Introduction
Radiation therapy has long been an indispensable
treatment for head and neck cancer, and primary and secondary brain
tumors, which may provide long-term survival benefits for patients
(1–3).
However, acute and chronic radiation-induced cognitive impairment
is a major reason for limiting radiotherapeutic dosage (2,4). This
cognitive impairment has a diverse character, but is typically
comprised of deficits in hippocampal-dependent functions, such as
learning, memory and spatial information processing (5). Numerous studies have demonstrated that
irradiation-induced cognitive impairments are associated with
decreases in neurogenesis within the hippocampus (5–7).
Granule neurons in the dentate gyrus, which are
generated throughout life, subsequently become functionally
integrated into the hippocampal circuitry (8). Axons and dendrites are the anatomical
bases of synaptic contact (9,10). In humans, progenitor cells in the
dentate gyrus are particularly vulnerable to ionizing radiation,
even at low doses (11). Irradiation
has been demonstrated to reduce the number of proliferating cells
in the dentate gyrus of rodents (12); however, the mechanism underlying the
neurotoxic effects of radiation has not been definitively
identified.
Epigenetic mechanisms, including histone
modifications and DNA methylation, appear to contribute to the
expression of neuronal genes involved in learning and memory within
mouse models; however, there is evidence that histone deacetylase
inhibitors promote the recovery of learning and memory (13). A previous study determined that
whole-brain irradiation (WBI) was associated with cognitive deficit
in Sprague Dawley rats, a reduction in histone H3 acetylation in
the hippocampus and the long-term impairment of neurogenesis in the
dentate gyrus (7). However, the
function of DNA methylation in the adult nervous system remains
unclear.
Epigenetic alterations via DNA methylation are
associated with synaptic plasticity, learning and memory (14). DNA methylation, which is catalyzed by
DNA methyltransferase 1 (DNMT1), DNMT3A and DNMT3B, prevents the
binding of transcription factors to promoter sequences (15). DNA methylation in adult neurons may be
crucial for the transcriptional regulation of genes involved in
memory formation (16). Evidence
indicates that DNA methylation in neurons regulates synaptic
plasticity, as well as neuronal network activity. A prior study
demonstrated that DNMT1 and DNMT3A double-knockout mice revealed
abnormal long-term synaptic plasticity in the hippocampal CA1
region, leading researchers to conclude that these DNA
methyltransferases are required for synaptic plasticity, learning
and memory (17). In addition, DNMT3A
overexpression increased the dendritic spine density of nucleus
accumbens neurons in mice (18).
These findings indicated that DNMTs may be crucial to
hippocampal-dependent memory consolidation and neurogenesis in the
dentate gyrus. However, alterations in DNMT1 and DNMT3A that may
occur following radiation require further investigation.
The present study evaluated the effects of WBI on
the protein expression levels of DNMT1, DNMT3A and DNMT3B in the
hippocampus, and investigated whether these effects were associated
with the radiation-induced impairment of neurogenesis.
Materials and methods
Study design
Sprague Dawley rats were randomly apportioned to the
following treatment groups (n=10/group): Control, radiation only,
zebularine only, or radiation combined with zebularine (radiation +
zebularine). A total of 5 rats were used for immunofluorescence
staining and 5 rats were used for western blot.
Each rat received a twice-daily intraperitoneal
injection of bromo-deoxyuridine (BrdU; 50 mg/kg body weight;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 4 days prior to
radiation exposure. Then, rats in the radiation only group and the
radiation + zebularine group received whole brain irradiation. Rats
of the zebularine-only and radiation with zebularine groups
received zebularine (20 mg/kg, Sigma-Aldrich; Merck KGaA)
intracoelomic injection with a fine needle via the skin following
irradiation. All rats were sacrificed on day 7 following radiation
exposure. Immunofluorescence staining and western blotting were
used to investigate the effects of WBI on cell proliferation,
dendritic growth, and DNMT1 and DNMT3A protein levels.
Animals
A total of 40 healthy male Sprague-Dawley rats
(150–200 g) were obtained from the Medical Experimental Animal
Center of Soochow University (Suzhou, China). All the rats were
housed together, 3–4 animals per cage, at ~24°C, with ad libitum
access to tap water and food. They were kept under natural light in
12 h cycles. The procedures involving animals and their care were
conducted in accordance with the Soochow University Medical
Experimental Animal Care Guidelines, which comply with national
policies for the ethical use of animals (Regulation of the
Administration of Laboratory Animals, order no. 638 of the State
Council). The present study was approved by the Ethics Committee of
the National Drug Clinical Trial Institution of The Second
Affiliated Hospital of Soochow University (Suzhou, China).
