Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of malignant lymphoma in adults, accounting for 31% of
all non-Hodgkin's lymphoma (NHL) in western countries (1). DLBCL can be divided into two main
subtypes, germinal center B-cell-like (GCB) and activated
B-cell-like (non-GCB), based on evaluation of the cell of origin
using gene expression profiling (2).
Non-GCB DLBCL tends to have an inferior prognosis compared to GCB
DLBCL, with a 3-year progression-free survival (PFS) rate of 40%
compared to 75% in GCB DLBCL (3).
The combination chemotherapy regimen with
cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)
plus rituximab (R-CHOP) is the new standard in first-line therapy
for DLBCL, which can significantly improve overall survival (OS) in
both GCB and non-GCB DLBCL. Previous studies have shown that 76% of
DLBCL patients acquire complete response (CR) with R-CHOP, while
~40% of patients will have an initial response followed by
refractory or relapsed disease and most of these patients will
eventually succumb to disease (4,5).
Therefore, researchers are studying the molecular biology and
genetics of tumor cells in order to discover novel biomarkers,
provide new therapeutic targets, and develop new ideas to improve
prognosis.
Myeloid differentiation factor 88 (MYD88) is
the first identified member of the Toll-interleukin-1 (IL-1)
receptor (TIR) family, an adaptor protein that mediates toll and
interleukin receptor signaling and activates nuclear factor-κB
(NF-κB) pathways (6). The
constitutive activation of NF-κB pathways is a distinguishing
feature of non-GCB DLBCL (7,8). Ngo et al identified that the
MYD88 signaling pathway is essential for the pathogenesis of
non-GCB DLBCL. Among mutations affecting this pathway, the
MYD88 L265P mutation is the most frequent and has the most
severe oncogenic effects through its alteration of NF-κB signaling
pathways (9). This mutation was
identified in 29% of non-GCB DLBCL but is rare in GCB DLBCL
(9).
To our best knowledge, there are seven studies
investigated the prognosis value of MYD88 L265P in DLBCL.
Three studies reported that the MYD88 L265P mutation was not
a significant prognostic indicator for DLBCL and primary breast
diffuse large B-cell lymphoma (PBDLBCL) (10–12).
Nevertheless, the other four studies found that MYD88 L265P
mutation was associated with poor prognosis of DLBCL, primary
cutaneous diffuse large B-cell lymphoma, and primary central
nervous system lymphoma (13–16). Researchers have not reached a
consensus regarding the role of MYD88 L265P as a prognostic
factor for this subset of DLBCL patients.
With the arrival of various targeted therapeutic
agents acting on NF-κB pathways, mutational analysis of a limited
number of genes in these pathways could help in selecting an
optimal treatment strategy in DLBCL (17,18). The
majority of non-GCB DLBCL patients treated with the R-CHOP regimen
have poor outcomes, which raises concerns regarding the
MYD88 L265P mutation. To the best of our knowledge, there
has been no analysis regarding the association between treatment
response to R-CHOP and the MYD88 L265P mutation in DLBCL
patients. Therefore, in our study we investigated the prevalence of
the MYD88 L265P mutation in patients with DLBCL and
evaluated its association with the response to R-CHOP and other
clinicopathologic characteristics, including patient outcome.
Materials and methods
Patients and sample collection
This study was retrospective in nature and included
53 patients who were newly diagnosed with DLBCL between January
2007 and January 2015 in the Sichuan Cancer Hospital based on the
current World Health Organization classifications (19). Inclusion criteria were as follows: i)
Available clinical and follow-up data; ii) CD20-positive; iii)
undergoing R-CHOP chemotherapy for at least 3 continuous cycles;
and iv) tumor samples available at diagnosis for DNA analysis.
Classification into the GCB/non-GCB subgroups by
immunohistochemistry followed the algorithm of Hans (20). Overall survival (OS) was defined as
the period from clear diagnosis to death, lost follow-up or
deadline. Progression-free survival (PFS) was defined as the period
from clear diagnosis of the tumor to first tumor progression,
death, lost follow-up or deadline.
DNA was extracted from 4% formalin-fixed
paraffin-embedded tissues with the QIAamp DNA FFPE Tissue kit
