Introduction
Gastric cancer is the fourth most common type of
cancer and the second leading cause of cancer-associated mortality
globally, and its incidence was estimated to be approximately one
million per year worldwide (1).
According to the multi-factorial and multi-step model in the
pathogenesis of gastric cancer, apart from genetic alterations,
environmental factors are also markedly involved in different
stages of carcinogenesis (2,3). Recently, cumulating evidence reported
that high intake of heme iron (e.g., fresh and processed red meat)
or low body iron store (e.g., iron-deficiency anemia) was
correlated with an increased risk of gastric cancer, indicating
that altered iron metabolism may be mediated in the development of
gastric cancer (4–7). Furthermore, novel findings indicated
that iron-chelating agents, including deferoxamine and deferasirox
might potentially exert anti-proliferative effects on gastric
cancer cells by inducing apoptosis (8). Accordingly, the altered iron metabolism
may have an extensive role in the development of gastric
carcinogenesis.
Hepcidin is the peptide hormone, which is primarily
synthesized by hepatocytes in the liver and secreted into the
circulation to effectively regulate systemic iron homeostasis
(9). It is generally believed that,
by binding to its target receptor, ferroportin, hepcidin would
inhibit iron absorption from duodenal enterocytes and iron release
from macrophages and hepatocytes, which is mediated by rapid
endocytosis and degradation of the hepcidin-ferroportin complex
(10). Disordered hepcidin signaling
may lead to several iron-restrictive and iron-overload diseases,
including iron deficiency anemia and hereditary hemochromatosis
(11,12). Previously, it has been reported that
increased circulating levels of hepcidin are associated with a
range of malignancies (13–15). Of note, apart from the liver as the
major place for hepcidin synthesis, various other organs including
cancer tissues may locally synthesize and secret hepcidin (16). Notably, studies have demonstrated that
altered expression of hepcidin in tumor tissues may serve as a
predictive biomarker in assessing the clinical outcomes of several
types of cancer (13,17). Furthermore, it was indicated that
aberrant hepcidin signaling might promote tumor growth in breast
cancer (18). However, in human
gastric cancer, there remain to be limited data on the expression
profile of hepcidin in tumor tissues and its correlation with the
clinicopathological characteristics in gastric cancer.
Previous studies have indicated that hepcidin is a
mature defensin-like peptide containing 25 amino acid residues and
4 disulfide bonds, which is cleaved intracellularly from the
preprohormone encoded by the gene named hepcidin antimicrobial
peptide (HAMP), which is located at the locus 19q13 in the human
genome (19). It is widely considered
that the production of hepcidin is predominately controlled at the
level of transcription, which is rapidly increased by various
inflammatory stimuli (20). In
particular, in response to pro-inflammatory cytokines, including
interleukin (IL)-6, the stimulatory effects on hepcidin expression
is largely mediated through the activation of Janus kinase/signal
transducer and activator of transcription 3 (JAK/STAT3) signaling.
Therefore, to promote the transcriptional activity of hepcidin,
this would depend on the interaction between STAT3 and the related
STAT3-binding element in the promoter region of the HAMP gene
(21–23). Indeed, regardless of underlying
etiologies, including diet and Helicobacter pylori
infection, the association between chronic inflammation and gastric
cancer has been well established (24). Nevertheless, whether and to what
extent the inflammation-induced JAK/STAT3 signaling would be
involved in the regulation of hepcidin expression in human gastric
cancer remains to be investigated.
The aim of the present study was to detect the
expression of hepcidin and then to assess its correlation with the
clinicopathological characteristics in human gastric cancer. It was
further determined whether altered hepcidin expression in human
gastric cancer might be associated with the status of the JAK/STAT3
signaling pathway as this may regulate the expression of hepcidin
at the transcriptional level. Consequently, the prognostic value of
hepcidin for gastric cancer was evaluated. Additionally, the
mechanistic underpinnings affecting hepcidin expression at the
cellular and molecular level were preliminarily investigated so as
to provide a potential target to correct aberrant local expression
of hepcidin in gastric cancer.
Materials and methods
Patients and tissue specimens
A total of 62 gastric cancer patients, who were
treated by curative gastrectomy at Taicang Affiliated Hospital of
Soochow University (Suzhou, China) from February 2009 to February
2014, were enrolled in the present study. The characteristics of
the 62 gastric cancer patients were summarized in Table I. All patients did not receive
chemotherapy and/or radiotherapy prior to surgery and did not have
a history of concurrent tumors. The 62 archived formalin-fixed
paraffin-embedded tumor tissue blocks of the aforementioned
patients, which were obtained by biopsy, were collected for the
experiments. Additionally, 15 randomly selected tissues adjacent to
the tumor confirmed by pathological diagnosis as normal gastric
mucosal tissues were selected as the non-tumor group. The tumor
grade and clinical stage were determined according to the tumor
node metastasis (TNM) classification for gastric cancer (7th
Edition of the AJCC Cancer Staging Manual) (25). Anemia was defined according to the
following criteria for hemoglobin concentration: <120.0 g/l for
males and <110.0 g/l for females. Written informed consent was
obtained from all patients and approved by the Committee on Human
Rights in Research. The present study was conducted in accordance
with the Declaration of Helsinki, and ethical approval was obtained
from the Institutional Review Board at the Taicang Affiliated
Hospital of Soochow University.
 | Table I.Clinicopathological characteristics
of patients with gastric cancer. |
Table I.
Clinicopathological characteristics
of patients with gastric cancer.
|
Characteristics | Number of cases, n
(%) |
|---|
| Age, years |
|
|
≤60 | 28 (45.2) |
|
>60 | 34 (54.8) |
| Gender |
|
|
Male | 40 (64.5) |
|
Female | 22 (35.5) |
| Anemia |
|
|
Negative | 33 (53.2) |
|
Positive | 29 (46.8) |
| T categories |
|
| T1 and
T2 | 23 (37.1) |
| T3 and
T4 | 39 (62.9) |
| Lymph node
metastasis |
|
|
Negative | 27 (43.5) |
|
Positive | 35 (56.5) |
| Other
metastasis |
|
|
Negative | 53 (85.5) |
|
Positive | 9 (14.5) |
Immunohistochemical analysis
All paraffin-embedded tissues were consecutively cut
into 4 sections (thickness, 5 µm). For each sample, one section was
used to confirm the pathological diagnosis by hematoxylin-eosin
staining (hematoxylin-stained for ~10 min and eosin-stained for ~2
min at 30°C). The other three sections were detected by
immunohistochemical analysis according to the following procedures.