Radiation procedure
For radiation treatment, rats were anesthetized with
an intraperitoneal injection of 1 ml/100 g (360 mg/kg body weight)
chloral hydrate, and placed in a prone position in a linear
accelerator (SL 18, Philips UK Ltd., Guildford, UK). Each rat in
the radiation-only group and the radiation + zebularine group
received a single dose of 10 Gy from a 4-MeV electron beam, as
described previously (7,19).
Drug treatments
Prior to radiation treatments, each rat received an
intraperitoneal injection of BrdU; 50 mg/kg body weight;
Sigma-Aldrich; Merck KGaA), twice daily at 8 h intervals, for 4
days. The DNA methylation inhibitor zebularine was first dissolved
in dimethyl sulfoxide, and then diluted in saline. A dose of 20
mg/kg was administered by intracoelomic injection with a fine
needle via the skin; this dose was non-toxic and did not affect the
general condition of the rats in the present study. All the animals
were sacrificed 7 days after WBI.
Immunohistochemistry
Rats were deeply anaesthetized with an
intraperitoneal injection of 1 ml/100 g (360 mg/kg body weight)
chloral hydrate 7 days after WBI. Then, they were treated with 100
ml 0.9% NaCl via transcardial reperfusion at 4°C to flush out the
blood, immediately followed by 500 ml 4% paraformaldehyde (PFA) in
PBS at 4°C for 30 min (pH 7.4). Brains were then carefully
extracted, placed in 4% PFA for overnight fixation at 4°C and then
transferred to 30% sucrose at 4°C until embedding. Subsequently,
brain tissues (30-mm-thick frozen sections) were cut using a
cryostat. Immunofluorescence staining was conducted in accordance
with previously described procedures (7). Briefly, the sections were treated with 2
M HCl at 37°C to denature DNA and then washed in 1X Tris-buffered
saline pH 8.5 to neutralize the acid. The sections were then
incubated in 5% bovine serum albumin (Beyotime Institute of
Biotechnology, Haimen, China) and 5% Triton X-100 in PBS for 1 h at
room temperature.
Tissue sections were incubated with primary
antibodies at 4°C for 24 h, followed by a fluorescent secondary
antibody for 2 h; the tissues were then washed in PBS 3 times. Cell
nuclei were stained with DAPI (Beyotime Institute of Biotechnology)
at room temperature for 5 min prior to mounting on glycerol-treated
slides. Analysis of tissue staining was performed using a confocal
laser-scanning microscope (Olympus Corporation, Tokyo, Japan) with
×10 objective. The excitation wavelength was as follows: Green, 495
nm; red, 590 nm and blue, 358 nm.
The antibodies and working concentrations of primary
antibodies were: Rat anti-doublecortin (DCX; 1:100; cat no. D9818;
Sigma-Aldrich; Merck KGaA); mouse anti-neuronal nuclear antigen
(NeuN; 1:50; cat no. mab377; Chemicon; Merck KGaA); and rat
anti-BrdU (1:500; cat no. B8434; Merck KGaA). The fluorescent
secondary antibodies and working concentrations were: Alexa
Fluor-488 goat anti-mouse (1:500; cat no. A11001; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and cy3 goat
anti-rat (1:500; cat no. 111-165-144; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA).
Western blotting
Rats were sacrificed 7 days post-WBI. Western
blotting was conducted by deeply anesthetizing rats with an
intraperitoneal injection of 1 ml/100 g (360 mg/kg body weight)
chloral hydrate. Following the loss of the righting reflex, animals
were stunned and the carotid artery and spinal cord was severed
using sharp scissors. Brains were excised and transferred to
ice-cold PBS, rinsed carefully, and dissected under a
stereomicroscope under cold conditions with ice-cold PBS. The
hippocampus of each hemisphere was separately analyzed, gently
rinsed in ice-cold PBS and snap-frozen in liquid nitrogen.
Western blotting was performed as previously
described (7). The integrated
densities of each band were quantified using ImageJ Software
(version 2006.02.01; National Institutes of Health, Bethesda, MD,
USA). Numerous exposures were obtained for each immunoblot to
ensure that densitometry was performed on images captured within
the linear exposure range. The primary antibodies and their
dilutions were as follows: Mouse anti-DNMT 1 (1:1,000; cat no.
ab13537; Abcam, Cambridge, UK); mouse anti-DNMT3A (1:1,000; cat no.
ab13888; Abcam); mouse anti-DNMT3B (1:2,000; cat no. ab13604;
Abcam) and anti-β-actin (1:10,000; cat no. A5316; Sigma-Aldrich;
Merck KGaA). The secondary antibodies used were goat anti-mouse
horseradish-peroxidase HRP (1:10,000; cat no. 58307; Jackson
ImmunoResearch Laboratories, Inc.).
Quantitative analysis
Stained tissue sections were evaluated by confocal
microscopy, with split panel and z-axis analysis. Cell counts were
evaluated in 5–10 tissue sections per rat using a multi-channel
configuration with a ×10 objective lens and ×10 eyepiece. The cell
counts were limited to regions in the granule cell layer and hilus.
