Introduction
In previous decades, colon cancer has become one of
the leading causes of cancer-associated mortality (1). Although traditional treatments including
surgery, radiation therapy and chemotherapy have been improved
(2), the evaluation and development
of new effective agents or phytochemicals is still required to
improve the survival rate.
With the development of natural medicinal chemistry
and molecular biology, new antitumor substances obtained from
traditional Chinese herbs are topics of much debate. In cellular
studies, luteolin (3′,4′,5,7-tetrahydroxyflavone), a common
constituent of flavone, identified in medicinal plants as well as
specific vegetables and spices, has been reported to possess
anti-inflammatory, antioxidant, anti-cancer and a number of other
activities (3–5). It has been demonstrated in a previous
study that luteolin delayed or blocked the development of cancer
cells in vitro and in vivo by providing protection
from carcinogenic stimuli, owing to inhibition of tumor cell
proliferation, induction of cell cycle arrest and induction of
apoptosis via intrinsic and extrinsic signaling pathways (6). However, the underlying mechanism of the
effects of luteolin on human colon cancer cells has not been
previously addressed. In the present study, LoVo cells were
therefore used as an appropriate model to evaluate the activity of
luteolin against human colon cancer using in vitro and in
vivo systems, and to provide further information regarding the
molecular mechanism of luteolin-mediated apoptosis and cell cycle
modulation. The results from the present study suggest that
luteolin may be a potential agent for the prevention and treatment
of human colon cancer.
Materials and methods
Main reagents
Luteolin was purchased from Sigma-Aldrich (EMD
Millipore, Billerica, MA, USA); and was dissolved in dimethyl
sulphoxide and its concentration was adjusted to 100 mmol/l, as a
stock solution. The Cell Counting kit-8 was supplied by Beyotime
Institute of Biotechnology (Haimen, China). Annexin V-fluorescein
isothiocyanate (FITC) apoptosis and cell cycle detection kits were
obtained from BD Biosciences (Franklin Lakes, NJ, USA). A
bicinchoninic acid protein assay kit was purchased from
Biosynthesis Biotechnology Co., Ltd. (Beijing, China) and
monoclonal antibodies, including rabbit anti-human cell division
cycle 2 (CDC2), cyclin-dependent kinase 2 (CDK2), cyclin B1, cyclin
A, apoptotic protease activating factor 1 (APAF-1), cytochrome c,
caspase-3, mouse anti-human procaspase-9, mouse anti-human
caspase-9 and mouse anti-human β-actin, were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Cell line and cell culture
The human colon cancer cell line, LoVo, was obtained
from the Institute of Biochemistry and Cell Biology, Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium supplemented with 10% (v/v) fetal calf serum, 100
U/ml penicillin, 100 µg/ml streptomycin and 1 mmol/l HEPES buffer
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) at 37°C in humidified air containing 5% CO2 until
they reached ~80% confluency, and the cells were used in subsequent
experiments.
Cell counting kit-8 (CCK8) assay
LoVo cells were trypsinized and plated at
4×103 cells/well in 96-well plates. Following incubation
for 24 h, various concentrations of luteolin (0, 10, 20, 40, 60 and
80 µmol/l) were added and cells were incubated for 12, 24, 48 and
72 h, respectively. Next, 10 µl CCK8 solution (5 g/l) in
phosphate-buffered saline (PBS) was added to each well. Following
incubation for an additional 3 h, the optical density for each well
was measured using a microculture plate reader (BioTek Instruments,
Inc., Winooski, VT, USA) at a wavelength of 450 nm.
Cell cycle analysis
A total of 4×105 LoVo cells per well were
seeded in six-well plates for 24 h at 37°C. The cells were washed,
replaced with fresh medium and subsequently incubated with various
doses of luteolin (0, 20, 40 and 60 µmol/l) for 12, 24 and 48 h.
The cells were then trypsinized, washed with PBS and stained with
50 µg/ml cold propidium iodide (PI) solution containing 0.1 mg/ml
RNase A in PBS (pH 7.4) for 30 min in the dark at room temperature.
Thereafter, cell cycle data analysis was performed using a
FACSCalibur flow cytometer with CellQuest V.3.3 software
(Becton-Dickinson; BD Biosciences, Franklin Lakes, NJ, USA).
Flow cytometric apoptosis assay
Following incubation with 0, 20, 40 and 60 µmol/l
luteolin for either 24 or 48 h. A total of 1×105 LoVo
cells were harvested, washed and resuspended with PBS. Apoptotic
cells were then identified using the FACSCalibur flow cytometer
(Becton-Dickinson) according to the manufacturer's protocol.
Briefly, the cells were washed and subsequently incubated for 15
min at room temperature in the dark in 100 µl 1X binding buffer
containing 5 µl Annexin V-FITC and 5 µl PI. Thereafter, the total
apoptosis rate was examined by flow cytometry.
Western blot analysis
Western blot analysis was performed as described
previously (7). Briefly, aliquots of
cell lysates containing 25 µg protein were separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis. Then,
electrophoresed proteins were transferred onto nitrocellulose
membranes and detected with specific primary and secondary
antibodies. Thereafter, the blots were visualized using an enhanced
chemiluminescence system (GE Healthcare Life Sciences, Little
Chalfont, UK), and the density of β-actin served as an internal
loading control.