According to the streptavidin-biotin-peroxidase complex
immunohistochemical protocol (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), the paraffin-embedded sections were
deparaffinized with xylene and rehydrated in the decreasing
concentrations of ethanol, followed by incubating in 3%
H2O2 for 30 min at room temperature. Then,
the slides were incubated in 10 mM citrate buffer (pH 6.0) for 20
min for antigen retrieval, and immersed in phosphate-buffered
saline (PBS) containing 15% goat serum (Thermo Fisher Scientific,
Inc.) for 30 min at room temperature. The rabbit anti-human
hepcidin polyclonal primary antibody (1:200; catalog no. ab30760;
Abcam, Cambridge, MA, USA) was added and incubated overnight at
4°C. Following washing with PBS, goat anti-rabbit horseradish
peroxidase-labeled secondary antibody (1:200; catalog no. BA1088;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) was added
and incubated for 30 min at room temperature. By rinsing with PBS,
the slides were then counterstained using hematoxylin for~10 min at
30°C. Following dehydrating in increasing concentrations of
ethanol, the slides were mounted, and cover slips were placed for
the next microscopic evaluation.
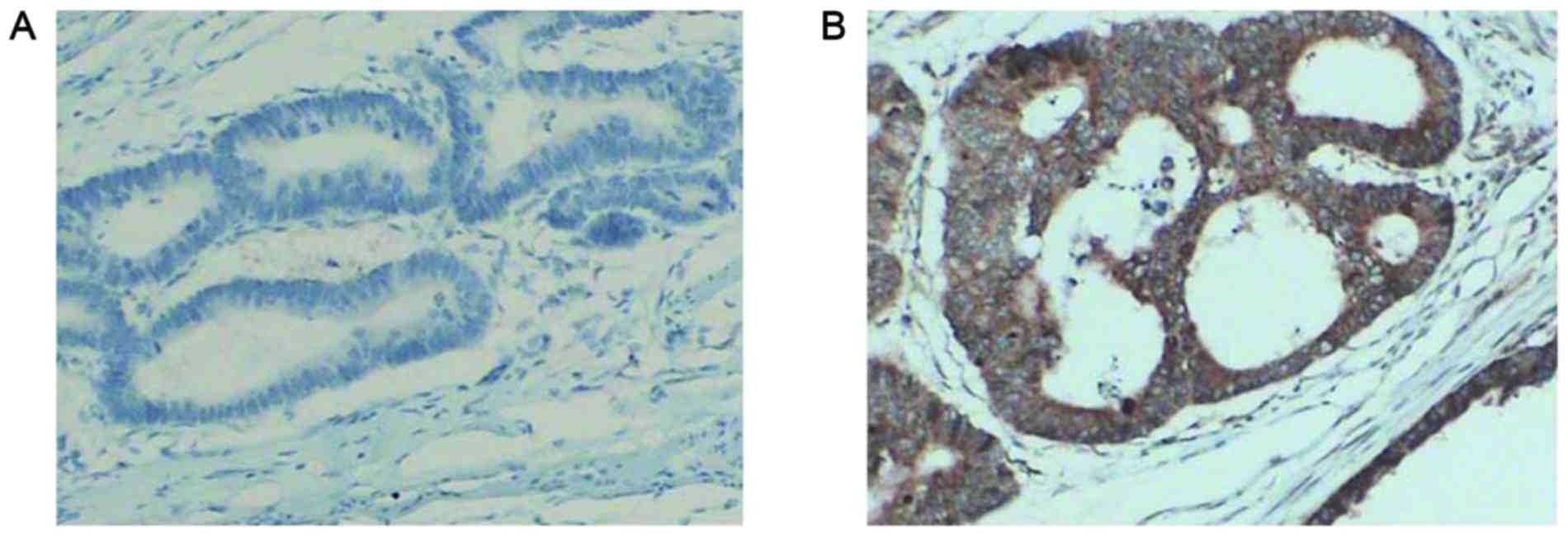
Immunohistochemical staining was scored
independently by three researchers who were blinded to the
clinicopathological data. Microscopic evaluation was performed
under five random visual fields at a magnification of ×200 (Leica
Microsystems GmbH, Wetzlar, Germany). Staining results for hepcidin
were classified by estimating the percentage of epithelial cells
exhibiting specific immunoreactivity (Fig. 1). Immunoreactivity was scored as
follows: Negative, no immunoreactivity; weak, <33% positive
cells; moderate, 33–67% positive cells; and strong, >67%
positive cells. Samples that exhibited negative and weak
immunoreactivity were considered as negative, and those exhibited
moderate and strong immunoreactivity were considered as
positive.
Western blot analysis
The protein samples were routinely extracted from
formalin-fixed paraffin-embedded tissue blocks using liquid tissue
buffer (Expression Pathology Inc., Rockville, MD, USA) for
homogenizing in dry ice, then incubated at 95°C for 90 min. Using
the protein extraction NP-40 lysis buffer (Thermo Fisher Scientific
Inc.) at 100°C for 20 min followed by a 2 h incubation at 80°C, the
protein concentration was determined using the Bradford method
(Quick Start™ Bradford Protein Assay; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Samples with equal
quantities of protein (80 µg) were then loaded in each lane for
electrophoresis using 0.1% SDS, 10% polyacrylamide gel and 4%
polyacrylamide stacking gel. Proteins were subsequently transferred
to polyvinyl difluoride membranes (EMD, Billerica, MA, USA). Each
membrane was treated with Tris-buffered PBS containing 5% bovine
serum albumin (BSA) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
and 0.1% v/v Tween-20 (Sigma-Aldrich; Merck KGaA) with gentle
shaking for 1 h at room temperature. This was followed by
incubation overnight at 4°C with primary antibodies against
interleukin 6 signal transducer (gp130; 1:1,000; catalog no.
