Introduction
Pedunculated hepatocellular carcinoma (P-HCC) is a
rare type of HCC, which is defined as a carcinoma protruding from
the liver with or without a pedicle, and with a low degree of liver
invasion (1). P-HCC was first
described by Roux in 1897 (2), and
represents 0.24–3.00% of all cases of HCC in Japan (3,4).
Additionally, to the best of our knowledge, <200 cases have been
reported in previous studies (4–6).
The majority of P-HCC cases are treated surgically,
with higher operability rates and better survival than conventional
HCC, which may be due to its unique growth pattern, high rate of
tumor capsule formation and lower vascular invasion (4–6). However,
up to 39.4% of patients with P-HCC are unable to undergo surgical
resection (7). Therefore, palliative
treatments may serve a central function in the treatment of
unresectable P-HCC. Transcatheter arterial chemoembolization (TACE)
is the most widely used locoregional therapy for patients with
intermediate HCC, who cannot be treated surgically (8–11).
However, the blood supply to P-HCC is complicated, arising from
hepatic arteries and extrahepatic collateral vessels (3). P-HCC tumor lesions protruding from the
liver may receive an extrahepatic blood supply from adjacent
vessels, despite the presence of a patent hepatic artery. In
addition, repeated TACE results in the attenuation of hepatic
arterial circulation and causes the development of extrahepatic
collaterals (12). Therefore, the
characteristics of the blood supply of P-HCC may aid the
improvement of the therapeutic effect of transcatheter management
and determine the selection of subsequent treatment schemes for
patients with P-HCC following TACE.
The aim of the present study was to retrospectively
analyze angiographic findings in 39 patients with P-HCC treated
with TACE, and to evaluate the blood supply characteristics of
P-HCC prior to and following TACE treatment.
Patients and methods
Patients
Between January 2003 and February 2016, 39 patients
(male, 37; female, 2; mean age, 49 years; age range, 19 to 70
years) with histologically proven P-HCC, out of a total of 1,238
patients with HCC, were treated in the Department of Interventional
Radiology, from the Nanfang Hospital of Southern Medical University
and The Second Affiliated Hospital of Shantou University Medical
College (Guangdong, China). Exclusion criteria were as follows: i)
Karnofsky performance score of <80; ii) hepatic function
analyzed using the Child-Pugh classification C (8); iii) vascular tumor thrombus; iv)
extrahepatic metastases (not including regional lymph node
involvement); v) received previous treatment for this type of
tumor. P-HCC was defined, using computed tomography (CT) scanning
or magnetic resonance imaging (MRI), as HCC with tumor lesions
protruding from the liver with or without a pedicle. A total of 39
patients with P-HCC were treated with TACE (between 2 and 9
sessions) in the present study. The P-HCC tumor lesions ranged
between 4.2 and 22.1 cm (mean, 10.2 cm) in diameter, including
<5 cm, n=2; 5–10 cm, n=19; and >10 cm, n=18, and were
localized within the right diaphragmatic surface (n=2), the right
visceral surface (n=20), the left diaphragmatic surface (n=2) and
the left visceral surface (n=15) (Table
I).
 | Table I.Blood supply, tumor size, and tumor
location in 39 patients with pedunculated hepatocellular carcinoma
prior to transcatheter arterial chemoembolization. |
Table I.
Blood supply, tumor size, and tumor
location in 39 patients with pedunculated hepatocellular carcinoma
prior to transcatheter arterial chemoembolization.
| Parameter | Only from
intrahepatic arteriesa,
n | Coupling with
extrahepatic collateral arteriesb, n | χ2 | P-value |
|---|
| Tumor size, cm |
|
| 164.000 | <0.001 |
|
<5 | 2 | 0 |
|
|
| 5–10 | 19 | 5 |
|
|
|
>10 | 18 | 18 |
|
|
| Tumor location |
|
| 7.358 | 0.061 |
| Right
diaphragmatic surface | 2 | 0 |
|
|
| Right visceral
surface | 20 | 13 |
|
|
| Left diaphragmatic
surface | 2 | 0 |
|
|
| Left visceral
surface | 15 | 10 |
|
|
CT scanning or MRI examination and laboratory tests,
including quantification of routine blood test, liver function,
coagulation function and α-fetoprotein (AFP) levels, were regularly
performed prior to angiography. Written informed consent was
obtained from all patients prior to treatment.