The volumes of the granule cell layer and hilus were used to
normalize the numbers of Brdu+/NeuN+ cells.
For dendritic growth analysis, a series of images were acquired at
1-µm intervals along the z-section using a ×10 lens and ×10
eyepiece with a digital zoom of 2–3. Maximum intensity projections
of the z-series were applied to create merged images for the
analysis.
Statistical analysis
All procedures were repeated 3 times. Data are
presented as the mean ± standard error of the mean. Statistical
analyses were performed using one-way analysis of variance to
compare the groups followed by a Kruskal-Wallis post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. All data were analyzed using SPSS 20.0 (IBM Corp.,
Armonk, NY, USA).
Results
WBI inhibits the proliferation of
progenitor cells of hippocampus
Rats were sacrificed 7 days following WBI.
Proliferating neurons were labeled with BrdU and NeuN
(BrdU+NeuN+); confocal microscopy was used to
determine the number of BrdU+NeuN+ cells.
Irradiated rats exhibited a markedly lower number of new neurons
compared with the control group. The number of
BrdU+NeuN+ cells was reduced by 71.8%
(P<0.01) in the radiation group compared with in the control
group (Fig. 1).
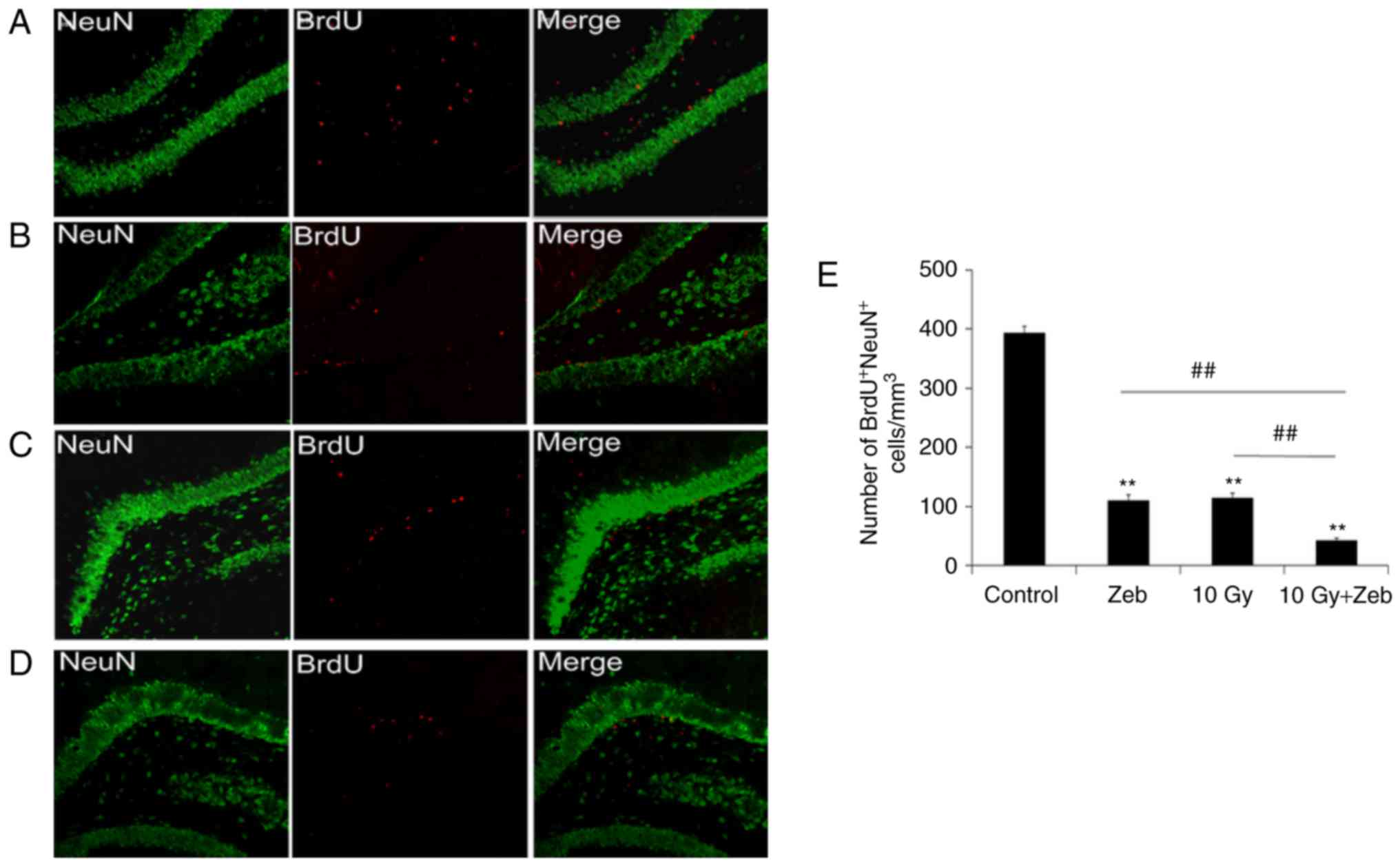 | Figure 1.Proliferation analysis of neural
precursor cells in the hippocampus. Confocal micrographs
(magnification, ×40) revealed novel neurons labeled for NeuN
(green) and BrdU (red). (A) Control, (B) Zeb, (C) 10 Gy radiation,
(D) 10 Gy radiation and Zeb and (E) quantification of the number of
BrdU+NeuN+ cells in different groups.