sc-9045; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), JAK1
(1:1,000; catalog no. sc-7228; Santa Cruz Biotechnology, Inc.) and
STAT3 (1:1,000; catalog no. sc-482; Santa Cruz Biotechnology,
Inc.), respectively. The membranes were washed for 10 min for three
times in TBST solution and further incubated at room temperature
for 10 min with the secondary horseradish peroxidase-conjugated
goat anti-rabbit antibody (1:5,000). Proteins were then visualized
using ECL reagent (Amersham; GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA) and then exposed to X-ray film. The β-actin
protein (1:1,000; catalog no. sc-47778; Santa Cruz Biotechnology,
Inc.) was used as the internal control for normalizing the relative
density. Results were quantified and analyzed (three repeats
performed for each sample) with Kodak electrophoresis documentation
and analysis system, and Kodak ID image analysis software (Kodak,
Rochester, NY, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total tissue RNA was routinely isolated from
formalin-fixed paraffin-embedded tissue blocks. Reverse
transcription and first strand cDNA synthesis was performed using
MMLV-RT reverse transcriptase (three repeats performed for each
sample) (Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR
analysis was employed to analyze the gene expression of HAMP, IL-6,
gp130, JAK1 and STAT3. The GAPDH gene was used as an internal
reference, and serial dilutions of the positive control were
performed on each plate to create a standard curve. The primer
sequences for the genes are as follows: HAMP forward,
5′-TCTGCTTTCACAGACGGGAC-3′, and reverse 5′-CTTAGCACAGACACTCGGCA-3′;
IL-6 forward, 5′-AACCTGAACCTTCCAAAGATGG-3′, and reverse
5′-TCTGGCTTGTTCCTCACTACT-3′; gp130 forward,
5′-TGAAGCCATAGTCGTGCCTG-3′, and reverse 5′-ACTGGACAGTGCTCGAAGTG-3′;
JAK1 forward 5′-TCTATGAAAGCCGGTGCAGG-3′, and reverse
5′-CCTGTATTGTCTTCGGGGTCA-3′; STAT3 forward
5′-GCCCTTTGGAACGAAGGGTA-3′, and reverse 5′-ATGGTATTGCTGCAGGTCGT-3′;
GAPDH forward 5′-GCATCTTCTTTTGCGTCGCC-3′, and reverse
5′-AGTGATGGCATGGACTGTGG-3′. RT-qPCR was performed with a 25 µl
reaction mixture in a 96-well plate (Takara, Japan) and a
thermocycler (iCycler iQ; Bio-Rad Laboratories, Inc.). The
expression of target gene was normalized to the reference GAPDH to
obtain the relative threshold cycle (ΔCq) and 2−ΔΔCq was
subsequently used to determine the relative abundance of target
gene expression between each group.
Chromatin immunoprecipitation assay
(ChIP)
With the use of commercially available kit
(CHIP-IT® FFPE; Active Motif, Carlsbad, CA, USA), ChIP
was performed on formalin-fixed paraffin-embedded gastric cancer
tissues, according to the manufacturer's instructions.
Specifically, the sonicated chromatin was used for the
immunoprecipitation reaction. Sonicated chromatin (≥200 ng per
reaction), ChIP buffer and protease inhibitor cocktail were added
in order in the 1.5 ml microcentrifuge tube. The rabbit monoclonal
anti-STAT3 (1:100; catalog no. ab68153; Abcam, Cambridge, UK) was
transferred and incubated overnight at 4°C. Protein G agarose beads
were subsequently added and incubated at room temperature for 3 h
following extensive blocking in 0.5% BSA for 2 h at room
temperature. Subsequently, following washing and reversing the
cross-links, DNA was recovered and purified. Finally, the
commercially available CYBR Green (Bio-Rad Laboratories, Inc.)
quantitative PCR was performed with primers (forward,
5′-GAGGGTGACACAACCCTGTT-3′, and reverse, 5′-CGAGTGACAGTCGCTTTT-3′)
flanking the 155 bp region containing the putative STAT3 binding
site in the promoter region of the human HAMP gene. The data was
expressed as the percent of input.
Statistical analysis
For the categorical variables, differences between
groups were calculated using χ2 test or Fisher's exact
test. Bivariate correlations between variables were examined by
Spearman's correlation analysis. For the continuous variables, all
data are expressed as the mean ± standard deviation. One-way
analysis of variance with Tukey's post hoc test was used to
evaluate the differences between certain groups in western blotting
experiments. With regards to the differences of mRNA levels between
each group, P<0.05 was considered to indicate a statistically
significant difference when the ratio of 2−ΔΔCq >1.7.
Statistical significance was accepted at a level of P<0.05. SPSS
statistical software (version 18.0; SPSS, Inc., Chicago, IL, USA)
was used for these statistical analyses.
Results
Local expression of hepcidin in human
gastric cancer
Using immunohistochemical analysis, the local
production of hepcidin was extensively evaluated in tumor and
adjacent non-tumor tissues of gastric cancer. The local positive
expression rate of hepcidin in tumor tissues (35/62, 56.5%) was
significantly higher compared with adjacent non-tumor tissues
(3/15, 20.0%) (P<0.05; data not shown). With respect to the T
categories in tumor tissues, the local positive expression rate of
hepcidin in T3 and T4 stages (27/39, 69.2%) was significantly
elevated compared with T1 and T2 stages (8/23, 34.8%) (P<0.05;
Table II). In addition, with respect
to lymph node metastasis, there was no significant difference
between negative (14/27, 51.9%) and positive (21/35, 60.0%) groups.
Furthermore, with regards to metastasis (other than lymph node
metastasis), there was no significant difference between negative
(29/53, 54.7%) and positive (6/9, 66.7%) groups (Table II).
 | Table II.Correlation between local expression
of hepcidin and clinicopathological characteristics. |
Table II.
Correlation between local expression
of hepcidin and clinicopathological characteristics.