(Qiagen, Ltd., Sussex, UK) following the manufacturer's
instructions. The L265P mutant of MYD88 was prepared by PCR
with site-directed mutagenic primers using DNA from a healthy
individual as a positive control, and the wild-type MYD88
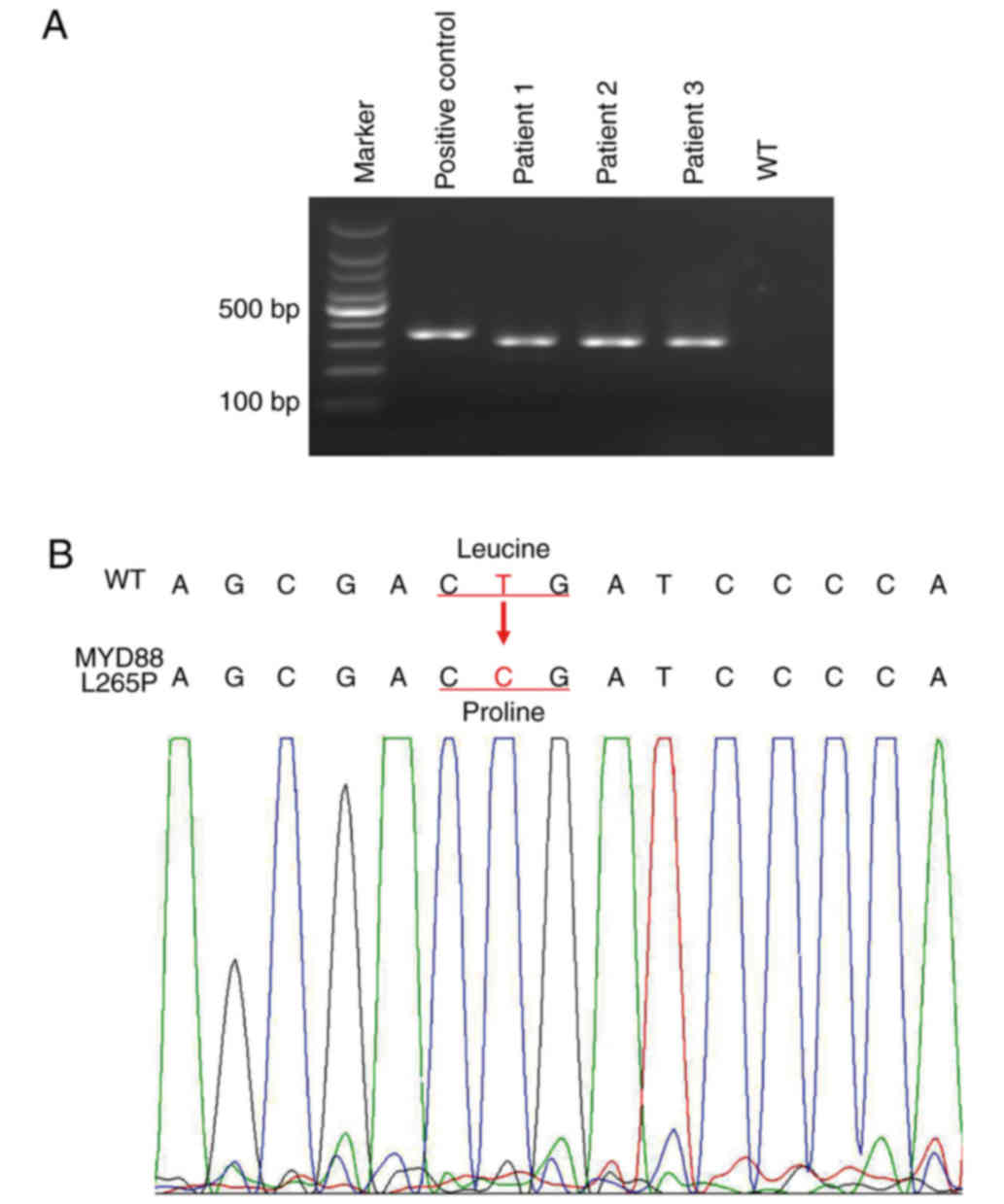
allele from a healthy person was used as a reference (Table I). L265P mutant DNA and wild-type DNA
was validated by examination of agarose gels and Sanger sequencing
(Fig. 1). The standards for
MYD88 L265P were generated by a serial dilution of the
mutant DNA with the wild-type DNA (10−3,
10−4, 10−5, 10−6, 10−7,
10−8, 10−9, 10−10,
10−11, 10−12, 10−13). All primers
were designed using Primer Premier 5.0 (Premier Biosoft
International, Palo Alto, CA, USA). Primer synthesis and Sanger
sequencing were conducted by Tsingke (Chengdu, China).
 | Table I.Primers of PCR in this study. |
Table I.
Primers of PCR in this study.
| Variable | Primers |
|---|
| Site-directed
mutagenesis primers (340 bp) | F:
5′-CAGCCTCTCTCCAGGTAAGCTCAACC-3′ |
|
| R:
5′-ATTGCCTTGTACTTGATGGGGATCGGTCGCTTCTG-3′ |
|
| (containing
mutation L265P base) |
| First round general
PCR (604 bp) | F:
5′-CAGCCTCTCTCCAGGTAAGCTCAACC-3′ |
|
| RW:
5′-ATTGCCTTGTACTTGATGGGGATCA-3′ |
|
| RM:
5′-CCTTGTACTTGATGGGGAAGG-3′ |
|
| (two internal
mismatches in the 2nd and 3rd position from the 3′-end) |
| Second real-time
PCR (304 bp) | F:
5′-GGCAAGAGAATGAGGGAATGTG-3′ |
|
| RW:
5′-GCCTTGTACTTGATGGGGAACA-3′ |
|
| RM:
5′-CCTTGTACTTGATGGGGAACG-3′ |
|
| (an internal
mismatch in the 3rd position from the 3′-end) |
The study was performed after patients signed
informed consent, and it was approved by the Ethics Committee of
the Sichuan Cancer Hospital in accordance with the Declaration of
Helsinki.
Development of allele-specific
semi-nested PCR (ASSN-PCR) assay for MYD88 L265P assessment
The ASSN-PCR method included two steps of PCR. The
first round was a conventional AS-PCR assay. We designed two
reverse primers to separate the mutant and wild-type alleles of
MYD88 L265P and one common primer to amplify large fragments
to improve the sensitivity of the ASSN-PCR. To increase the
specificity of the ASSN-PCR, we introduced two internal mismatches
in the second and third positions from the 3′-end in the reverse
primer (Table I) (21).
PCR was performed in a total reaction volume of 25
ml, including 50 nM of each primer, 15 ng DNA and 2X master mix
(Tsingke). Thermal cycling conditions consisted of the following:
Five minutes of preheating at 95°C, followed by 40 cycles of 30 sec
at 95°C, 45 sec at 56°C, and 1 min at 72°C. The final step was an
extension step for 5 min at 72°C.
The second round was a quantitative AS-(q)PCR assay
for the assessment of MYD88 L265P. After the first round of
conventional PCR, we obtained two PCR products for each specimen
(wild-type products, W; likely mutation products, M). To optimize
the real-time PCR, we diluted W and M to 10−8 and
10−4, respectively. Then, the second round of real-time
AS-PCR was developed using specific primers (Table I) with diluted W, diluted M and
standards as templates. Power SYBR Green PCR Master Mix was applied
following the manufacturer's instructions and reactions were run on
the ABI Prism 7500 Sequence Detection system (Applied Biosystems,
Foster City, CA, USA). The contents of the PCR reactions were the
same as in the first round of AS-PCR. Thermal cycling conditions
were: Two minutes of preheating at 95°C, followed by 40 cycles of
30 sec at 95°C, 45 sec at 62°C, and routine melt curve cycling
conditions. The products of the second round of real-time AS-PCR
were confirmed by Sanger sequencing.
Interpretation of AS-qPCR results
The CT (MYD88 L265P)
represents the amount of mutated MYD88 L265P within the
sample, while the CT (wild-type) reflects the
total amount of MYD88 allelic template in the sample.
ΔCT cut-off value was measured using the formula
below:
ΔCT=CT(MY88L265P)–CT(wild-type)ΔCTcut-off=CT(10–8)–CT(AR-W)
where CT (10−8) is the average
CT (MY88 L265P) value of the 10−8 dilution of
positive control template mixed into a normal DNA template, and
CT(AR-W) is the average CT(wild-type) value
of allelic reference.
A positive result for the MYD88 L265P
mutation is defined as a mean ΔCT value less than
ΔCT cut-off value for each sample, while a negative
mutation result (i.e., no mutation detected) is defined as a mean
ΔCT exceeding the ΔCT cut-off value.