Establishment and treatment of human
colon cancer xenografts
Six-week old BALB/C nude mice (18–22 g) were
obtained from Shanghai National Center for Laboratory Animals
(Shanghai, China). In the present study the nude mice, which were
fed with sterilized food and water ad libitum, were
maintained at a temperature of 22°C and a humidity environment
approximately 40–50% with a light-dark cycle of 12:12 h. All
research procedures carried out in the present study were approved
by the Medical Ethics Committee of Southeast University (Nanjing,
China).
To assess the effect of luteolin on tumorigenicity,
BALB/C nude mice were inoculated with LoVo cells for formatting
LoVo colon cancer xenografts. In brief, 30 nude mice were
inoculated subcutaneously into the flank with 100 ml exponentially
growing LoVo cells at a concentration of 5×106 cells/ml,
and allowed to proliferate for ~1 week. When the tumor volume of
mice reached ~100 mm3, they were randomly divided into
five groups with six mice in each group. Three of these groups were
administered 10, 20 or 40 mg/kg luteolin intraperitoneally on
alternate days for a month. The other groups were administered
either normal saline or 15 mg/kg 5-fluorouracil (5-FU)
intraperitoneally as controls. During the whole experimental
period, the feed intake and motor activity of mice were carefully
observed, their body weights were measured, and the tumor volumes
were calculated every 5 days using the following formula: Tumor
volume (mm3)=(1/2)xaxb2, where a is the
largest diameter (length) and b is the smallest diameter (width) of
the tumor. At the end of the experiments, the mice were sacrificed,
the excised primary tumor mass was weighed and the tumor volume was
calculated. Thereafter, the relative tumor volume (RTV) was
calculated as RTV=Vday X/Vfirst day, and the
inhibitory rate was calculated using the formula: Inhibitory rate
(%)=(1-RTVexperimental group/RTVcontrol
group)×100.
Statistical analysis
All data are expressed as the means ± standard
deviation for experiments performed in triplicate, and the data
were analyzed using the Statistical Package for the Social Sciences
(SPSS version 18.0; SPSS Inc., Chicago, IL, USA). Comparisons
between two groups were performed with unpaired Student's t-test