|
| Local expression of
hepcidin in tumor tissues |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
|
Characteristics | Number of negative
cases | Number of positive
cases | χ2 | P-value | Spearman's
correlation coefficient | P-value |
|---|
| Age, years |
|
| 0.172 | 0.678 | 0.053 | 0.684 |
|
≤60 | 13 | 15 |
|
|
|
|
|
>60 | 14 | 20 |
|
|
|
|
| Gender |
|
| 0.051 | 0.822 | −0.029 | 0.826 |
|
Male | 17 | 23 |
|
|
|
|
|
Female | 10 | 12 |
|
|
|
|
| T stages |
|
| 6.984 | 0.008a | 0.336 | 0.008a |
| T1 and
T2 | 15 | 8 |
|
|
|
|
| T3 and
T4 | 12 | 27 |
|
|
|
|
| Lymph node
metastasis |
|
| 0.412 | 0.521 | 0.081 | 0.529 |
|
Negative | 13 | 14 |
|
|
|
|
|
Positive | 14 | 21 |
|
|
|
|
| Other
metastasis |
|
| 0.447 | 0.503 | 0.085 | 0.512 |
|
Negative | 24 | 29 |
|
|
|
|
|
Positive | 3 | 6 |
|
|
|
|
| Anemia |
|
| 1.821 | 0.177 | 0.171 | 0.183 |
|
Negative | 17 | 16 |
|
|
|
|
|
Positive | 10 | 19 |
|
|
|
|
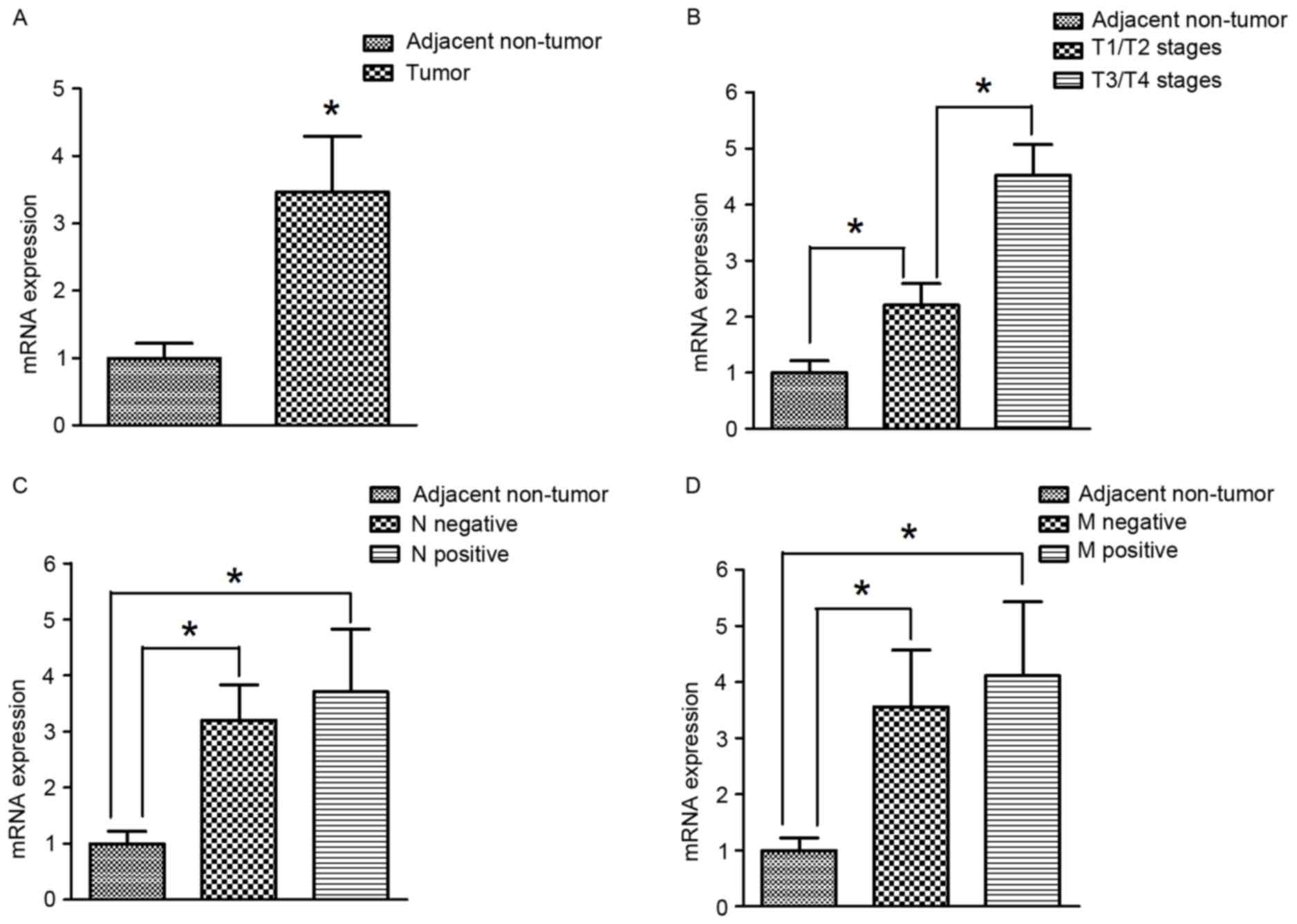
HAMP mRNA expression was extensively evaluated in
tumor and adjacent non-tumor gastric cancer tissues using RT-qPCR.
The mRNA expression of HAMP in tumor tissues was significantly
higher compared with adjacent non-tumor tissues (P<0.05;
Fig. 2A). With respect to the T
stages, the mRNA expression of HAMP in tumor tissues at T3/T4
stages was significantly elevated compared with T1/T2 stages
(P<0.05; Fig. 2B). Additionally,
there were no significant differences in HAMP expression between
positive and negative lymph node metastasis groups (Fig. 2C). Similarly, with regards to
metastasis (excluding lymph node metastasis), there were also no
significant differences between in HAMP expression between negative
and positive groups (Fig. 2D).
Expression of gp130, JAK1, and STAT3
proteins in human gastric cancer tissues
In order to compare the differences in JAK/STAT3
signaling in response to inflammatory stimuli mediated by IL-6 in
human gastric cancer, protein expression of the associated
ligand-receptor and intracellular regulators were detected in the
tumor and adjacent non-tumor tissues. Western blot analyses
indicated that the expression of gp130, JAK1, and STAT3 proteins
were significantly higher in gastric cancer tumor tissues compared
with adjacent non-tumor tissues (Fig.
3A-C). In addition, the local protein expression of JAK1 and
STAT3 in T3/T4 stages was significantly elevated in gastric cancer
tumor tissues compared with T1/T2 stages (Fig. 3B and C). However, there were no
significant differences in gp130 expression between T1/T2 and T3/T4
(Fig. 3A).
mRNA expression of IL-6, gp130, JAK1
and STAT3 in human gastric cancer tissues
Similar to the results for protein expression,
RT-qPCR results indicated that the mRNA expression of IL-6, gp130,
JAK1, and STAT3 was significantly higher in tumor tissues compared
with adjacent non-tumor tissues (Fig.
4A-D). In addition, the expression of JAK1 and STAT3 in T3/T4
stage gastric cancer tumor tissues was significantly increased
compared with expression in T1/T2 gastric cancer tumor tissues
(Fig. 4C and D). However, in tumor
tissues, there were no significant differences in IL-6 and gp130
expression between T3/T4 and T1/T2 stages (Fig. 4A and B).