Statistical analysis
All statistical analyses were conducted using SPSS
version 20.0 (IBM Corp., Armonk, NY, USA). We used a Chi-square or
Fisher's exact test to analyze the association between categorical
variables and the MYD88 L265P mutation, and the Mann-Whitney
U test to evaluate the association between continuous variables and
the MYD88 L265P mutation. The association between
MYD88 L265P and patient survival (OS and PFS) was evaluated
by survival curves using the Kaplan-Meier method and the log-rank
(Mantel-Cox test). Cox regression was applied to evaluate the
independent factors for OS and PFS. Two-sided P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Specificity and sensitivity of AS-qPCR
assay
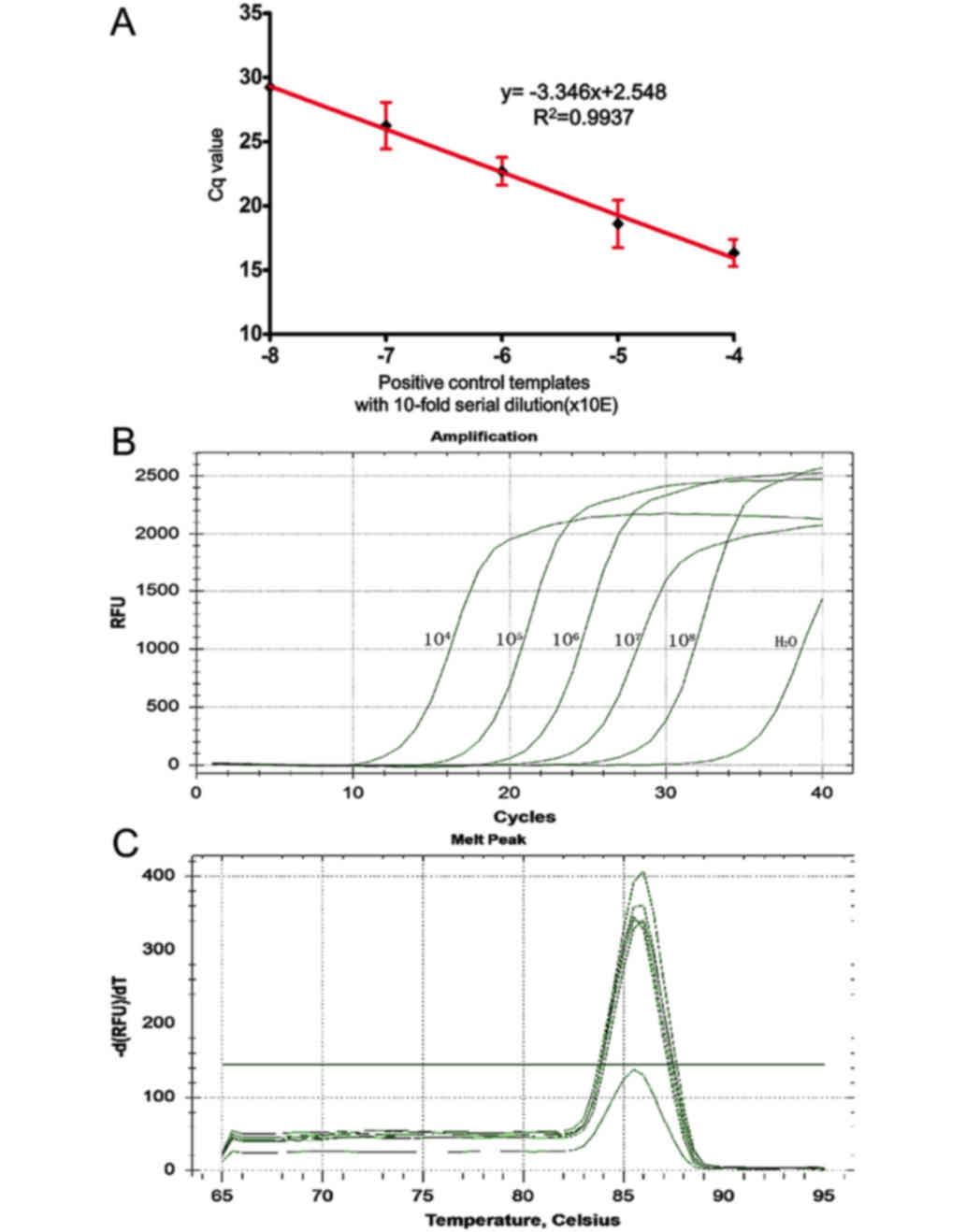
We analyzed the sensitivity and specificity of the
AS-qPCR assay in detecting the MYD88 L265P mutation using a
standard curve. The standard curve amplification plot and linear
regression (the standards diluted from 10−4 to
10−8) generated a correlation coefficient of 0.9937,
with a y-intercept value of 2.548 and a slope of −3.346. The
calculated amplification efficiency was 99% (Fig. 2A and B). This method was determined to
be suitable for the detection and quantitative assessment of
MYD88 L265P and is capable of detecting MYD88 L265P
at a lower limit of 10−12. Analysis of the melt curves
showed that the PCR assay had good specificity (Fig. 2C). ΔCT cut-off value has a
value of 6.01±0.076. Thus, the sample ΔCT value of all
mutant specimens for each assay ≤6 or >6 was interpreted as
positive or negative for the MYD88 L265P mutation,
respectively.
Correlation between MYD88 L265P status
and clinical characteristics
The clinicopathologic characteristics of the 53
DLBCL patients are listed in Table
II and associations between clinicopathologic factors and the
MYD88 mutation status are summarized in Table III. Among 53 DLBCL patients, 28
cases presented with extranodal invasion, and mutation statuses of
the DLBCL patients are listed in Table
IV. Using the ASSN-PCR assay, we detected the MYD88
L265P mutation in 16 out of 53 R-CHOP-treated DLBCL patients
(30.19%). The MYD88 L265P mutation rate in the central
nervous system (CNS) and testicular DLBCLs is 60% (3/5) (Table IV). Further, by excluding the CNS and
testicular DLBCLs, the MYD88 L265P mutation ratio is 27.08%
(13/48). We discovered that the MYD88 L265P mutation was not
statistically significantly associated with treatment response or
tumor recurrence (P>0.05). However, the MYD88 L265P
mutational status showed a significant association with age
(P=0.025) and location (P=0.033).
 | Table II.Clinicopathologic characteristics of
DLBCL cases. |
Table II.