**P<0.01 vs. the control group. ##P<0.01 vs. the
10 Gy + Zeb group. BrdU, bromo-deoxyuridine; control, no radiation
exposure or Zeb injections; Zeb, zebularine; 10 Gy, irradiated
group; 10 Gy + Zeb, irradiated group treated with zebularine. NeuN,
neuronal nuclear antigen. |
WBI inhibits dendritic growth of novel
neurons
The dendritic development of granule cells in the
dentate gyrus is crucial for integrating into hippocampal circuits
(8,9).
Thus, the length of dendrites was analyzed via immunofluorescence
staining for DCX. The results of the present study indicated that,
compared with that in the control rats, the total dendritic length
of novel neurons was significantly less (52.6%) in the irradiated
group (P<0.05; Fig. 2).
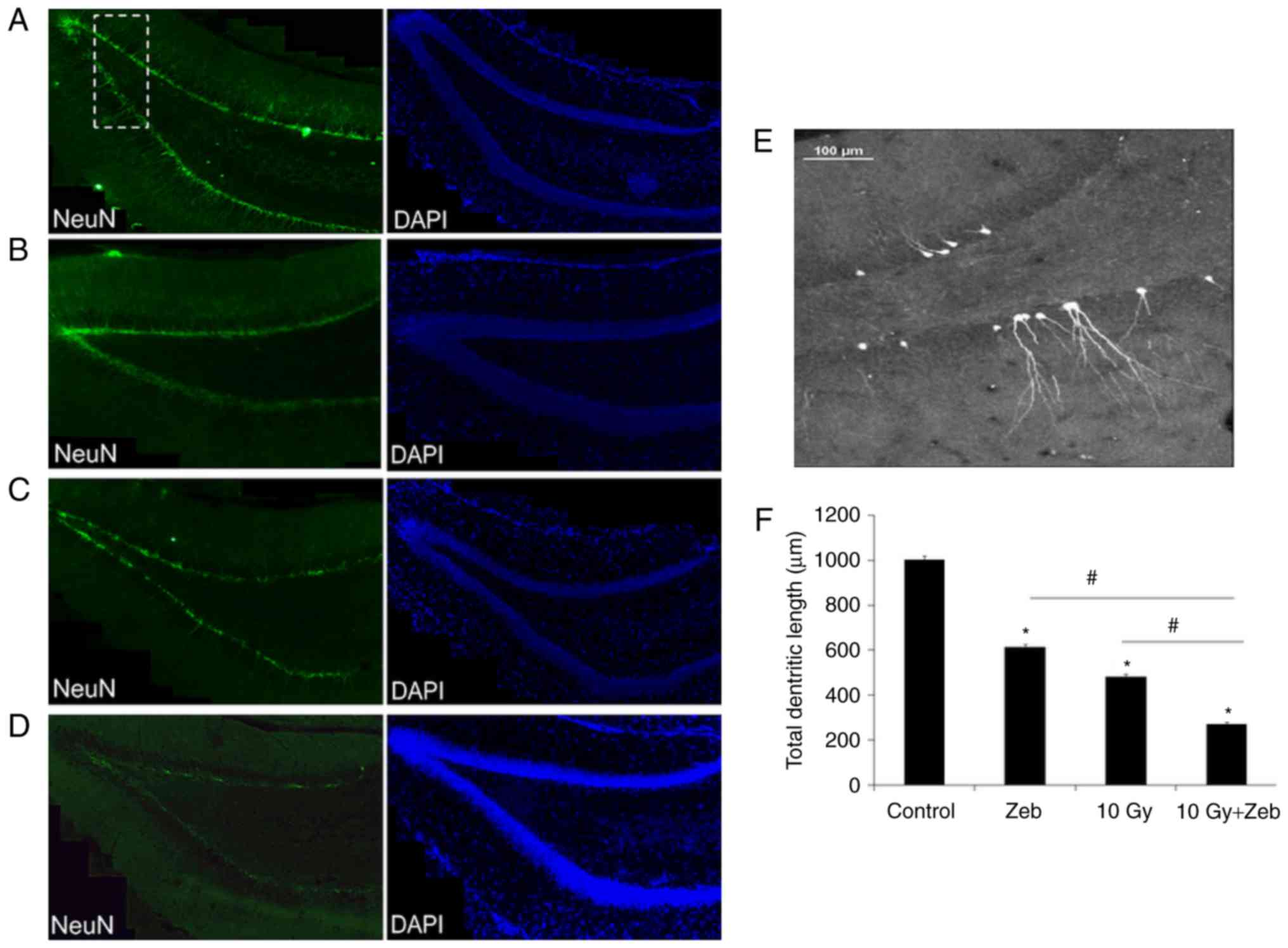 | Figure 2.Dendrite length of newly formed
neurons decreases following irradiation. DCX was used to label
dendrites of novel neurons in the hippocampus (magnification, ×20).