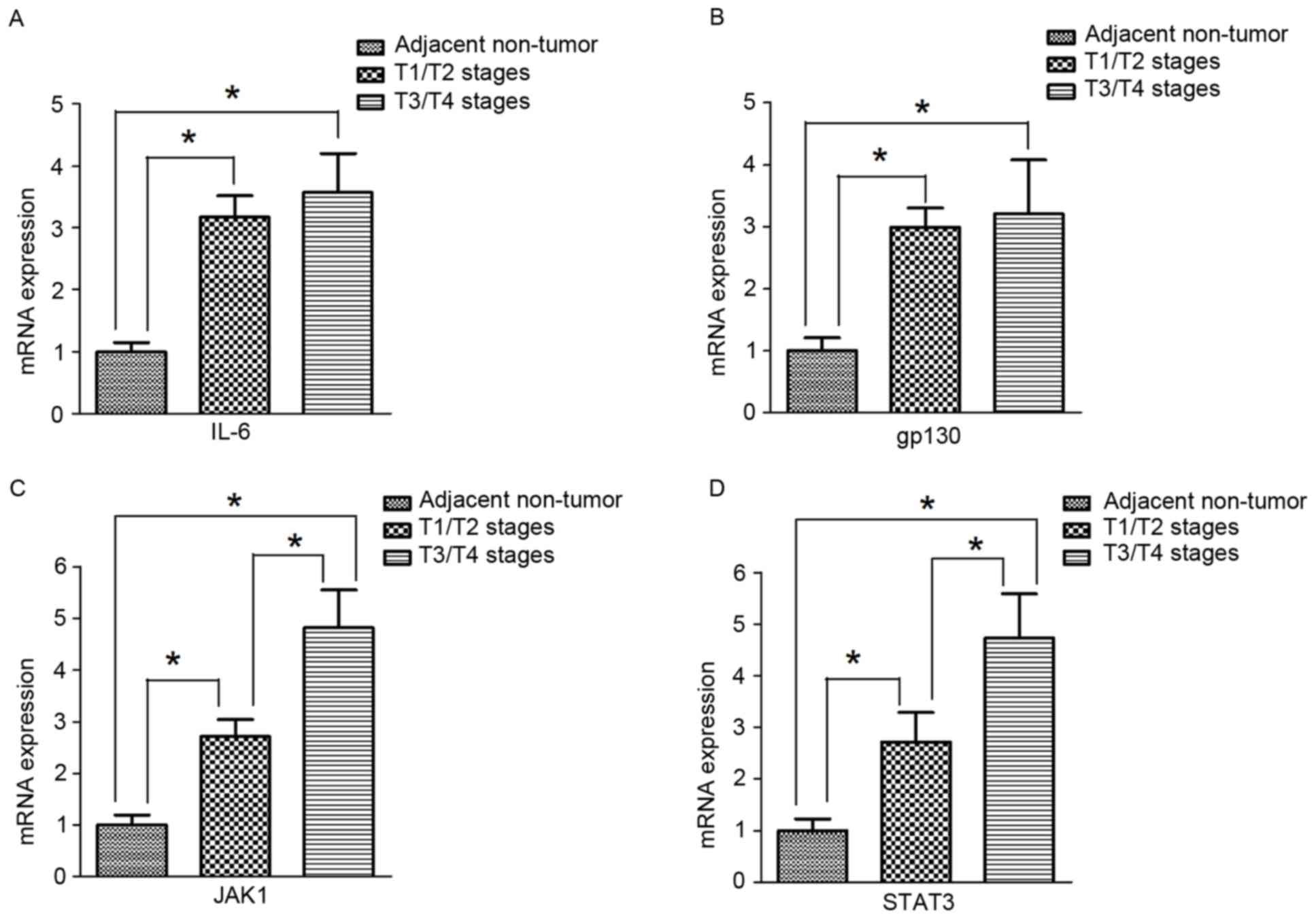 | Figure 4.mRNA expression of IL-6, gp130, JAK1
and STAT3 in human gastric cancer tissues. (A) The mRNA expression
of IL-6 in tumor tissues from patients at different T stages were
significantly higher compared with adjacent non-tumor tissues
(n=15). However, there were no significant differences in IL-6
expression between T1/T2 (n=23) and T3/T4 (n=39). (B) The mRNA
expression of gp130 in tumor tissues of different T stages were
significantly higher compared with adjacent non-tumor tissues
(n=15). However, there were no significant differences in gp130
expression between T1/T2 (n=23) and T3/T4 (n=39). (C) The mRNA
expression of JAK1 in tissues from patients with tumors at T3/T4
stages (n=39) was significantly increased compared with the
expression in tumor tissues at T1/T2 stages (n=23), which were both
significantly higher compared with adjacent non-tumor tissues
(n=15). (D) The mRNA expression of STAT3 in tumor tissues at T3/T4
stages (n=39) was significantly increased compared with the
expression in tumor tissues at T1/T2 stages (n=23), which were both
significantly higher compared with adjacent non-tumor tissues
(n=15). Values are expressed as the mean ± standard deviation.
*P<0.05. gp130, interleukin 6 signal transducer; IL-6,
interleukin-6; JAK1, Janus kinase 1; STAT3, signal transducer and
activator of transcription 3. |
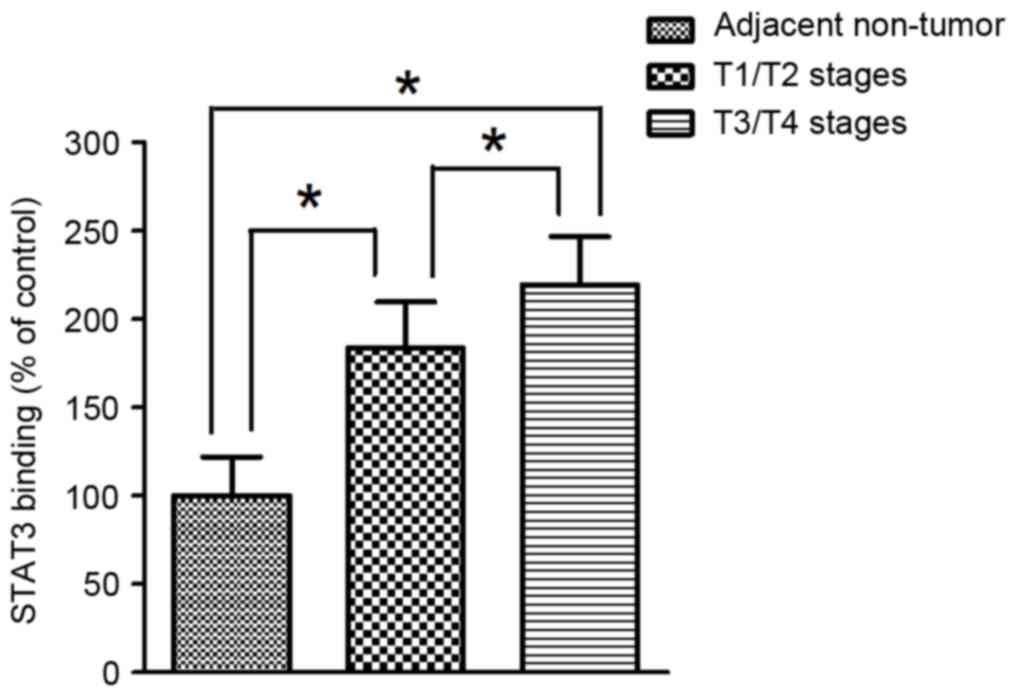
Binding capacity of STAT3 to the HAMP
gene promoter of in human gastric cancer
To further evaluate the function of STAT3 as a
trans-acting regulator that stimulate the transcriptional activity
of the HAMP gene, the binding affinity of STAT3 on the concerned
element in the promoter region of the HAMP gene was determined by
ChIP assay in paraffin-embedded gastric cancer tissues. ChIP
analyses indicated that the binding affinity of STAT3 to the
promoter region of HAMP gene was significantly higher in tumor
tissues compared with adjacent non-tumor tissues in human gastric
cancer. In addition, the binding affinity of STAT3 to the promoter
region in the HAMP gene was significantly more elevated in tumor
tissues at T1/T2 stages compared with T3/T4 (Fig. 5).