Clinicopathologic characteristics of
DLBCL cases.
| Clinicopathologic
parameters | No. of patients
(n=53) | Proportion (%) |
|---|
| Age (years) |
|
|
|
<60 | 32 | 56.604 |
|
≥60 | 21 | 43.396 |
| Sex |
|
|
|
Male | 31 | 58.491 |
|
Female | 22 | 41.509 |
| Location |
|
|
|
Nodal | 25 | 47.169 |
|
Extranodal | 28 | 52.831 |
| B symptom |
|
|
|
Absent | 41 | 77.358 |
|
Present | 12 | 22.642 |
| Clinical stage |
|
|
| Low
(I–II) | 26 | 49.057 |
| High
(III–IV) | 27 | 50.943 |
| Subgroup |
|
|
|
GCB | 11 | 20.755 |
|
Non-GCB | 42 | 79.245 |
| IPI score |
|
|
| Low
(0–2) | 35 | 66.038 |
| High
(3–5) | 18 | 33.962 |
| ECOG score |
|
|
| Low
(0–1) | 45 | 84.906 |
| High
(2–4) | 8 | 15.094 |
| LDH |
|
|
|
Normal | 24 | 45.283 |
|
High | 29 | 54.717 |
| Ki-67 |
|
|
|
≤50 | 42 | 79.25 |
|
>50 | 11 | 20.75 |
| Treatment
response |
|
|
|
CR/PR | 47 | 88.679 |
|
PD/SD | 6 | 11.321 |
| Recurrence |
|
|
|
Absent | 28 | 52.83 |
|
Present | 25 | 47.17 |
 | Table III.The association analysis between
clinical characters and MYD88 mutation in DLBCL cases. |
Table III.
The association analysis between
clinical characters and MYD88 mutation in DLBCL cases.
|
|
| MYD88
mutation (%) |
|
|---|
|
|
|
|
|
|---|
| Clinicopathologic
parameters | No. | WT | L265P | P-value |
|---|
| Age (years) |
|
|
| 0.025 |
|
<60 | 32 | 26 (70.03) | 6 (37.5) |
|
|
≥60 | 21 | 11 (29.7) | 10 (62.5) |
|
| Sex |
|
|
| 0.828 |
|
Male | 31 | 22 (59.5) | 9 (56.2) |
|
|
Female | 22 | 15 (40.5) | 7 (43.8) |
|
| Location |
|
|
| 0.033 |
|
Nodal | 25 | 21 (56.8) | 4 (25.0) |
|
|
Extranodal | 28 | 16 (43.2) | 12 (75.0) |
|
| B symptom |
|
|
| 0.787 |
|
Absent | 41 | 29 (78.4) | 12 (75.0) |
|
|
Present | 12 | 8 (21.6) | 4 (25.0) |
|
| Clinical stage |
|
|
| 0.611 |
| Low
(I–II) | 26 | 19 (51.4) | 7 (43.8) |
|
| High
(III–IV) | 27 | 18 (48.6) | 9 (56.2) |
|
| Subgroup |
|
|
| 0.275 |
|
GCB | 11 | 6 (16.2) | 5 (31.2) |
|
|
Non-GCB | 42 | 31 (83.8) | 11 (68.8) |
|
| IPI score |
|
|
| 0.322 |
| Low
(0–2) | 35 | 26 (70.3) | 9 (56.2) |
|
| High
(3–5) | 18 | 11 (29.7) | 7 (43.8) |
|
| ECOG score |
|
|
| 0.729 |
| Low
(0–1) | 45 | 31 (83.8) | 14 (87.5) |
|
| High
(2–4) | 8 | 6 (16.2) | 2 (12.5) |
|
| LDH |
|
|
| 0.454 |
|
Normal | 24 | 18 (48.6) | 6 (37.5) |
|
|
High | 29 | 19 (51.4) | 10 (62.5) |
|
| Ki-67 |
|
|
| 0.632 |
|
<50 | 5 | 3 (8.1) | 2 (12.5) |
|
|
≥50 | 48 | 34 (91.1) | 14 (87.5) |
|
| Treatment
response |
|
|
| 0.655 |
|
CR/PR | 47 | 32 (86.5) | 15 (93.8) |
|
|
PD/SD | 6 | 5 (23.5) | 1 (6.2) |
|
| Recurrence |
|
|
| 0.743 |
|
Absent | 28 | 19 (51.4) | 9 (56.2) |
|
|
Present | 25 | 18 (48.6) | 7 (43.8) |
|
 | Table IV.The mutation status of enrolled
patients. |
Table IV.
The mutation status of enrolled
patients.