and those between three or more groups were done using one way
analysis of variance followed by the Student-Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of luteolin on growth of LoVo
cells
The growth inhibitory potential of luteolin was
determined in cultured LoVo cells by CCK8 assay at various
intervals (12, 24, 48 and 72 h) of treatment. As a result, luteolin
exhibited a significant growth inhibitory effect against LoVo
cells, and the concentration of luteolin required to yield a 50%
inhibitory concentration (IC50) of the proliferation, as
measured at the 24, 48 and 72 h time points, was 66.70, 41.49 and
30.47 µmol/l, respectively (Fig. 1).
Therefore, these results demonstrated that luteolin inhibited the
proliferation of LoVo cells significantly in a time- and
dose-dependent manner.
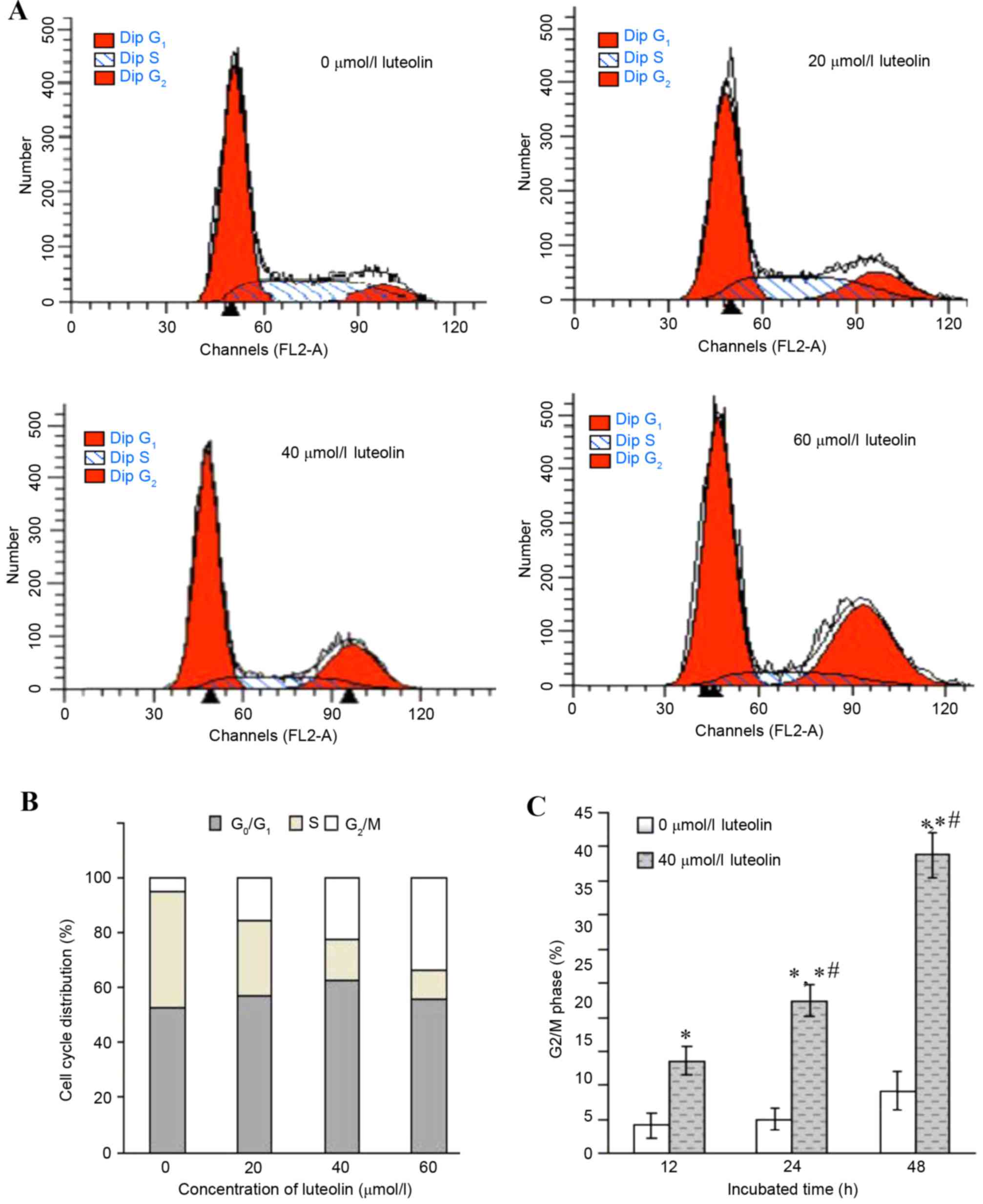
Effect of luteolin on cell cycle in
colon tumor cells
The inhibition of cell proliferation may be a result
of the induction of apoptosis, which may be mediated by cell cycle
arrest. Therefore, the cell cycle distribution in the LoVo cells
treated with luteolin was further analyzed for various times. An
increased percentage of cells in the G2/M phase together
with a decrease in the S phase was observed to occur in a
dose-dependent manner (Fig. 2A and
B), while the percentages of G0/G1 phase
cells remained at almost the same levels. When exposed to 40 µmol/l
luteolin for various times, the cell population of LoVo cells in
the G2/M phase was 13.62±2.15% at 12 h, 22.35±2.43% at
24 h and 43.76±3.21% at 48 h, respectively, and there were
significant differences compared with the control group (Fig. 2C; 5.07±1.64%; P<0.05).
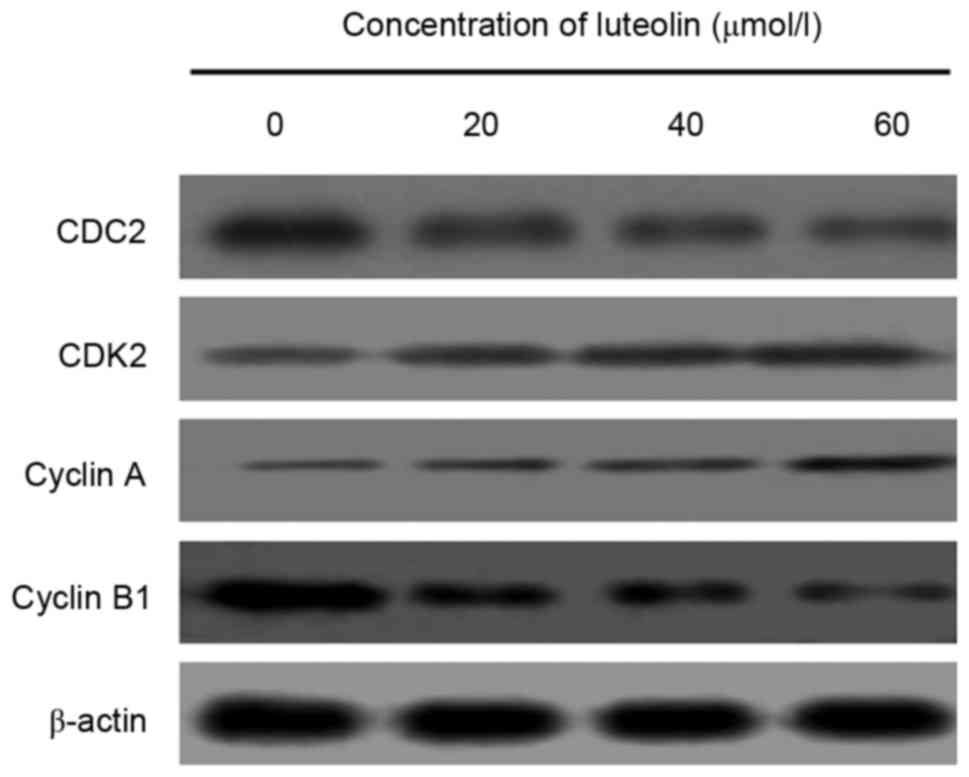
To investigate the apoptotic mechanisms through
which luteolin interferes with cell cycle progression, the
expression of cell cycle-associated proteins was measured following
treatment with various concentrations of luteolin for 48 h. The
measurement of cell cycle-associated protein markers revealed that
the protein expression levels of CDC2 and cyclin B, which regulate
G2/M transition in luteolin-treated LoVo cells, were
downregulated, whereas those of cyclin A and CDK2 were upregulated
in a dose-dependent manner (Fig.
3).