Green, DCX+ cells Blue, DAPI+ cells. (A)
Control, (B) Zeb, (C) 10 Gy radiation, (D) 10 Gy radiation and Zeb.
(E) Magnified structure presented within the white dotted box in
Fig. 2A. (F) Quantification.
*P<0.05 vs. the control group. #P<0.05 vs. the 10
Gy + Zeb group. Control, no radiation exposure; DCX, doublecortin;
Zeb, zebularine; 10 Gy, irradiated group; 10 Gy + Zeb, irradiated
group treated with zebularine. |
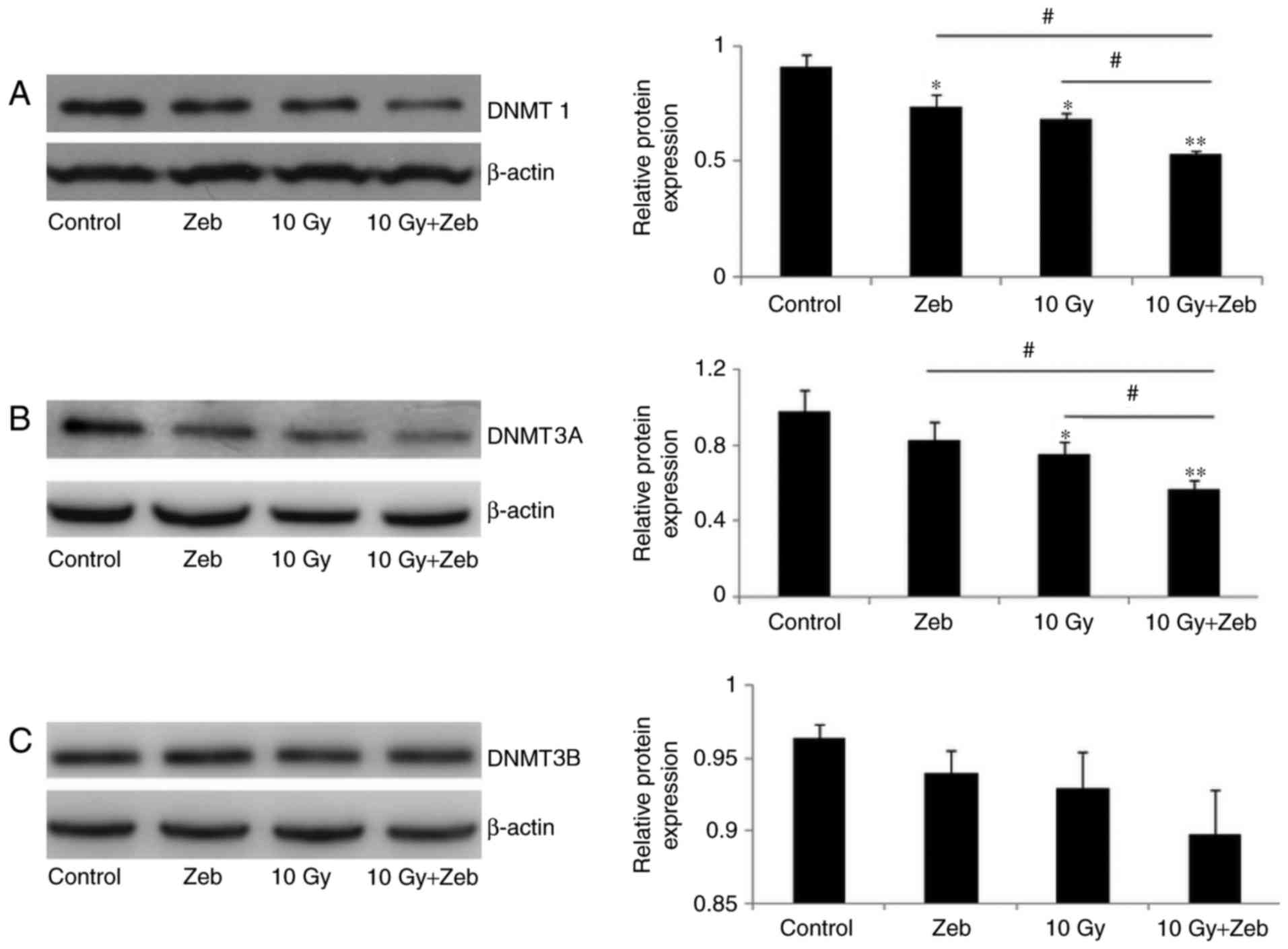
DNMT1 and DNMT3A protein levels in the
hippocampus following WBI
Western blotting revealed a 26.0 and 22.8% reduction
in the protein levels of DNMT1 and DNMT3A, respectively, following
irradiation (P<0.05; Fig. 3).