| ID | MYD88 | Sex | Age (years) | Extranodal sites
(NO.; location) |
|---|
| 1 | Wide-type | Male | 60 | 1; testis |
| 2 | Wide-type | Female | 48 | 3; lung, liver,
bone marrow |
| 3 | Wide-type | Male | 68 | 0 |
| 4 | Wide-type | Male | 68 | 2; Liver, CNS |
| 5 | L265P | Male | 55 | 0 |
| 6 | Wide-type | Male | 48 | 2; Oropharynx,
stomach |
| 7 | Wide-type | Female | 58 | 1; Left frontal
lobe |
| 8 | Wide-type | Male | 26 | 0 |
| 9 | Wide-type | Female | 41 | 1; stomach |
| 10 | L265P | Female | 61 | 0 |
| 11 | L265P | Male | 70 | 1; testis |
| 12 | Wide-type | Male | 25 | 0 |
| 13 | L265P | Female | 62 | 3; Bone marrow,
iliac, calf skin |
| 14 | L265P | Female | 40 | 4; Breast, CNS,
spinal cord, pelvic cavity |
| 15 | Wide-type | Male | 61 | 1; thyroid |
| 16 | Wide-type | Female | 61 | 0 |
| 17 | Wide-type | Male | 27 | 1; stomach |
| 18 | Wide-type | Male | 78 | 1; bone |
| 19 | Wide-type | Female | 74 | 1; skin |
| 20 | Wide-type | Female | 79 | 1; thyroid |
| 21 | Wide-type | Male | 49 | 2; bone marrow,
bone |
| 22 | Wide-type | Female | 53 | 0 |
| 23 | L265P | Female | 46 | 1; Bone |
| 24 | Wide-type | Female | 32 | 1; Breast |
| 25 | Wide-type | Male | 73 | 0 |
| 26 | Wide-type | Female | 20 | 0 |
| 27 | Wide-type | Female | 56 | 2; Psoas muscle,
vertebral body |
| 28 | Wide-type | Female | 44 | 0 |
| 29 | L265P | Male | 57 | 1; lung |
| 30 | L265P | Male | 40 | 1; CNS |
| 31 | L265P | Male | 34 | 0 |
| 32 | Wide-type | Male | 56 | 0 |
| 33 | Wide-type | Male | 54 | 1; lung |
| 34 | L265P | Male | 67 | 0 |
| 35 | L265P | Male | 62 | 1; thyroid |
| 36 | Wide-type | Female | 53 | 0 |
| 37 | Wide-type | Male | 52 | 0 |
| 38 | Wide-type | Male | 64 | 0 |
| 39 | L265P | Female | 75 | 1; thyroid |
| 30 | L265P | Male | 63 | 1; lung |
| 41 | Wide-type | Male | 43 | 0 |
| 42 | Wide-type | Male | 75 | 0 |
| 43 | Wide-type | Female | 49 | 0 |
| 44 | Wide-type | Male | 47 | 0 |
| 45 | Wide-type | Female | 55 | 0 |
| 46 | Wide-type | Male | 66 | 0 |
| 47 | Wide-type | Female | 43 | 0 |
| 48 | L265P | Male | 72 | 1; stomach |
| 49 | Wide-type | Male | 60 | 0 |
| 50 | Wide-type | Male | 41 | 0 |
| 51 | L265P | Female | 66 | 1; thyroid |
| 52 | Wide-type | Male | 45 | 1; stomach |
| 53 | L265P | Female | 64 | 2; lung, bone |
MYD88 L265P mutation and survival
analysis
The median follow-up time across the entire cohort
was 18 months (range, 3–80 months), with 3-year OS and PFS rates of
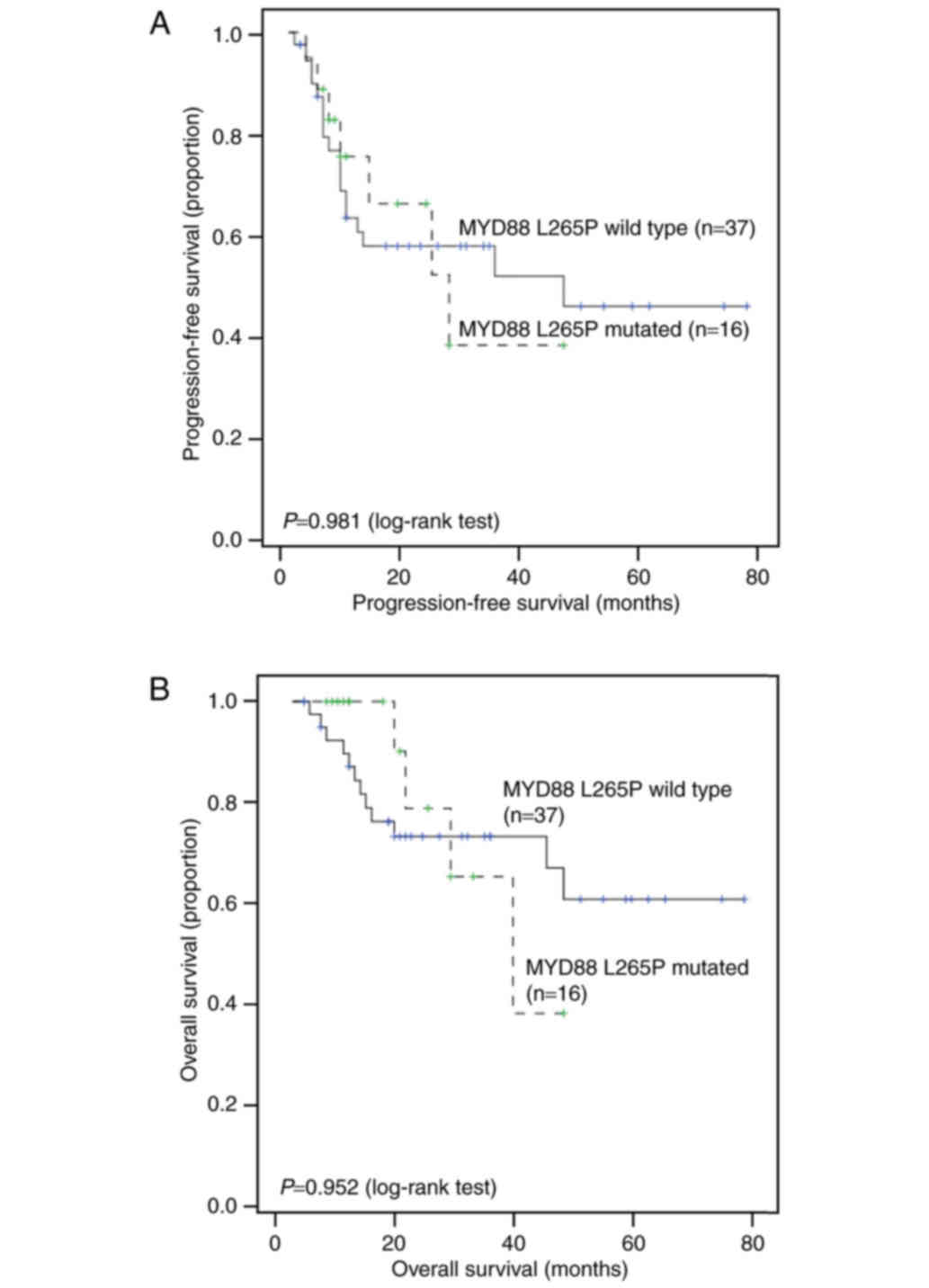
56 and 42%, respectively. Univariate analysis showed that B
symptoms (P=0.004) and Ki-67 (P=0.03) were significantly associated
with PFS. However, the MYD88 mutation status and other
factors showed no association with OS or PFS (Fig. 3). Cox regression showed that B
symptoms remained a significant risk factor for PFS (P=0.012,
hazard ratio (HR) = 3.08; 95% CI = 1.28–7.41) (Table V) after controlling for other factors.