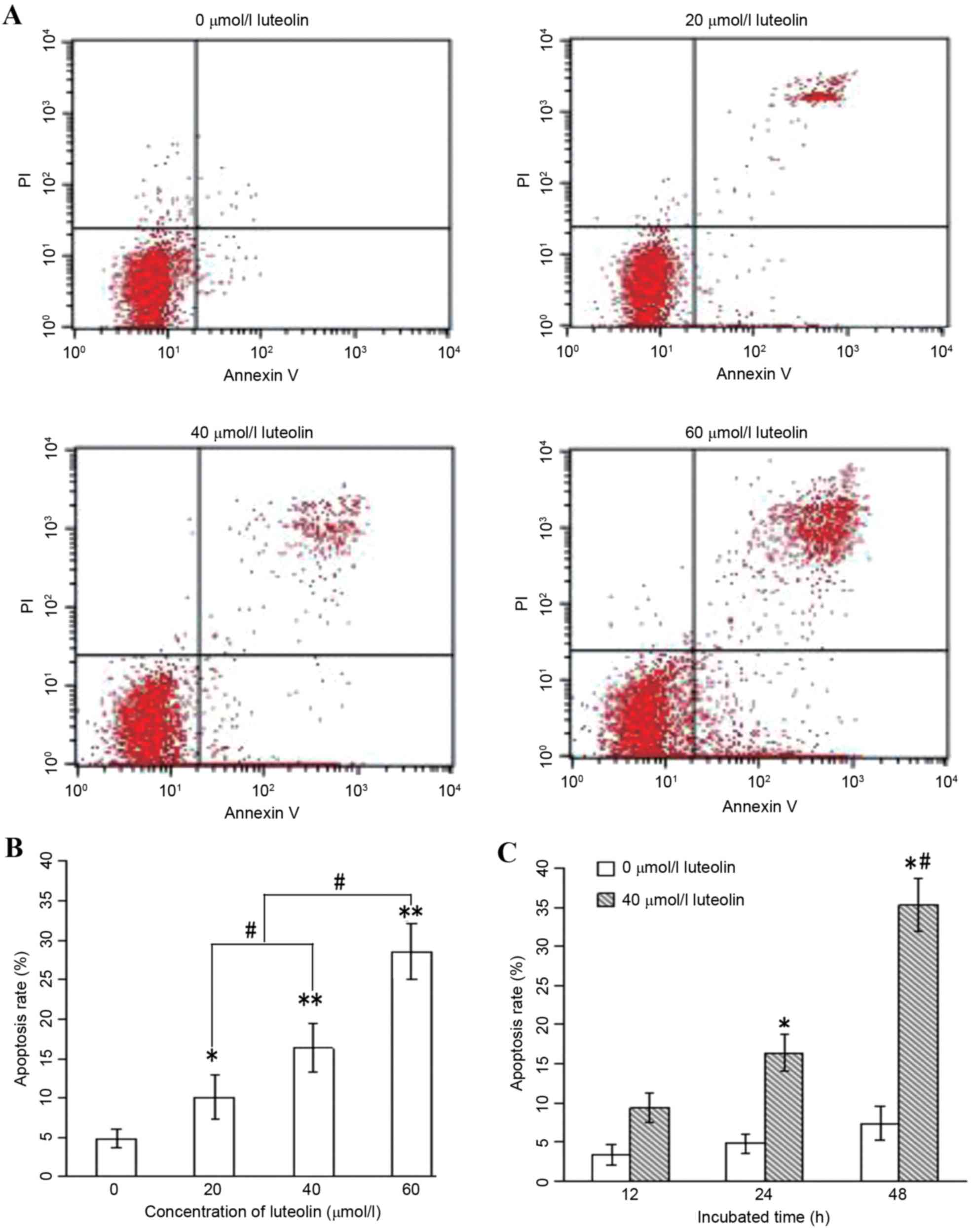
Effect of luteolin on apoptotic death
in colon cancer cells
Annexin V/PI analysis was applied to quantify the
percentage of cells undergoing apoptosis. Following treatment with
luteolin for 24 h, the total percentages of cells undergoing early
(Annexin-positive/PI-negative) and late
(Annexin-positive/PI-positive) apoptosis were measured, and the
results are shown in Fig. 4A and B.
These results indicate that luteolin induced apoptosis in the LoVo
cells in a dose-dependent manner. Furthermore, when the LoVo cells
were incubated with 40 µmol/l luteolin for 12, 24 and 48 h, the
apoptotic rate increased significantly with the prolonged duration
of the experiment (Fig. 4C;
P<0.05).