DNMT3B levels were unaltered following irradiation.
Zebularine decreases DNMT protein
expression levels and inhibits precursor cell development
Zebularine is a DNA methylation inhibitor that
increases the affinity of DNA-methyltransferase binding with DNA so
that the former becomes inactive. To assess the effects of DNMTs on
dendritic development, zebularine was utilized to determine whether
the decrease in DNMT resulted in reduced cell proliferation. The
results of the present study revealed that there were no variations
between the zebularine-only and the radiation-only groups. Compared
with that in the control group, radiation together with zebularine
decreased DNMT1 (45.0%) and DNMT3A (41.7%) expression levels
(P<0.05; Fig. 3). Additionally,
compared with that in the control group, radiation together with
zebularine decreased the percentage of proliferative cells (90.3%,
P<0.05; Fig. 1D and E) and the
total dendritic length (73.0%, P<0.05; Fig. 2D and F).
Compared with the radiation-only group, the
radiation and zebularine group exhibited lower DNMT1 protein levels
(24.7%) and DNMT3A (23.5%). A more significant reduction in the
percentage of proliferative cells (62.6%; Fig. 1C and D) and total dendritic length
(43.6%; Fig. 2) was observed in the
radiation + zebularine group than the radiation-only group
(P<0.05).
Discussion
The present study investigated the effects of WBI on
DNMT1, DNMT3A, and DNMT3B protein expression levels in the
hippocampus and the function of these DNA methyltransferases in
radiation-induced neurogenesis impairment. The findings of the
present study revealed that radiation inhibited cellular
proliferation, dendritic growth, and DNMT1 and DNMT3A protein
expression levels in the hippocampus. Radiation and zebularine
treatment exhibited a highly significant inhibitory effect compared
with radiation treatment alone. These results indicated that DNMT1
and DNMT3A may be involved in the pathogenesis of WBI-induced
neurogenesis. To the best of our knowledge, this is the first
report of alterations in DNMT expression in the hippocampus of a
rat model of radiation brain injury.
Cognitive impairment has been reported in patients
irradiated for the treatment of head and neck cancer, as well as
primary and secondary brain tumors (4). Irradiation of the temporal lobe,
including the hippocampus, cannot usually be avoided during
radiation therapy. Thus, patients are at an increased risk of
hippocampal injury and impairment of complex cognitive processes,
including spatial recognition (20)
and declarative memory, which depend on the integrity of the
hippocampus (21). The hippocampus
has been recognized as a region associated with radiation-induced
cognitive dysfunction (5,20).
Gene expression studies have demonstrated that DNMTs
in the central nervous system may be distinguished by their
expression profiles. The presence of DNMT3B is only observed in
neural progenitor tissue during early embryogenesis (15,22).
However, DNMT1 and DNMT3A expression levels are high within neurons
during embryogenesis and throughout adulthood. This suggests that
DNMTs and dynamic methylation states retain functional importance
in the adult brain (22–24). Evidence revealed by Maddox et
al (23) strongly indicates that
DNMT activity regulates the consolidation and post-retrieval
retention of alterations associated with conditioning in the
tone-evoked neural activity of the lateral amygdala. In the
developing mouse brain, deletion of DNMT1 in progenitor cells was
associated with the inhibition of neuronal maturation and survival
(25). Singh and Thakur (24) demonstrated that age-associated memory
decline was associated with lower levels of DNMT1 and higher levels
of histone deacetylase 2. In the postnatal forebrain, DNMT3A is
expressed in the subventricular zone and the hippocampal dentate
gyrus (26,27). Morris et al (26) demonstrated that DNMT3A-knockout mice
exhibited synaptic alterations and learning deficits. These mutant
mice lacking DNMT3A exhibited a loss of motor neurons in the
hypoglossal nucleus and morphological defects in the neuromuscular
junctions of the diaphragm. This indicated that DNMT3A could
contribute to the survival of motor neurons and the maintenance of
the neuromuscular endplate structure. Although the function of DNA
demethylation in post-mitotic neurons remains uncertain, the
possibility of an epigenetic mechanism that regulates cellular and
behavioral activities requires further investigation.