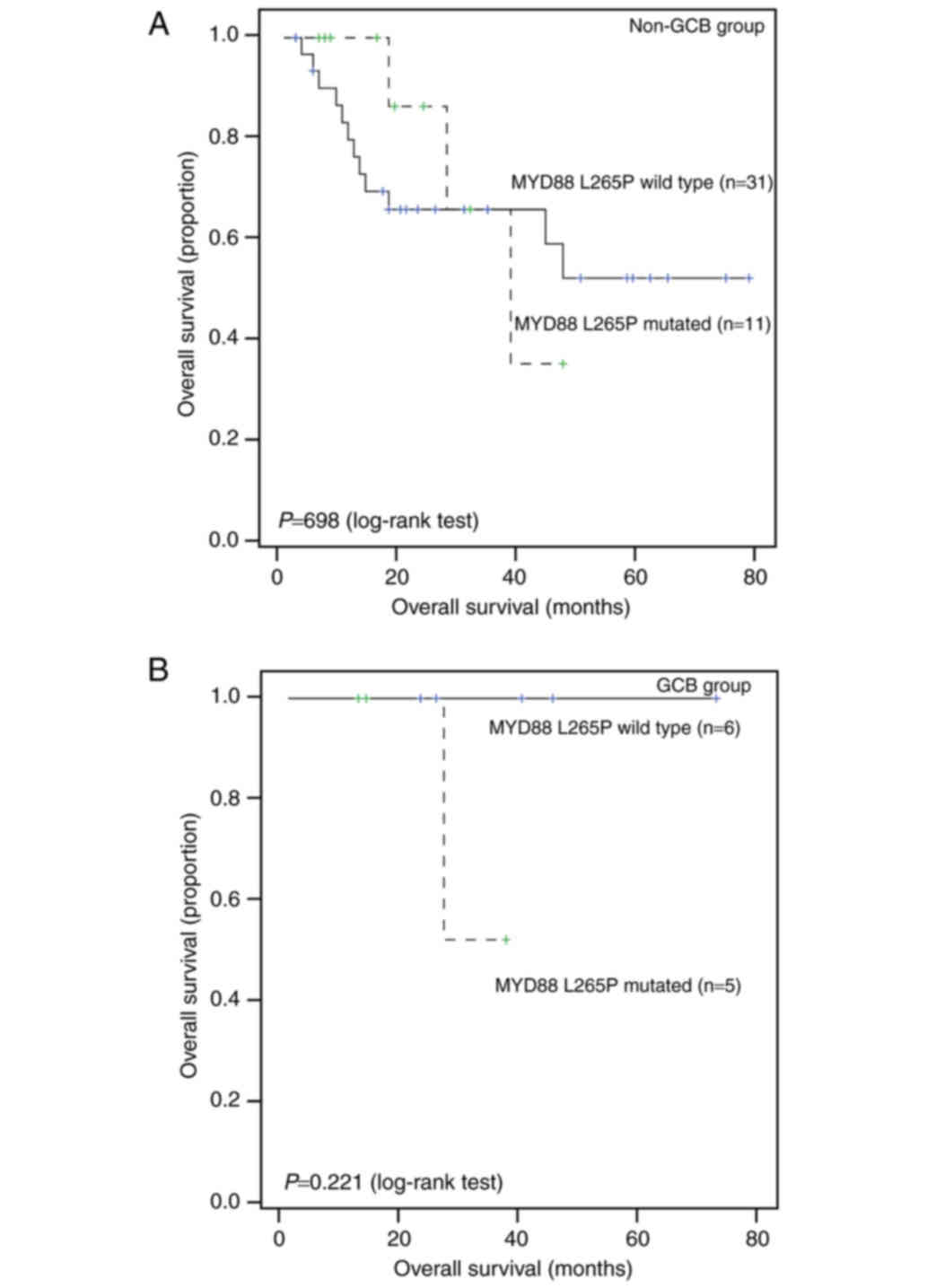
Further subgroup analysis showed that MYD88 mutation status
is not significantly associated with survival in either the Non-GCB
group or the GCB group (all P>0.05; Fig. 4).
 | Table V.Clinical characters affecting
progression-free and overall survival. |
Table V.
Clinical characters affecting
progression-free and overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| OS | PFS | PFS |
|---|
|
|
|
|
|
|---|
| Clinicopathologic
parameters | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| MYD88 (WT vs.
L265P) | 0.97
(0.31–3.04) | 0.952 | 0.99
(0.41–2.39) | 0.981 |
|
|
| Age (<60 vs. ≥60
years) | 1.16
(0.43–3.12) | 0.766 | 0.83
(0.37–1.86) | 0.645 |
|
|
| Sex (male vs.
female) | 1.31
(0.48–3.56) | 0.596 | 0.93
(0.42–2.04) | 0.848 |
|
|
| Location (nodal vs.
extranodal) | 0.66
(0.25–1.76) | 0.401 | 1.39
(0.61–3.15) | 0.423 |
|
|
| B symptom (absent
vs. present) | 2.04
(0.69–5.97) | 0.184 | 3.29
(1.39–7.80) | 0.004 | 3.08
(1.28–7.41) | 0.012 |
| Clinical stage
(I–II vs. III–IV) | 2.50
(0.80–7.77) | 0.102 | 1.45
(0.64–3.30) | 0.370 |
|
|
| Subgroup (GCB vs.
non-GCB) | 3.47
(0.46–26.40) | 0.200 | 2.12
(0.63–7.08) | 0.207 |
|
|
| IPI score (0–2 vs.
3–5) | 1.67
(0.62–4.46) | 0.304 | 1.14
(0.51–2.55) | 0.754 |
|
|
| ECOG score (0–1 vs.
2–4) | 2.18
(0.70–6.80) | 0.166 | 2.02
(0.30–5.05) | 0.122 |
|
|
| LDH (normal vs.
high) | 1.50
(0.54–4.15) | 0.429 | 0.93
(0.42–2.04) | 0.845 |
|
|
| Ki-67 (<50 vs.
≥50) | 2.02
(0.27–15.42) | 0.487 | 0.32
(0.11–0.96) | 0.030 | 0.38
(0.12–1.15) | 0.085 |
Discussion
In our study, we developed the ASSN-PCR to detect
the MYD88 L265P mutation and successfully revealed a high
prevalence of the MYD88 L265P mutation in DLBCL patients
undergoing R-CHOP treatment. However, we did not have enough
evidence to conclude that there was a significant association
between the MYD88 L265P mutation and treatment response or
tumor recurrence. The MYD88 L265P mutation may not be a
significant prognostic factor for DLBCL patients undergoing R-CHOP
treatment.