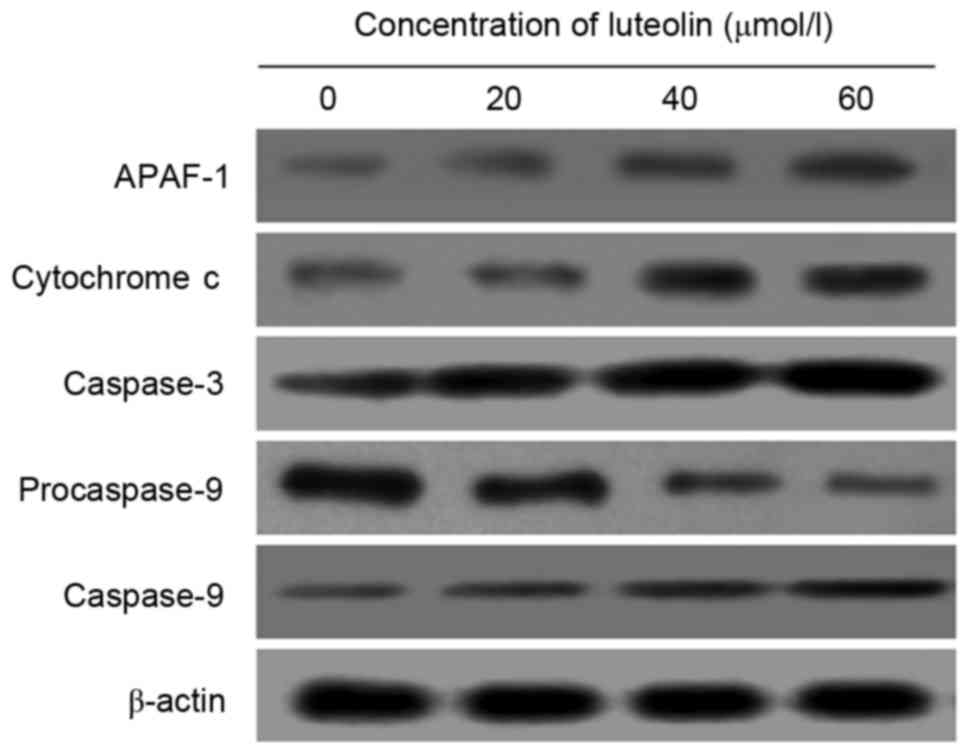
To explore the molecular mechanisms of luteolin on
apoptotic proteins, Western blot analysis was conducted to evaluate
the expression of APAF-1, cytochrome c, procaspase-9,
caspase-9 and caspase-3 proteins. Following treatment with luteolin
for 48 h, a significant decrease of procaspase-9 in LoVo cells was
observed in the groups treated with 20, 40 and 60 µmol/l luteolin
compared with the control group (P<0.05). By contrast, the
protein expression of APAF-1, cytochrome c, caspase-9 and
caspase-3 was significantly increased compared with the control
group (Fig. 5).
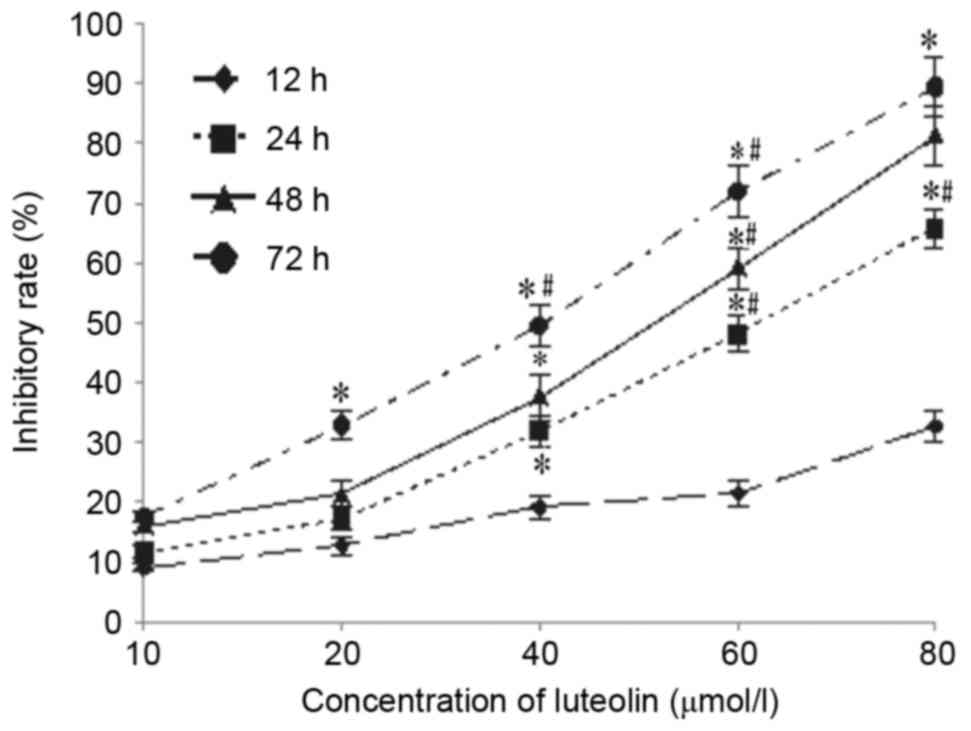
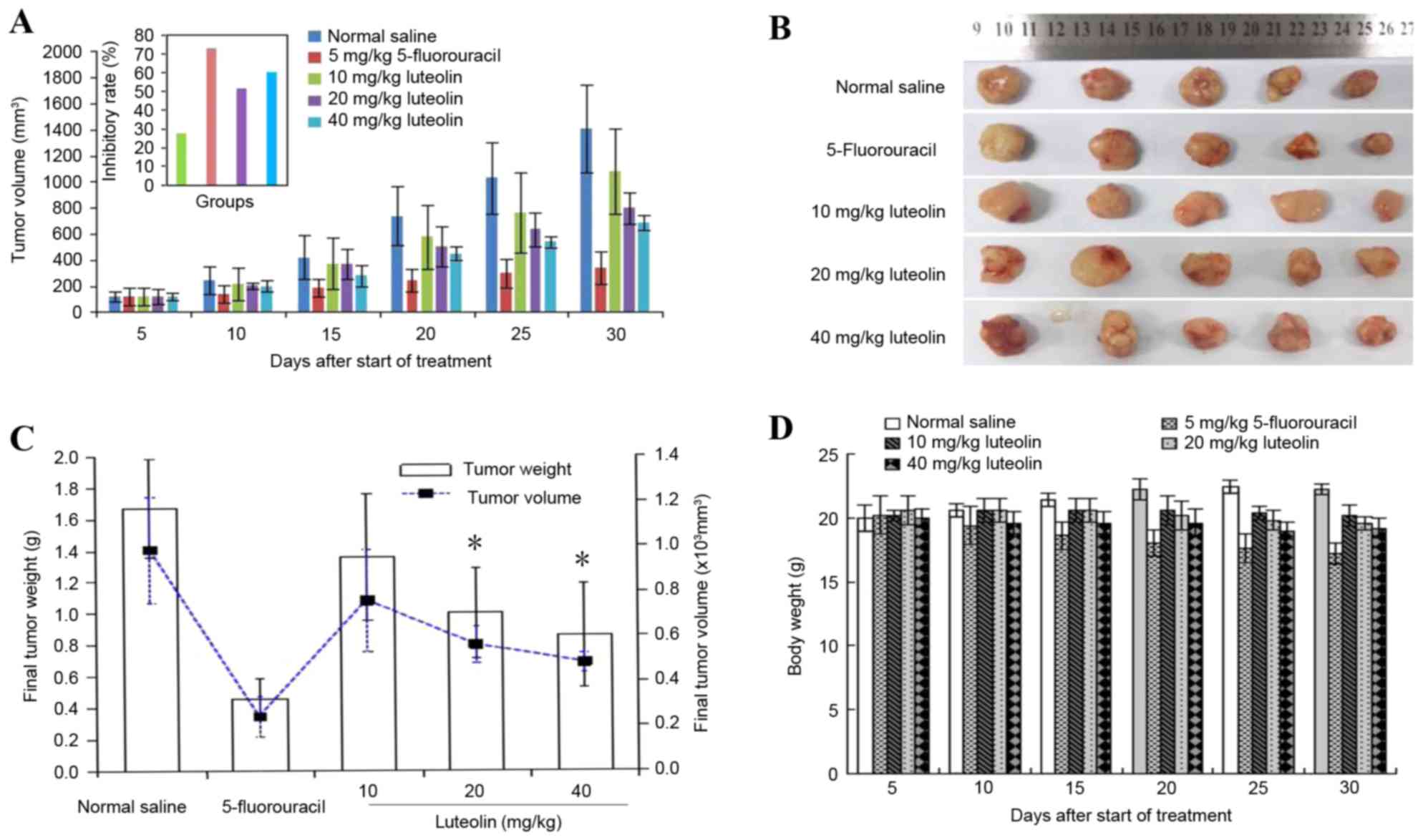
Effect of luteolin on tumorigenicity
in vivo
The incidence of subcutaneous tumors derived from
LoVo cells was 100%. Luteolin inhibited tumor growth in a dose- and
time-dependent manner (Fig. 6A and
B). On the final day of the experiment, the excised primary
tumor mass was 0.32±0.09 g for 15 mg/kg 5-FU, 0.70±0.20 g for 20
mg/kg luteolin, and 0.60±0.23 g for 40 mg/kg luteolin, which was
lower than that of the control group (1.17±0.29 g). Similar results
were obtained for the tumor volume, as shown in Fig. 6C, but there was no significant
difference between the group receiving a low dose of luteolin (10
mg/kg) and the control group (0.95±0.45 g vs. 1.17±0.29 g, and
1405.8±574.84 mm3 vs. 1081.39±794.58 mm3).
The tumor inhibition rate was 72.60% in the 5-FU group, 51.28% in
the 20 mg/kg luteolin group and 59.83% in the 40 mg/kg luteolin
group, which was higher than that in the 10 mg/kg luteolin group
(27.35%; P<0.05). In addition, mice treated with 20 and 40 mg/kg