Certain mental disorders in humans have been
associated with DNA methylation (28). Immunodeficiency-centromere
instability-facial anomalies syndrome is caused by a recessive
mutation in the DNMT3B gene (28).
Additionally, methyl CpG binding protein 2 gene mutations have been
reported in Rett syndrome (29). The
results of the present study demonstrated that DNMT1 and DNMT3A
levels declined in the hippocampus following WBI, which may result
in defective neurogenesis. The use of DNMT inhibitors also
demonstrated that decreased expression of DNMTs inhibited the
development of precursor cells. This finding is consistent with
numerous studies (17,18,26,30).
Nelson et al (30) reported
that DNMT inhibitors may cause deficits in excitatory synaptic
transmission and reduce spontaneous network activity. Previously,
the results of studies using DNMT inhibitors have indicated that
DNA methylation may target specific genes involved in synaptic
plasticity, as well as learning and memory (17,23,31).
Therefore, identifying the genes that are crucial to learning and
memory that are also regulated by DNMT1 and DNMT3A may be
beneficial to the understanding of human disorders.
All animals in the present study were male
Sprague-Dawley rats; as it is commonly accepted that the emotions
of female animals vary markedly during the different phases of the
estrous cycle (32,33). Furthermore, chromatin-modifying
enzymes are regulated by estrogen (32). Tsai et al (33) demonstrated that epigenetic
modifications, including DNA methylation and histone acetylation,
varied between male and female mouse brains. Therefore, male rats
were used throughout the present study to ensure consistency and
provide data with high validity.
The present study demonstrated that WBI led to the
impairment of neurogenesis and decreased the protein levels of
DNMT1 and DNMT3A, and may be involved in the pathogenesis of
WBI-induced cognitive deficits via the regulation of neuronal
proliferation and dendritic growth.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81402517 and
81372411), the Suzhou Science and Technology Project (grant no.
SYS201651), the Suzhou Cancer Clinical Medical Center (grant no.
Szzx201506), the Jiangsu Provincial Medical Youth Talent (grant no.
QNRC2016234) and the Natural Science Foundation of Jiangsu Province
(grant no. BK20171224).
References
|
1
|
Cairncross G, Wang M, Shaw E, Jenkins R,
Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W
and Mehta M: Phase III trial of chemoradiotherapy for anaplastic
oligodendroglioma: Long-term results of RTOG 9402. J Clin Oncol.
31:337–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu T, Zhu G, He X, Ying H and Hu C: A
phase III randomized study comparing neoadjuvant chemotherapy with
concurrent chemotherapy combined with radiotherapy for
locoregionally advanced nasopharyngeal carcinoma: Updated long-term
survival outcomes. Oral Oncol. 50:71–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis MA, Tyrrell J, Slotman GJ, Sudhindra
R, Sachdeva K, Fanelle J, Smith G, Wurzer JV, Cassir J and Nazha
NT; Southern New Jersey Head and Neck Cancer Treatment Network, :
Preoperative simultaneous fractionated cisplatin and radiation
therapy in the treatment of advanced operable stage III and IV
squamous cell carcinoma of the head and neck. Am J Surg.
209:575–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douw L, Klein M, Fagel SS, van den Heuvel
J, Taphoorn MJ, Aaronson NK, Postma TJ, Vandertop WP, Mooij JJ,
Boerman RH, et al: Cognitive and radiological effects of
radiotherapy in patients with low-grade glioma: Long-term
follow-up. Lancet Neurol. 8:810–818. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Greene-Schloesser D, Moore E and Robbins
ME: Molecular pathways: Radiation-induced cognitive impairment.
Clin Cancer Res. 19:2294–2300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monje M and Dietrich J: Cognitive side
effects of cancer therapy demonstrate a functional role for adult
neurogenesis. Behav Brain Res. 227:376–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji S, Tian Y, Lu Y, Sun R, Ji J, Zhang L
and Duan S: Irradiation-induced hippocampal neurogenesis impairment
is associated with epigenetic regulation of bdnf gene
transcription. Brain Res. 1577:77–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bond AM, Ming GL and Song H: Adult
mammalian neural stem cells and neurogenesis: Five decades later.
Cell Stem Cell. 17:385–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park H and Poo MM: Neurotrophin regulation
of neural circuit development and function. Nature Rev Neurosci.
14:7–23. 2013. View
Article : Google Scholar
|
|
10
|
Cheng Z, Li YQ and Wong CS: Effects of
aging on hippocampal neurogenesis after irradiation. Int J Radiat
Oncol Biol Phys. 94:1181–1189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monje ML, Mizumatsu S, Fike JR and Palmer
TD: Irradiation induces neural precursor-cell dysfunction. Nat Med.