Previous studies have shown that MYD88 L265P
was a key player in the constitutive activation of NF-κB pathways
in lymphomagenesis. It was frequently detected in non-GCB type
DLBCL (21.6–32.5%), as well as extranodal DLBCL, such as in the
central nervous system and testes (50 and 90%, respectively)
(22–24). In our study, MYD88 L265P was
identified in 30.19% of all DLBCL patients treated with R-CHOP. The
MYD88 L265P mutation was predominantly detected in non-GCB
type DLBCL (68.8 vs. 31.2% GCB type), as was previously reported
(22,25). It was reported that excessive
activation of NF-κB pathways frequently existed in non-GCB type
DLBCL, which may explain the predominant existence of MYD88
L265P in this subtype (21,26).
In our study, 5 out of 7 primary extranodal DLBCL
patients harboring MYD88 L265P were in the advanced stage.
This result is consistent with a previous study (27) that suggests that the MYD88
L265P gene mutation may be an early molecular change in DLBCL
tumorigenesis (28). Moreover, we
observed a significant association between the MYD88 L265P
mutation and age as well as location, which is consistent with the
previous study (15). With increasing
age, the incidence of poor prognosis factors, such as various
genetic features, non-GCB subtype, and BCL2 expression will
increase for DLBCL (29). This may
explain the predominance of MYD88 L265P in elderly DLBCL
patients. Meanwhile, we discovered that the MYD88 L265P
mutation was not significantly associated with treatment response
or tumor recurrence.
To evaluate the prognostic value of MYD88
L265P for DLBCL, we conducted univariate analyses and multivariate
Cox regression analyses. The presence of B symptoms and
Ki-67>50% indicated poor prognosis. After controlling for other
factors and conducting the cox regression analysis, Ki-67 lost its
prognostic significance. Our results suggest that MYD88
L265P does not affect the outcome of R-CHOP-treated DLBCL patients.
Recent meta-analysis study revealed that the MYD88 L265P mutation
was associated with a low survival rate, except for individual
studies (30). However, since the
study didn't enrolled all published data into pooled analysis, and
pathological type and clinical treatment is various, additional
studies are required with increased number of patients and
differential patient stratification to determine the role of this
mutation.
Limitations of our study include that patients had a
relatively short period of follow-up and the sample size was
relatively small. Therefore, further large-scale, multi-center,
prospective studies with longer follow-up periods are warranted.
Although there are some limitations, the treatment of enrolled
patients was homogeneous; moreover, it is worth noting that we are
the first group to use semi-nested PCR to detect the MYD88
L265P mutation and that the prevalence of detected MYD88
L265P mutations in our study was 30.19%, which is higher than the
prevalence seen when using previously reported methods (13,31).
Excluding the CNS and testicular DLBCLs, the MYD88 L265P
mutation ratio is 27.08%, which is higher than the pooled published
data (16.5%) (30).
As far as we know, some investigators used general
PCR and sequencing to detect MYD88 L265p mutations (10,11,27). MYD88
L265P mutation rate is low and heterogeneous (6.5–19.3%). Since
Sanger sequencing might be unable to detect lower frequency
mutations in FFPE samples with fragmented nucleic acids, AS-PCR was
applied to detect the MYD88 L265P mutation, which is a highly
sensitive and cost-effective (24).
Two powerful studies utilized this method and detected relative
high rate of MYD88 L265P mutation (22–22.3%) (32,33).
Nested PCR is a modification of polymerase chain reaction intended
to reduce non-specific binding in products due to the amplification
of unexpected primer binding sites. In this study, we combined
AS-PCR and semi-nested PCR to assess the MYD88 L265P status.
This method can overcome the issues involved with DNA extraction
from paraffin wax, such as poor quality and low concentration, thus
improving the sensitivity and specificity of PCR. This method can
detect MYD88 L265P at a lower limit of 10−12,
which is more sensitive than the 0.1% previously published for
allele-specific oligonucleotide PCR alone (34).
In conclusion, this study indicates that the
MYD88 L265P mutation is not associated with treatment
response or tumor recurrence and that MYD88 L265P does not
affect patient outcomes and may not be a prognostic factor for
DLBCL patients undergoing R-CHOP treatment. Current data should be
validated in further studies.
Acknowledgements
This study was funded by the Department of Medical
Oncology of Sichuan Cancer Hospital and Institute.
References
|
1
|
Martelli M, Ferreri AJ, Agostinelli C, Di
Rocco A, Pfreundschuh M and Pileri SA: Diffuse large B-cell
lymphoma. Crit Rev Oncol Hematol. 87:146–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lenz G, Wright G, Dave SS, Xiao W, Powell
J, Zhao H, Xu W, Tan B, Goldschmidt N, Iqbal J, et al: Stromal gene
signatures in large-B-cell lymphomas. N Engl J Med. 359:2313–2323.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coiffier B, Lepage E, Briere J, Herbrecht
R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G,
Gaulard P, et al: CHOP chemotherapy plus rituximab compared with
CHOP alone in elderly patients with diffuse large-B-cell lymphoma.
N Engl J Med. 346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowakowski GS and Czuczman MS: ABC, GCB
and double-hit diffuse large B-cell lymphoma: Does subtype make a
difference in therapy selection? Am Soc Clin Oncol Educ Book.
e449–e457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim KH, Yang Y and Staudt LM: Pathogenetic
importance and therapeutic implications of NF-κB in lymphoid
malignancies. Immunol Rev. 246:359–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davis RE, Brown KD, Siebenlist U and
Staudt LM: Constitutive nuclear factor kappaB activity is required
for survival of activated B cell-like diffuse large B cell lymphoma
cells. J Exp Med. 194:1861–1874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ngo VN, Young RM, Schmitz R, Jhavar S,
Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, et al:
Oncogenically active MYD88 mutations in human lymphoma. Nature.
470:115–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim Y, Ju H, Kim DH, Yoo HY, Kim SJ, Kim
WS and Ko YH: CD79B and MYD88 mutations in diffuse large B-cell
lymphoma. Hum Pathol. 45:556–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi JW, Kim Y, Lee JH and Kim YS: MYD88
expression and L265P mutation in diffuse large B-cell lymphoma. Hum
Pathol. 44:1375–1381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taniguchi K, Takata K, Chuang SS,
Miyata-Takata T, Sato Y, Satou A, Hashimoto Y, Tamura M, Nagakita
K, Ohnishi N, et al: Frequent MYD88 L265P and CD79B mutations in
primary breast diffuse large B-cell lymphoma. Am J Surg Pathol.