luteolin consumed slightly more food than those in the control
group, but there was no mortality or significant change in mice
body weight observed throughout the experimental period in the
control group or luteolin-treated groups (Fig. 6D). These results suggest that luteolin
treatment significantly decreases colon tumor size and tumor weight
without having a significant effect on the food intake or total
body weight of the mice.
Discussion
Cancer is a multistep process that typically occurs
over an extended period of time, beginning with initiation followed
by promotion and progression (8).
Recently, there have been concentrated efforts to develop novel
dietary substances as cancer preventive and/or therapeutic agents
(9). Understanding how these natural
and synthetic compounds inhibit cell proliferation and cell
survival may play a critical role in the development of new agents
that prevent and treat cancer with low toxicity.
A growing body of evidence suggests that a number of
herbal medicines provide a significant curative effect by
inhibiting tumor cell proliferation and inducing apoptosis in tumor
cells (7,10,11). As
with numerous other flavonoids, luteolin is capable of inhibiting
the proliferation of cancer cells, inducing tumor cell apoptosis
and influencing tumor cell cycle distribution, as well as
inhibiting the formation of new blood vessels in tumors. In the
present study, the results of CCK8 assay demonstrated that luteolin
exerted significant cytotoxicity on LoVo cells, and that the
concentration of luteolin required to yield IC50 of the
proliferation decreased with the prolonged incubation time.
