Liu J and Jia X: Oxidative damage and apoptosis induced by
microcystin-LR in the liver of Rana nigromaculata in vivo. Aquat
Toxicol. 140–141, 1 18. 2013.PubMed/NCBI
|
|
6
|
Zegura B, Volcic M, Lah TT and Filipic M:
Different sensitivities of human colon adenocarcinoma (CaCo-2),
astrocytoma (IPDDC-A2) and lymphoblastoid (NCNC) cell lines to
microcystin-LR induced reactive oxygen species and DNA damage.
Toxicon. 52:518–525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gaudin J, Huet S, Jarry G and Fessard V:
In vivo DNA damage induced by the cyanotoxin microcystin-LR:
Comparison of intra-peritoneal and oral administrations by use of
the comet assay. Mutat Res. 652:65–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puerto M, Pichardo S, Jos A, Prieto AI,
Sevilla E, Frías JE and Cameán AM: Differential oxidative stress
responses to pure Microcystin-LR and Microcystin-containing and
non-containing cyanobacterial crude extracts on Caco-2 cells.
Toxicon. 55:514–522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoon SS and Tanabe KK: Surgical treatment
and other regional treatments for colorectal cancer liver
metastases. Oncologist. 4:197–208. 1999.PubMed/NCBI
|
|
10
|
Wheelock MJ and Johnson KR: Cadherins as
modulators of cellular phenotype. Annu Rev Cell Dev Biol.
19:207–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berx G and van Roy F: Involvement of
members of the cadherin superfamily in cancer. Cold Spring Harb
Perspect Biol. 1:a0031292009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Margulis A, Zhang W, Alt-Holland A,
Crawford HC, Fusenig NE and Garlick JA: E-cadherin suppression
accelerates squamous cell carcinoma progression in
three-dimensional, human tissue constructs. Cancer Res.
65:1783–1791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andreeva AV and Kutuzov MA: Cadherin 13 in
cancer. Gene Chromosome Cancer. 49:775–790. 2010.
|
|
14
|
Van Roy F: Beyond E-cadherin: Roles of
other cadherin superfamily members in cancer. Nat Rev Cancer.
14:121–134. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang CF, Lira C, Chu K, Bilen MA, Lee YC,
Ye X, Kim SM, Ortiz A, Wu FL, Logothetis CJ, et al: Cadherin-11
increases migration and invasion of prostate cancer cells and
enhances their interaction with osteoblasts. Cancer Res.
70:4580–4589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pishvaian MJ, Feltes CM, Thompson P,
Bussemakers MJ, Schalken JA and Byers SW: Cadherin-11 is expressed
in invasive breast cancer cell lines. Cancer Res. 59:947–952.
1999.PubMed/NCBI
|
|
17
|
Kaur H, Phillips-Mason PJ, Burden-Gulley
SM, Kerstetter-Fogle AE, Basilion JP, Sloan AE and Brady-Kalnay SM:
Cadherin-11, a marker of the mesenchymal phenotype, regulates
glioblastoma cell migration and survival in vivo. Mol Cancer Res.
10:293–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miao C, Ren Y, Chen M, Wang Z and Wang T:
Microcystin-LR promotes migration and invasion of colorectal cancer
through matrix metalloproteinase-13 up-regulation. Mol Carcinog.
55:514–524. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu PF, Zhang XX, Miao C, Fu Z, Li Z, Zhang
G, Zheng M, Liu Y, Yang L and Wang T: Promotion of melanoma cell
invasion and tumor metastasis by microcystin-LR via
phosphatidylinositol 3-Kinase/AKT pathway. Environ Sci Technol.
47:8801–8808. 2013.PubMed/NCBI
|
|
20
|
Zhang XX, Fu Z, Zhang Z, Miao C, Xu P,
Wang T, Yang L and Cheng S: Microcystin-LR promotes melanoma cell
invasion and enhances matrix metalloproteinase-2/−9 expression
mediated by NF-kappaB activation. Environ Sci Technol.
46:11319–11326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao H, Miura Y, Yoshioka S, Miura M,
Hayashi Y, Tamura A, Iwasa M, Sato A, Hishita T, Higashi Y, et al:
Parathyroid hormone enhances hematopoietic expansion via
upregulation of cadherin-11 in bone marrow mesenchymal stromal
cells. Stem Cells. 32:2245–2255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Liu JH, Huang P, Guo ZL and Xu LH:
Alterations of tau and VASP during microcystin-LR-induced
cytoskeletal reorganization in a human liver cell line. Environ
Toxicol. 30:92–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brasch J, Harrison OJ, Honig B and Shapiro
L: Thinking outside the cell: How cadherins drive adhesion. Trends
Cell Biol. 22:299–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cavallaro U and Dejana E: Adhesion
molecule signalling: Not always a sticky business. Nat Rev Mol Cell
Biol. 12:189–197. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harris TJ and Tepass U: Adherens
junctions: From molecules to morphogenesis. Nat Rev Mol Cell Biol.
11:502–514. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Guo Z, Chen H, Dong Z, Pan ZK, Ding
H, Su SB and Huang S: HOXC8-dependent cadherin 11 expression
facilitates breast cancer cell migration through Trio and Rac.
Genes Cancer. 2:880–888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
28
|
Li Y, Chao F, Huang B, Liu D, Kim J and
Huang S: HOXC8 promotes breast tumorigenesis by transcriptionally
facilitating cadherin-11 expression. Oncotarget. 5:2596–2607. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ortiz A, Lee YC, Yu G, Liu HC, Lin SC,
Bilen MA, Cho H, Yu-Lee LY and Lin SH: Angiomotin is a novel
component of cadherin-11/β-catenin/p120 complex and is critical for
cadherin-11-mediated cell migration. FASEB J. 29:1080–1091. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Noss EH, Chang SK, Watts GF and Brenner
MB: Modulation of matrix metalloproteinase production by rheumatoid
arthritis synovial fibroblasts after cadherin 11 engagement.
Arthritis Rheum. 63:3768–3778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roeb E, Arndt M, Jansen B, Schumpelick V
and Matern S: Simultaneous determination of matrix
metalloproteinase (MMP)-7, MMP-1, −3, and −13 gene expression by
multiplex PCR in colorectal carcinomas. Int J Colorectal Dis.
19:518–524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Monahan TS, Andersen ND, Panossian H,
Kalish JA, Daniel S, Shrikhande GV, Ferran C and Logerfo FW: A
novel function for cadherin 11/osteoblast-cadherin in vascular
smooth muscle cells: Modulation of cell migration and
proliferation. J Vasc Surg. 45:581–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Locksley RM, Killeen N and Lenardo MJ: The
TNF and TNF receptor superfamilies: Integrating mammalian biology.
Cell. 104:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujiki H and Suganuma M: Tumor
promoters-microcystin-LR, nodularin and TNF-alpha and human cancer
development. Anticancer Agents Med Chem. 11:4–18. 2011. View Article : Google Scholar : PubMed/NCBI
|