40:324–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernándezrodríguez C, Bellosillo B,
Garcíagarcía M, Sánchez-González B, Gimeno E, Vela MC, Serrano S,
Besses C and Salar A: MYD88 (L265P) mutation is an independent
prognostic factor for outcome in patients with diffuse large B-cell
lymphoma. Leukemia. 28:2104–2106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pham-Ledard A, Beylot-Barry M, Barbe C,
Leduc M, Petrella T, Vergier B, Martinez F, Cappellen D, Merlio JP
and Grange F: High frequency and clinical prognostic value of MYD88
L265P mutation in primary cutaneous diffuse large B-cell lymphoma,
leg-type. JAMA Dermatol. 150:1173–1179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rovira J, Karube K, Valera A, Colomer D,
Enjuanes A, Colomo L, Martínez-Trillos A, Giné E, Dlouhy I, Magnano
L, et al: MYD88 L265P mutations, but no other variants, identify a
subpopulation of DLBCL patients of activated B-cell origin,
extranodal involvement and poor outcome. Clin Cancer Res.
22:2755–2764. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hattori K, Sakata-Yanagimoto M, Okoshi Y,
Goshima Y, Yanagimoto S, Nakamoto-Matsubara R, Sato T, Noguchi M,
Takano S, Ishikawa E, et al: MYD88 (L265P) mutation is associated
with an unfavourable outcome of primary central nervous system
lymphoma. Br J Haematol. 177:492–494. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steinhardt JJ and Gartenhaus RB: Promising
personalized therapeutic options for diffuse large B-cell lymphoma
subtypes with oncogene addictions. Clin Cancer Res. 18:4538–4548.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dunleavy K, Pittaluga S, Czuczman MS, Dave
SS, Wright G, Grant N, Shovlin M, Jaffe ES, Janik JE, Staudt LM and
Wilson WH: Differential efficacy of bortezomib plus chemotherapy
within molecular subtypes of diffuse large B-cell lymphoma. Blood.
113:6069–6076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swerdllow SH, Campo E, Harris NL, Jaffe
ES, Pileri SA, Stein H, Thiele J and Vardiman JW: WHO
classification of tumours of haematopoietic and lymphoid tissues.
2. 4th. IARC Press; France: pp. 4392008
|
|
20
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu L, Hunter ZR, Yang G, Zhou Y, Cao Y,
Liu X, Morra E, Trojani A, Greco A, Arcaini L, et al: MYD88 L265P
in Waldenstrom macroglobulinemia, immunoglobulin M monoclonal
gammopathy, and other B-cell lymphoproliferative disorders using
conventional and quantitative allele-specific polymerase chain
reaction. Blood. 121:2051–2058. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kraan W, Horlings HM, van Keimpema M,
Schilder-Tol EJ, Oud ME, Scheepstra C, Kluin PM, Kersten MJ,
Spaargaren M and Pals ST: High prevalence of oncogenic MYD88 and
CD79B mutations in diffuse large B-cell lymphomas presenting at
immune-privileged sites. Blood Cancer J. 3:e1392013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossi D: Role of MYD88 in
lymphoplasmacytic lymphoma diagnosis and pathogenesis. Hematology
Am Soc Hematol Educ Program. 2014:113–118. 2014.PubMed/NCBI
|
|
24
|
Staiger AM, Ott MM, Parmentier S,
Rosenwald A, Ott G, Horn H and Griese EU: Allele-specific PCR is a
powerful tool for the detection of the MYD88 L265P mutation in
diffuse large B cell lymphoma and decalcified bone marrow samples.
Br J Haematol. 171:145–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fernández-Rodriguez C, Bellosillo B,
Garcia-Garcia M, Sánchez-González B, Gimeno E, Vela MC, Serrano S,
Besses C and Salar A: MYD88 (L265P) mutation is an independent
prognostic factor for outcome in patients with diffuse large B-cell
lymphoma. Leukemia. 28:2104–2106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eliopoulos AG, Stack M, Dawson CW, Kaye
KM, Hodgkin L, Sihota S, Rowe M and Young LS: Epstein-Barr
virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial
cells via an NF-kappaB pathway involving TNF receptor-associated
factors. Oncogene. 14:2899–2916. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caner V, Sen Turk N, Baris IC, Cetin GO,
Tepeli E, Hacioglu S, Sari I, Zencir S, Dogu MH, Bagci G and Keskin
A: MYD88 expression and L265P mutation in mature B-cell non-Hodgkin
lymphomas. Genet Test Mol Biomarkers. 19:372–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Landau DA, Carter SL, Stojanov P, et al:
Evolution and impact of subclonal mutations in chronic lymphocytic
leukemia. Cell. 152:714–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klapper W, Kreuz M, Kohler CW, Burkhardt
B, Szczepanowski M, Salaverria I, Hummel M, Loeffler M, Pellissery
S, Woessmann W, et al: Patient age at diagnosis is associated with
the molecular characteristics of diffuse large B-cell lymphoma.
Blood. 119:1882–1887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JH, Jeong H, Choi JW, Oh H and Kim YS:
Clinicopathologic significance of MYD88L265P mutation in diffuse
large B-cell lymphoma: A meta-analysis. Sci Rep. 7:17852017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim Y, Ju H, Kim DH, Yoo HY, Kim SJ, Kim
WS and Ko YH: CD79B and MYD88 mutations in diffuse large B-cell
lymphoma. Hum Pathol. 45:556–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kraan W, Horlings HM, van Keimpema M,
Schilder-Tol EJ, Oud ME, Scheepstra C, Kluin PM, Kersten MJ,
Spaargaren M and Pals ST: High prevalence of oncogenic MYD88 and
CD79B mutations in diffuse large B-cell lymphomas presenting at
immune-privileged sites. Blood Cancer J. 3:e1392013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rovira J, Karube K, Valera A, Colomer D,
Enjuanes A, Colomo L, Martínez-Trillos A, Giné E, Dlouhy I, Magnano
L, et al: MYD88 L265P mutations, but no other variants, identify a
subpopulation of DLBCL patients of activated B-cell origin,
extranodal involvement and poor outcome. Clin Cancer Res.
22:2755–2764. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu L, Hunter ZR, Yang G, Zhou Y, Cao Y,
Liu X, Morra E, Trojani A, Greco A, Arcaini L, et al: MYD88 L265P
in Waldenström macroglobulinemia, immunoglobulin M monoclonal
gammopathy, and other B-cell lymphoproliferative disorders using
conventional and quantitative allele-specific polymerase chain
reaction. Blood. 121:2051–2058. 2013. View Article : Google Scholar : PubMed/NCBI
|