Therefore, the results demonstrate that luteolin significantly
inhibited the proliferation of LoVo cells in a time- and
dose-dependent manner. Similar observations have also been made in
human colon carcinoma HCT-15 cells (12). Notably, it has been reported that
there was no significant cytotoxicity in luteolin-treated normal
human peripheral blood mononuclear cells (13). Despite previous findings that the
decrease in cell proliferation and cell viability following
treatment with luteolin may be associated with the effect of cell
cycle arrest and/or the induction of apoptosis (14), the molecular mechanisms remain
elusive. In the present study, the LoVo cell line was used as a
model to provide in vitro evidence that luteolin induced
G2/M phase arrest of cell cycle progression and
apoptotic cell death, thus demonstrating the effect of luteolin on
the decrease of cell viability and induction of cell death.
It is known that cell cycle check points and
apoptosis play critical roles in the molecular pathogenesis of
cancer, and influence the outcome of chemotherapy and radiotherapy
(8). In the present study, an
increased percentage of cells in the G2/M phase together
with a decrease in S-phase cells was observed to occur in a dose-
and time-dependent manner, while the percentages of
G0/G1 phase cells remained at almost the same
levels in the colon cancer cells. These results clearly confirm the
effect of luteolin on the induction of G2/M cell cycle
arrest in colon cancer cells, and these results are supported by
other published studies using several other types of human colon
cancer cells, including HCT-15 cells (4,12).
Evidence in the literature suggests that cycle progression is
controlled by several CDKs and their cyclin partners. Among the
CDKs that regulate cell cycle progression, CDK1 and CDK2 are
activated primarily in association with cyclin A and cyclin B in
the cell division cycle (15–17). It is also worth noting that a key
regulator of the G2/M transition of the cell cycle is a
complex of CDC1/CDK2 and a B-type cyclin (18). If CDC1/CDK2 were inhibited, one would
expect an arrest at the G2/M transition. In the present
study, the protein expression levels of CDC2 and cyclin B were
downregulated, whereas those of cyclin A and CDK2 were upregulated
in a dose-dependent manner in luteolin-treated LoVo cells. In a
previous study, Lim et al (4)
demonstrated that luteolin-mediated negative regulation of CDC2
contributed to increasing G2/M arrest. Taken together,
these results demonstrate that treatment of luteolin triggers a
dose-dependent accumulation of G2/M phase colon cancer
cells through the inactivation of cyclin B1/CDC2.
Based on its relevant effects on cell growth and
cell cycle progression, there was a need to examine whether
luteolin was capable of inducing apoptosis in LoVo cells. Annexin
V/PI analysis was applied to quantify the percentage of cells
undergoing apoptosis. Following treatment with luteolin for 24 h,
the total percentages of cells undergoing early and late apoptosis
indicated that luteolin induced apoptosis in the LoVo cells in a
dose-dependent manner. Furthermore, the apoptotic rate increased
significantly with the prolonged duration of the experiment. These
results indicate that the induction of apoptosis by luteolin is
involved in its antitumor activity. It is known that APAF-1
contains a caspase recruitment domain (CARD) at the N terminus, a
nucleotide-binding domain, a helical domain, a winged helix domain,
a second helical domain and 15 WD40 repeats at the C-terminal half
(19), and exerts a critical role in
apoptosis. Upon binding to cytochrome c and deoxyadenosine
triphosphate (dATP), APAF-1 ‘calls’ caspase-9 through its CARD
domain to form apoptotic bodies, activates caspase-3 and initiates
the caspase cascade reaction, thereby leading to apoptosis
(20). Thus, in the present study,
the expression of certain key apoptotic proteins was assayed to
investigate the possible mechanism of luteolin-induced apoptosis. A
significant decrease of procaspase-9 in LoVo cells was observed
with the increase of luteolin. By contrast, the protein expression
of APAF-1, cytochrome c, caspase-9 and caspase-3 increased
significantly compared with the control group. The results of the
present study are consistent with those of a previous in
vitro study demonstrating that apoptosis induction of luteolin
was a significant cellular mechanism in inhibiting cell
proliferation in various cancer types (3,14). These
results further demonstrate that induction of luteolin on apoptosis
of LoVo cells may be achieved by the molecular mechanism of the
cytochrome c- and dATP-mediated activation of APAF-1.
To confirm the above inhibitory effect of luteolin
on human colon cancer cells in culture, an in vivo study was
conducted by transplanting human colon carcinoma cells into BALB/C
nude mice. The effects of luteolin were assessed by measuring
changes in the tumor volumes over a one-month treatment period.
Supporting the in vitro results, in vivo experiments
in nude mice with xenografted tumors revealed that luteolin
suppressed the growth of tumors formed from human colon carcinoma
cells in a dose- and time-dependent manner (Fig. 6A). Luteolin also significantly
decreased the colon tumor size and tumor weight on the final day of
the experiment. The present results are consistent with those of
previous studies demonstrating that luteolin inhibited tumor growth
and angiogenesis in xenografted tumors (21). In addition, BALB/C nude mice treated
with 20 and 40 mg/kg luteolin consumed slightly more food than
those in the control group, but there was no mortality or
significant changes in mouse body weight observed during the
experimental period in either the control group or the
luteolin-treated groups, suggesting that the dose of luteolin used
in the present study was well tolerated by the BALB/C nude mice.
Additionally, other studies have demonstrated that luteolin may be
administered orally or topically without any adverse effects on
xenografts (22,23), and that it demonstrates potential
cancer preventative effects (24).
These results imply that luteolin is relatively safe when used as
an anticancer agent.