Discussion
Due to its capacity to generate deleterious free
radicals, the disturbed iron metabolism not only has the ability to
damage pivotal macromolecules, including DNA, but also mediate
diverse pathogenic signaling, such as hypoxia-inducible factor and
Wnt signaling pathways, which are toxic and carcinogenic (26–29).
Accruing epidemiological evidence has revealed that altered
hepcidin signaling is tightly associated with the clinical outcomes
in cancer patients and may be a prognostic biomarker in malignancy,
including breast cancer (30,31). In fact, a previous study by the
present authors demonstrated that the local production of hepcidin
in breast cancer tissue was prominently increased (15). Moreover, the expression of hepcidin
may be essentially regulated by inflammation in response to
pro-inflammatory cytokines, including IL-6 (10). Indeed, chronic inflammation has
critical roles in the carcinogenesis of gastric cancer (24). As such, the present authors
hypothesize that local hepcidin production may be altered in the
development of human gastric cancer and be associated with the
altered inflammatory responses mediated by IL-6. In the present
retrospective study on formalin-fixed paraffin-embedded patient
samples, it was demonstrated that the local production of hepcidin
was significantly elevated positively correlated with increasing
tumor stages. In addition, the local JAK/STAT3 signaling associated
with IL-6 was significantly increased in parallel with the
expression of hepcidin, which was able to stimulate the
transcriptional activity of the hepcidin gene.
Previous studies have demonstrated that the
intracellular iron regulation is modified in a range of
malignancies (28). In particular,
elevating intracellular iron levels may enhance Wnt signaling,
which is closely associated with increased cellular proliferation
in the pathogenesis of colorectal cancer (26). In general, the disorder of iron
homeostasis in cancer may occur through changes in iron flow
(uptake and efflux) and storage, both of which are controlled by
dozens of iron-regulatory proteins (28). Hepcidin, which can be locally produced
in the tumor, is the master protein for regulating cellular iron
flow (32). Together with the iron
efflux pump in vertebrates, ferroportin, the local
hepcidin-ferroportin axis has a key role in the regulation of
autocrine and/or paracrine iron regulatory loop in cancer (33). When intracellular iron storage and
systemic iron levels are elevated, hepcidin expression is induced
to bind with ferroportin and trigger its subsequent lysosomal
degradation (10,34). In cancer cells, the local expression
of hepcidin is elevated, together with the low levels of
ferroportin, to synergistically decrease the iron efflux to
generate the unstable iron pool to satisfy the increased metabolic
needs in cancer (33,35). Furthermore, a previous study indicated
that the increased expression of hepcidin in colorectal cancer
tissues was correlated with increasing T-stage, according to TNM
classification (36). In the present
study, results from immunohistochemistry and RT-qPCR showed that
local hepcidin production and mRNA expression of HAMP were
significantly increased in gastric cancer tumor tissues compared
with adjacent non-tumor tissues. In addition, the local hepcidin
production and mRNA expression of HAMP in tumor tissues at T3/T4
stages was significantly more elevated compared with T1/T2 stages.
However, with respect to the N and M categories in the TNM
classification system, there were no significant differences in
local hepcidin production and HAMP mRNA expression between
different N and M stages. Therefore, the local expression of
hepcidin was prominently elevated in tumor tissues and positively
correlated with invasive but not metastatic properties of human
gastric cancer. Of note, a previous study indicated that increased
hepcidin expression in tumor tissues was associated with increased
metastatic potential and shorter overall survival in renal cell
carcinoma (17). Due to the lack of
follow-up data, survival was not analyzed in the present study.
Therefore, the prognostic value of local expression of hepcidin in
human gastric cancer remains to be further evaluated.
It is generally thought that hepcidin production was
mainly regulated at the transcriptional level (19).
In general, the transcriptional activity of HAMP
gene may be extensively regulated by various stimuli, including
substrate (e.g., iron), erythropoietic signals (e.g.,
erythropoietin) and inflammatory stimuli (37). In particular, in response to
inflammation, the pro-inflammatory cytokines, such as IL-6 would
instigate the downstream signaling to positively upregulate the
expression of hepcidin (20).
Previous studies indicated that, IL-6 is able to bind to the gp130
protein receptor complex (IL-6 receptor), stimulate JAK tyrosine
kinas-mediated phosphorylation of the transcription factor STAT3
(22,38). Subsequently, the activated STAT3 would
translocate into the nucleus followed by binding to the
STAT3-responsive element on the proximal promoter (~0.6 kb fragment
of 5′upstream flanking sequence) of the HAMP gene, so as to enhance
the transcriptional activity of HAMP (23). In the present study, it was
demonstrated that IL-6 mRNA expression was significantly elevated,
and both mRNA and protein expressions gp130 were significantly
increased in gastric cancer tumor tissues compared with adjacent
non-tumor tissues. Additionally, mRNA and protein expression of
JAK1 and STAT3 were significantly increased in tumor tissues
compared with adjacent non-tumor tissue in human gastric cancer.
This indicated that JAK/STAT3 signaling in response to inflammation
mediated by IL-6 was prominently enhanced in gastric cancer tumor
tissues and may be involved in the pathogenesis of human gastric
cancer. In fact, a previous in vitro study in a range of
gastric cancer cell lines showed that the broadly expressed IL-6
and gp130 were able to promote proliferation, invasion and
lymphangiogenesis via the JAK/STAT3 signaling pathway (39). Furthermore, the present study showed
that, the local mRNA and protein expression of JAK1 and STAT3 in
gastric cancer tumor tissues at T3 and T4 stages were significantly
increased compared with tumor tissues at T1 and T2 stages. In the
light of these findings, the present authors hypothesize that the
local elevated expression of hepcidin gastric cancer tissues in
increasing T stages was closely associated with the upregulation of
IL-6-mediated JAK/STAT3 signaling pathway.