8:955–962. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marty VN, Vlkolinsky R, Minassian N, Cohen
T, Nelson GA and Spigelman I: Radiation-induced alterations in
synaptic neurotransmission of dentate granule cells depend on the
dose and species of charged particles. Radiat Res. 182:653–665.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fischer A, Sananbenesi F, Wang X, Dobbin M
and Tsai LH: Recovery of learning and memory is associated with
chromatin remodelling. Nature. 447:178–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zovkic IB, Guzman-Karlsson MC and Sweatt
JD: Epigenetic regulation of memory formation and maintenance.
Learn Mem. 20:61–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hon GC, Rajagopal N, Shen Y, McCleary DF,
Yue F, Dang MD and Ren B: Epigenetic memory at embryonic enhancers
identified in DNA methylation maps from adult mouse tissues. Nat
Genet. 45:1198–1206. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller CA, Gavin CF, White JA, Parrish RR,
Honasoge A, Yancey CR, Rivera IM, Rubio MD, Rumbaugh G and Sweatt
JD: Cortical DNA methylation maintains remote memory. Nature
Neurosci. 13:664–666. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng J, Zhou Y, Campbell SL, Le T, Li E,
Sweatt JD, Silva AJ and Fan G: Dnmt1 and Dnmt3a maintain DNA
methylation and regulate synaptic function in adult forebrain
neurons. Nat Neurosci. 13:423–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
LaPlant Q, Vialou V, Covington HE III,
Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iñiguez
SD, et al: Dnmt3a regulates emotional behavior and spine plasticity
in the nucleus accumbens. Nat Neurosci. 13:1137–1143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tian Y, Shi Z, Yang S, Chen Y and Bao S:
Changes in myelin basic protein and demyelination in the rat brain
within 3 months of single 2-, 10-, or 30-Gy whole-brain radiation
treatments. J Neurosurg. 109:881–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pereira Dias G, Hollywood R, Bevilaqua MC,
da Luz AC, Hindges R, Nardi AE and Thuret S: Consequences of cancer
treatments on adult hippocampal neurogenesis: Implications for
cognitive function and depressive symptoms. Neuro-oncol.
16:476–492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Snyder JS, Soumier A, Brewer M, Pickel J
and Cameron HA: Adult hippocampal neurogenesis buffers stress
responses and depressive behaviour. Nature. 476:458–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe D, Uchiyama K and Hanaoka K:
Transition of mouse de novo methyltransferases expression from
Dnmt3b to Dnmt3a during neural progenitor cell development.
Neurosci. 142:727–737. 2006. View Article : Google Scholar
|
|
23
|
Maddox SA, Watts CS and Schafe GE: DNA
methyltransferase activity is required for memory-related neural
plasticity in the lateral amygdala. Neurobiol Learn Mem.
107:93–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh P and Thakur MK: Reduced recognition
memory is correlated with decrease in DNA methyltransferase1 and
increase in histone deacetylase2 protein expression in old male
mice. Biogerontology. 15:339–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hutnick LK, Golshani P, Namihira M, Xue Z,
Matynia A, Yang XW, Silva AJ, Schweizer FE and Fan G: DNA
hypomethylation restricted to the murine forebrain induces cortical
degeneration and impairs postnatal neuronal maturation. Hum Mol
Genet. 18:2875–2888. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morris MJ, Adachi M, Na ES and Monteggia
LM: Selective role for DNMT3a in learning and memory. Neurobiol
Learn Mem. 115:30–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simmons RK, Stringfellow SA, Glover ME,
Wagle AA and Clinton SM: DNA methylation markers in the postnatal
developing rat brain. Brain Res. 1533:26–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hamidi T, Singh AK and Chen T: Genetic
alterations of DNA methylation machinery in human diseases.
Epigenomics. 7:247–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lombardi LM, Baker SA and Zoghbi HY: MECP2
disorders: From the clinic to mice and back. J Clin Invest.
125:2914–2923. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nelson ED, Kavalali ET and Monteggia LM:
Activity-dependent suppression of miniature neurotransmission
through the regulation of DNA methylation. J Neurosci. 28:395–406.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Meyer K and Korz V: Stress induced
hippocampal mineralocorticoid and estrogen receptor β gene
expression and long-term potentiation in male adult rats is
sensitive to early-life stress experience.
Psychoneuroendocrinology. 38:250–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao Z, Fan L and Frick KM: Epigenetic
alterations regulate estradiol-induced enhancement of memory
consolidation. Proc Natl Acad Sci USA. 107:pp. 5605–5610. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsai HW, Grant PA and Rissman EF: Sex
differences in histone modifications in the neonatal mouse brain.
Epigenetics. 4:47–53. 2009. View Article : Google Scholar : PubMed/NCBI
|