In summary, the present study provides evidence that
the inhibition of tumor growth by luteolin is significantly
associated with cell cycle arrest at the G2/M phase
transition with the inactivation of cyclin B1/CDC2 and cell
apoptosis in part via the cytochrome c- and dATP-mediated
activation of APAF-1. These findings provide a relevant basis for
developing luteolin as a potential chemopreventive and
chemotherapeutic agent against human colon cancer.
Acknowledgements
The present study was supported by a grant from the
Natural Science Foundation of China (grant no. 81372985).
References
|
1
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang HF and Yang LL: Gamma-Mangostin, a
micronutrient of mangosteen fruit, induces apoptosis in human colon
cancer cells. Molecules. 17:8010–8021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu T, Li D and Jiang D: Targeting cell
signaling and apoptotic pathways by luteolin: Cardioprotective role
in rat cardiomyocytes following ischemia/reperfusion. Nutrients.
4:2008–2019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim DY, Jeong Y, Tyner AL and Park JH:
Induction of cell cycle arrest and apoptosis in HT-29 human colon
cancer cells by the dietary compound luteolin. Am J Physiol
Gastrointest Liver Physiol. 292:G66–G75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xavier CP, Lima CF, Preto A, Seruca R,
Fernandes-Ferreira M and Pereira-Wilson C: Luteolin, quercetin and
ursolic acid are potent inhibitors of proliferation and inducers of
apoptosis in both KRAS and BRAF mutated human colorectal cancer
cells. Cancer Lett. 281:162–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seelinger G, Merfort I, Wölfle U and
Schempp CM: Anti-carcinogenic effects of the flavonoid luteolin.
Molecules. 13:2628–2651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu H, Gao F, Shu G, Xia G, Shao Z, Lu H
and Cheng K: Wogonin inhibits the proliferation of myelodysplastic
syndrome cells through the induction of cell cycle arrest and
apoptosis. Mol Med Rep. 12:7285–7292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pandurangan AK, Dharmalingam P, Sadagopan
SK, Ramar M, Munusamy A and Ganapasam S: Luteolin induces growth
arrest in colon cancer cells through involvement of
Wnt/β-catenin/GSK-3β signaling. J Environ Pathol Toxicol Oncol.
32:131–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suh Y, Afaq F, Johnson JJ and Mukhtar H: A
plant flavonoid fisetin induces apoptosis in colon cancer cells by
inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways.
Carcinogenesis. 30:300–307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turktekin M, Konac E, Onen HI, Alp E,
Yilmaz A and Menevse S: Evaluation of the effects of the flavonoid
apigenin on apoptotic pathway gene expression on the colon cancer
cell line (HT29). J Med Food. 14:1107–1117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Gong Y, Li L, Abdolmaleky HM and
Zhou JR: Bioactive tanshinone I inhibits the growth of lung cancer
in part via downregulation of aurora a function. Mol Carcinog.
52:535–543. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sulaiman GM: In vitro study of molecular
structure and cytotoxicity effect of luteolin in the human colon
carcinoma cells. Eur Food Res Technol. 241:83–90. 2015. View Article : Google Scholar
|
|
13
|
Horinaka M, Yoshida T, Shiraishi T, Nakata
S, Wakada M, Nakanishi R, Nishino H, Matsui H and Sakai T: Luteolin
induces apoptosis via death receptor 5 upregulation in human
malignant tumor cells. Oncogene. 24:7180–7189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai X, Ye T, Liu C, Lu W, Lu M, Zhang J,
Wang M and Cao P: Luteolin induced G2 phase cell cycle arrest and
apoptosis on non-small cell lung cancer cells. Toxicol In Vitro.
25:1385–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krasinska L, Cot E and Fisher D: Selective
chemical inhibition as a tool to study Cdk1 and Cdk2 functions in
the cell cycle. Cell Cycle. 7:1702–1708. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Canavese M, Santo L and Raje N: Cyclin
dependent kinases in cancer: Potential for therapeutic
intervention. Cancer Biol Ther. 13:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nigg EA: Cyclin-dependent protein kinases:
Key regulators of the eukaryotic cell cycle. Bioessays. 17:471–480.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou M, Li Y, Hu Q, Bai XC, Huang W, Yan
C, Scheres SH and Shi Y: Atomic structure of the apoptosome:
Mechanism of cytochrome c- and dATP-mediated activation of Apaf-1.
Genes Dev. 29:2349–2361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun KW, Ma YY, Guan TP, Xia YJ, Shao CM,
Chen LG, Ren YJ, Yao HB, Yang Q and He XJ: Oridonin induces
apoptosis in gastric cancer through Apaf-1, cytochrome c and
caspase-3 signaling pathway. World J Gastroenterol. 18:7166–7174.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bagli E, Stefaniotou M, Morbidelli L,
Ziche M, Psillas K, Murphy C and Fotsis T: Luteolin inhibits
vascular endothelial growth factor-induced angiogenesis; inhibition
of endothelial cell survival and proliferation by targeting
phosphatidylinositol 3′-kinase activity. Cancer Res. 64:7936–7946.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ueda H, Yamazaki C and Yamazaki M:
Inhibitory effect of perilla leaf extract and luteolin on mouse
skin tumor promotion. Biol Pharm Bull. 26:560–563. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiu FL and Lin JK: Downregulation of
androgen receptor expression by luteolin causes inhibition of cell
proliferation and induction of apoptosis in human prostate cancer
cells and xenografts. Prostate. 68:61–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin Y, Shi RX, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|