In order to further assess the trans-acting effects
of STAT3 on regulating the transcriptional activity of the HAMP
gene, ChIP assay was performed on paraffin-embedded tissue blocks
to identify the interactions of STAT3 with specific promoter loci
in the HAMP gene in the intact chromatin. The results showed that
the binding affinity of STAT3 was significantly increased in
gastric cancer tumor tissues compared with adjacent non-tumor
tissues. In addition, the binding affinity of STAT3 in tumor
tissues at T3/T4 stages was significantly elevated compared with
T1/T2 stages. As a consequence, the causal effects of enhanced
JAK/STAT3 signaling on the elevated expression of hepcidin in
increasing T stages of human gastric cancer were tentatively
verified in the present study. Furthermore, it was previously
reported that in human hepatocellular carcinoma, the HAMP gene was
transcriptionally repressed in tumor tissues, which was closely
associated with the hypermethylated signature in the promoter
region (40). However, whether the
epigenetic regulation through DNA methylation in the promoter
region in the HAMP gene may be altered in human gastric cancer was
not determined in the present study and should be further
elucidated. Moreover, whether and to what extent the binding
affinity of STAT3 would be affected by altered DNA methylation in
the promoter region of HAMP gene should be further
investigated.
Unlike JAK/STAT3 signaling regulators, there were no
significant differences in expression of IL-6 mRNA, gp130 mRNA and
protein in gastric cancer tumor tissues between different T stages.
This indicated that upregulation of JAK/STAT3 signaling in
increasing T stages may not be directly affected by the local IL-6
generated de novo in tumor tissues. In fact, previous
studies reported that high levels of pro-inflammatory cytokines,
such as IL-6 in serum were correlated with poor prognosis in human
gastric cancer (41). As such, it was
hypothesized that the systemic level of IL-6 in the T3 and T4
stages may be further increased compared with T1 and T2 stages,
which may lead to more upregulation of JAK/STAT3 signaling in the
increasing T stages of human gastric cancer. However, this
hypothesis was not verified in the present study and should be
further evaluated in future research.
Increasing evidence indicated that the incidence of
anemia in cancer patients including gastric cancer was frequent and
is tightly associated with the poor clinical outcomes (42,43).
Multifactorial pathogenesis is involved in the anemia of cancer
patients, of which disturbed iron homeostasis, probably due to the
inflammatory stimulus induced by the tumor, has a key role
(44,45). Moreover, novel studies indicated that
hemoglobin concentration is inversely correlated with the levels of
hepcidin in cancer of the upper gastrointestinal tract (46). In the present study, the association
of anemia in gastric cancer with the local expression of hepcidin
in tumor tissues was assessed. In order to avoid the effects of
chemotherapy/radiotherapy and surgery on anemia, the hemoglobin
concentrations were detected in gastric cancer patients not treated
by chemotherapy/radiotherapy prior to surgery. Results from
immunohistochemical analysis and RT-qPCR indicated that there were
no significant differences in local hepcidin production and HAMP
mRNA expression between anemic and non-anemic gastric cancer
patients. Therefore, in contrast to systemic hepcidin levels, the
local expression of hepcidin in tumor tissue may not serve as a
predictive biomarker for assessing anemia in human gastric
cancer.
In conclusion, the findings of the present study
showed that elevated local expression of hepcidin in tumor tissues
was closely correlated with increasing tumor stages in the
development of human gastric cancer, which provides a novel insight
into the potential prognostic value of tumor hepcidin expression in
clinical practice. Additionally, in the pathogenesis of human
gastric cancer, the increased tumor hepcidin expression was tightly
associated with the upregulation of the JAK/STAT3 signaling
pathway, which may be mediated by IL-6. As such, apart from
iron-chelating agents as the therapeutic candidates, drugs
targeting IL-6-mediated JAK/STAT3 signaling may also be a potential
strategy for correcting disturbed local iron homeostasis in gastric
cancer.
Acknowledgements
The present study was supported by research grants
from the Suzhou Science and Technology Development project (grant
no. SYSD2011035) and the Jiangsu Science and Research project
(grant no. YG201404).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Correa P: Gastric cancer: Overview.
Gastroenterol Clin North Am. 42:211–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
González CA, Sala N and Rokkas T: Gastric
cancer: Epidemiologic aspects. Helicobacter. 18 Suppl 1:S34–S38.
2013. View Article : Google Scholar
|
|
4
|
Epplein M, Zheng W, Li H, Peek RM Jr,
Correa P, Gao J, Michel A, Pawlita M, Cai Q, Xiang YB and Shu XO:
Diet, Helicobacter pylori strain-specific infection, and gastric
cancer risk among Chinese men. Nutr Cancer. 66:550–557. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fonseca-Nunes A, Agudo A, Aranda N, Arija
V, Cross AJ, Molina E, Sanchez MJ, Bueno-de-Mesquita HB, Siersema
P, Weiderpass E, et al: Body iron status and gastric cancer risk in
the EURGAST study. Int J Cancer. 137:2904–2914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jakszyn P, Agudo A, Lujan-Barroso L,
Bueno-de-Mesquita HB, Jenab M, Navarro C, Palli D, Boeing H, Manjer
J, Numans ME, et al: Dietary intake of heme iron and risk of
gastric cancer in the European prospective investigation into
cancer and nutrition study. Int J Cancer. 130:2654–2663. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noto JM and Peek RM Jr: Micronutrients: A
double-edged sword in microbial-induced gastric carcinogenesis.
Trends Cancer. 1:136–144. 2015. View Article : Google Scholar
|
|
8
|
Kim JL, Lee DH, Na YJ, Kim BR, Jeong YA,
Lee SI, Kang S, Joung SY, Lee SY, Oh SC and Min BW: Iron
chelator-induced apoptosis via the ER stress pathway in gastric
cancer cells. Tumour Biol. 37:9709–9719. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park CH, Valore EV, Waring AJ and Ganz T:
Hepcidin, a urinary antimicrobial peptide synthesized in the liver.
J Biol Chem. 276:7806–7810. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ganz T: Systemic iron homeostasis. Physiol
Rev. 93:1721–1741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Babitt JL and Lin HY: The molecular
pathogenesis of hereditary hemochromatosis. Semin Liver Dis.
31:280–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang DL, Senecal T, Ghosh MC,
Ollivierre-Wilson H, Tu T and Rouault TA: Hepcidin regulates
ferroportin expression and intracellular iron homeostasis of
erythroblasts. Blood. 118:2868–2877. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Q, Wang L, Ma Y, Wu X, Jin L and Yu
F: Increased hepcidin expression in non-small cell lung cancer
tissue and serum is associated with clinical stage. Thorac Cancer.
5:14–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisfeld AK, Westerman M, Krahl R, Leiblein
S, Liebert UG, Hehme M, Teupser D, Niederwieser D and Al-Ali HK:
Highly elevated serum hepcidin in patients with acute myeloid
leukemia prior to and after allogeneic hematopoietic cell
transplantation: Does this protect from excessive parenchymal iron
loading? Adv Hematol 2011. 4910582011.
|
|
15
|
Pan X, Lu Y, Cheng X and Wang J: Hepcidin
and ferroportin expression in breast cancer tissue and serum and
their relationship with anemia. Curr Oncol. 23:e24–e26. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miseta A, Nagy J, Nagy T, Poór VS, Fekete
Z and Sipos K: Hepcidin and its potential clinical utility. Cell
Biol Int. 39:1191–1202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamai T, Tomosugi N, Abe H, Arai K and
Yoshida K: Increased serum hepcidin-25 level and increased tumor
expression of hepcidin mRNA are associated with metastasis of renal
cell carcinoma. BMC Cancer. 9:2702009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Chen Y, Guo W, Yuan L, Zhang D,
Xu Y, Nemeth E, Ganz T and Liu S: Disordered hepcidin-ferroportin
signaling promotes breast cancer growth. Cell Signal. 26:2539–2550.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ganz T and Nemeth E: Hepcidin and iron
homeostasis. Biochim Biophys Acta. 1823:1434–1443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidt PJ: Regulation of iron metabolism
by hepcidin under conditions of inflammation. J Biol Chem.
290:18975–18983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawabata H, Uchiyama T, Sakamoto S, Kanda
J, Oishi S, Fujii N, Tomosugi N, Kadowaki N and Takaori-Kondo A: A
HAMP promoter bioassay system for identifying chemical compounds
that modulate hepcidin expression. Exp Hematol. 43:404–413.e5.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee P, Peng H, Gelbart T, Wang L and
Beutler E: Regulation of hepcidin transcription by interleukin-1
and interleukin-6. Proc Natl Acad Sci USA. 102:pp. 1906–1910. 2005;
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wrighting DM and Andrews NC: Interleukin-6
induces hepcidin expression through STAT3. Blood. 108:3204–3209.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Senol K, Ozkan MB, Vural S and Tez M: The
role of inflammation in gastric cancer. Adv Exp Med Biol.
816:235–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. Hoboken:
Wiley-Blackwell; 2009
|
|
26
|
Brookes MJ, Boult J, Roberts K, Cooper BT,
Hotchin NA, Matthews G, Iqbal T and Tselepis C: A role for iron in
Wnt signalling. Oncogene. 27:966–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gozzelino R and Arosio P: Iron homeostasis
in health and disease. Int J Mol Sci. 17:pii: E1302016. View Article : Google Scholar
|
|
28
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xue X, Taylor M, Anderson E, Hao C, Qu A,
Greenson JK, Zimmermann EM, Gonzalez FJ and Shah YM:
Hypoxia-inducible factor-2α activation promotes colorectal cancer
progression by dysregulating iron homeostasis. Cancer Res.
72:2285–2293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ciniselli CM, De Bortoli M, Taverna E,
Varinelli L, Pizzamiglio S, Veneroni S, Bonini C, Orlandi R,
Verderio P and Bongarzone I: Plasma hepcidin in early-stage breast
cancer patients: No relationship with interleukin-6, erythropoietin
and erythroferrone. Expert Rev Proteomics. 12:695–701. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orlandi R, De Bortoli M, Ciniselli CM,
Vaghi E, Caccia D, Garrisi V, Pizzamiglio S, Veneroni S, Bonini C,
Agresti R, et al: Hepcidin and ferritin blood level as noninvasive
tools for predicting breast cancer. Ann Oncol. 25:352–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tesfay L, Clausen KA, Kim JW, Hegde P,
Wang X, Miller LD, Deng Z, Blanchette N, Arvedson T, Miranti CK, et
al: Hepcidin regulation in prostate and its disruption in prostate
cancer. Cancer Res. 75:2254–2263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marques O, Porto G, Rema A, Rêma A, Faria
F, Paula A Cruz, Gomez-Lazaro M, Silva P, da Silva B Martins and
Lopes C: Local iron homeostasis in the breast ductal carcinoma
microenvironment. BMC Cancer. 16:1872016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nemeth E, Tuttle MS, Powelson J, Vaughn
MB, Donovan A, Ward DM, Ganz T and Kaplan J: Hepcidin regulates
cellular iron efflux by binding to ferroportin and inducing its
internalization. Science. 306:2090–2093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pinnix ZK, Miller LD, Wang W, D'Agostino R
Jr, Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, et al:
Ferroportin and iron regulation in breast cancer progression and
prognosis. Sci Transl Med. 2:43ra562010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ward DG, Roberts K, Brookes MJ, Joy H,
Martin A, Ismail T, Spychal R, Iqbal T and Tselepis C: Increased
hepcidin expression in colorectal carcinogenesis. World J
Gastroenterol. 14:1339–1345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ruchala P and Nemeth E: The
pathophysiology and pharmacology of hepcidin. Trends Pharmacol Sci.
35:155–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pietrangelo A, Dierssen U, Valli L, Garuti
C, Rump A, Corradini E, Ernst M, Klein C and Trautwein C: STAT3 is
required for IL-6-gp130-dependent activation of hepcidin in vivo.
Gastroenterology. 132:294–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao G, Zhu G, Huang Y, Zheng W, Hua J,
Yang S, Zhuang J and Ye J: IL-6 mediates the signal pathway of
JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis
in gastric cancer. Oncol Rep. 35:1787–1795. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Udali S, Guarini P, Ruzzenente A,
Ferrarini A, Guglielmi A, Lotto V, Tononi P, Pattini P, Moruzzi S,
Campagnaro T, et al: DNA methylation and gene expression profiles
show novel regulatory pathways in hepatocellular carcinoma. Clin
Epigenetics. 7:432015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ock CY, Nam AR, Bang JH, Kim TY, Lee KH,
Han SW, Im SA, Kim TY, Bang YJ and Oh DY: Signature of cytokines
and angiogenic factors (CAFs) defines a clinically distinct
subgroup of gastric cancer. Gastric Cancer. 20:164–174. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fraenkel PG: Understanding anemia of
chronic disease. Hematology Am Soc Hematol Educ Program.
2015:14–18. 2015.PubMed/NCBI
|
|
43
|
Knight K, Wade S and Balducci L:
Prevalence and outcomes of anemia in cancer: A systematic review of
the literature. Am J Med. 116 Suppl 7A:11S–26S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim A, Rivera S, Shprung D, Limbrick D,
Gabayan V, Nemeth E and Ganz T: Mouse models of anemia of cancer.
PLoS One. 9:e932832014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park S, Jung CW, Kim K, Kim SJ, Kim WS and
Jang JH: Iron deficient erythropoiesis might play key role in
development of anemia in cancer patients. Oncotarget.
6:42803–42812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maccio A, Madeddu C, Gramignano G, Mulas
C, Tanca L, Cherchi MC, Floris C, Omoto I, Barracca A and Ganz T:
The role of inflammation, iron, and nutritional status in
cancer-related anemia: Results of a large, prospective,
observational study. Haematologica. 100:124–132. 2015. View Article : Google Scholar : PubMed/NCBI
|