Angiography
Angiographies were performed using the Seldinger
technique (13). Subsequent to
introducing a 4- or 5-F catheter through the femoral artery,
arteriograms of the celiac, common hepatic and superior mesenteric
arteries were initially performed in all patients to localize
lesions and identify blood vessels feeding the tumor. During
selected catheterization, the catheter must be placed at the
arterial orifice; however, it cannot be entered too deeply into the
arteries orifice as this may omit extrahepatic collateral supplies.
A microcatheter was used for highly selective catheterization when
the tumor-feeding vessel was small and twisted. Extrahepatic
collateral pathways were sought when a tumor stain not
corresponding to P-HCC, as depicted by imaging modalities including
contrast-enhanced CT and MRI, was not identified on angiograms of
these arteries. CT and MRI results obtained prior to the TACE
procedure, which can depict the tumor and guide the TACE procedure.
The individual vessels, which may feed HCC, depending on the tumor
location, were selected to determine whether collateral supply to
the tumor was present. Aortography aided the location of the
individual vessels arising from the aorta, if required.
Statistical analysis
All data were presented as the percentage of
patients or the mean ± standard deviation. Data were compared using
Pearson's χ2 tests when appropriate. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS software (version
17.0; SPSS, Inc., Chicago, IL, USA).
Results
Angiographic results prior to
TACE
Angiographies at the first TACE session revealed 70
tumor-feeding arteries in the 39 patients, including 39 (56.0%)
intrahepatic arteries and 31 (44.0%) extrahepatic collateral
vessels in 23 cases (23/39), which consisted of 14 cases with a
tumor >10 cm in size (5/14 had two extrahepatic collateral
arteries) and 9 cases with a tumor 5–10 cm in size (3/9 had two
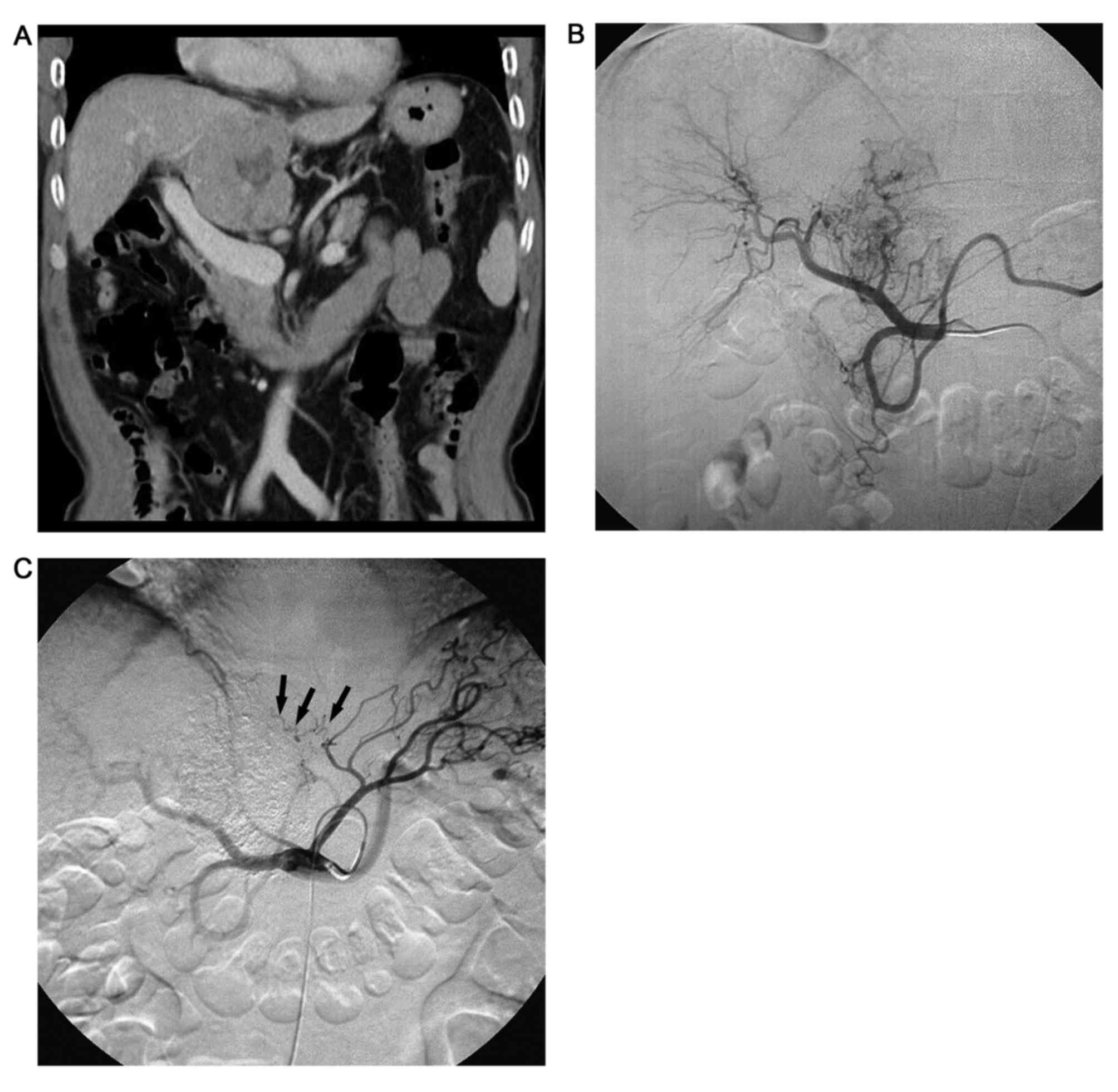
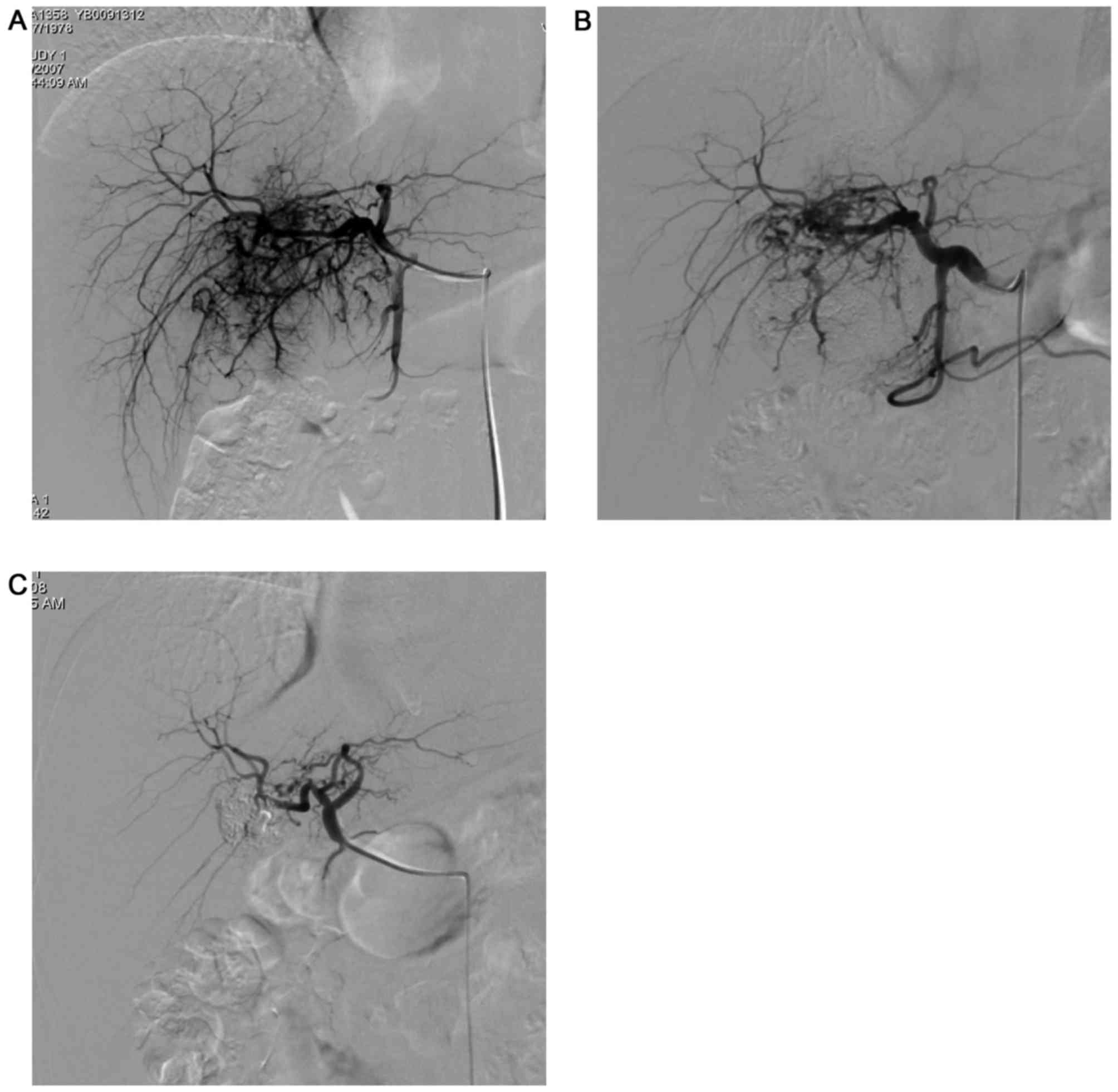
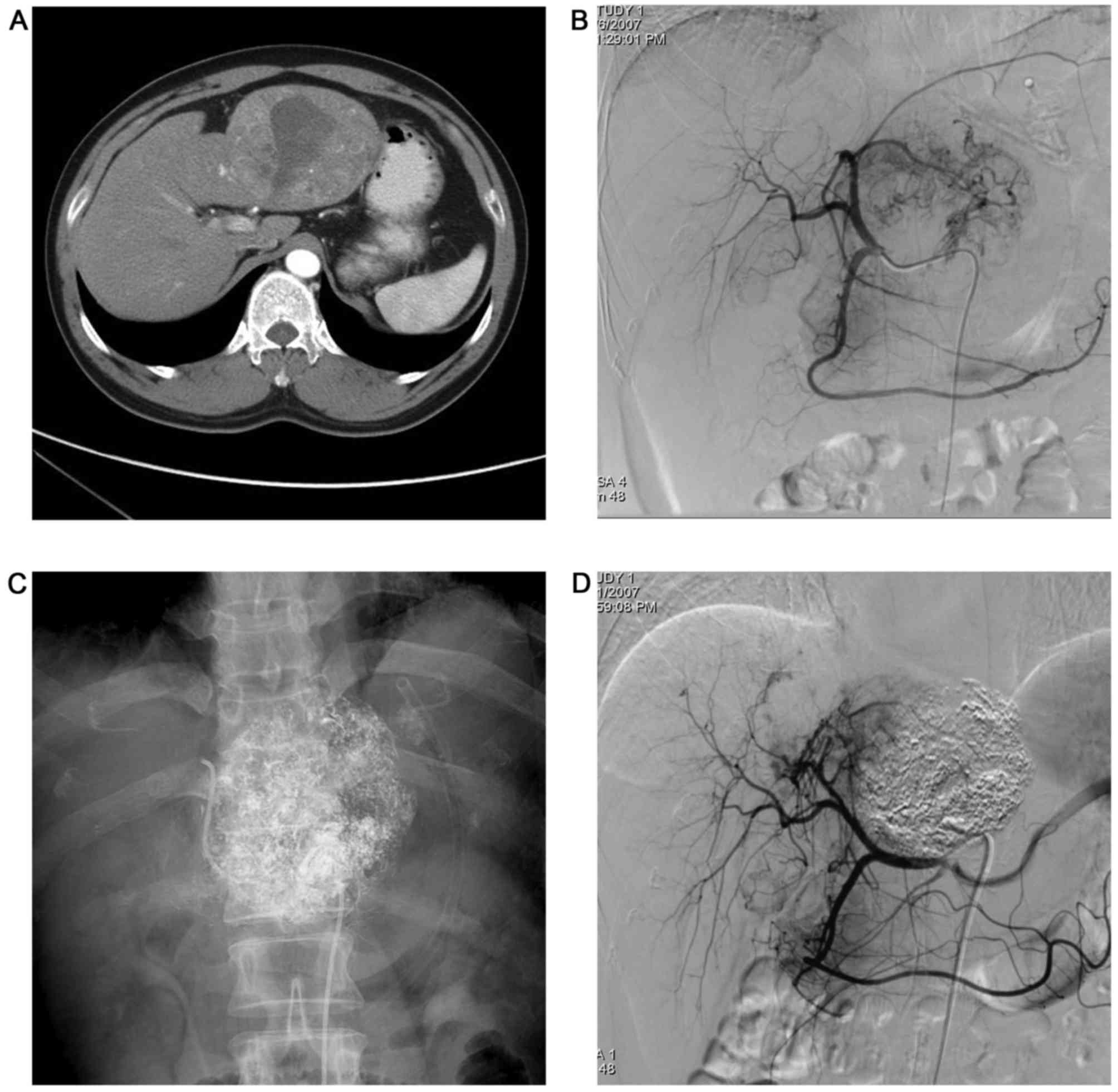
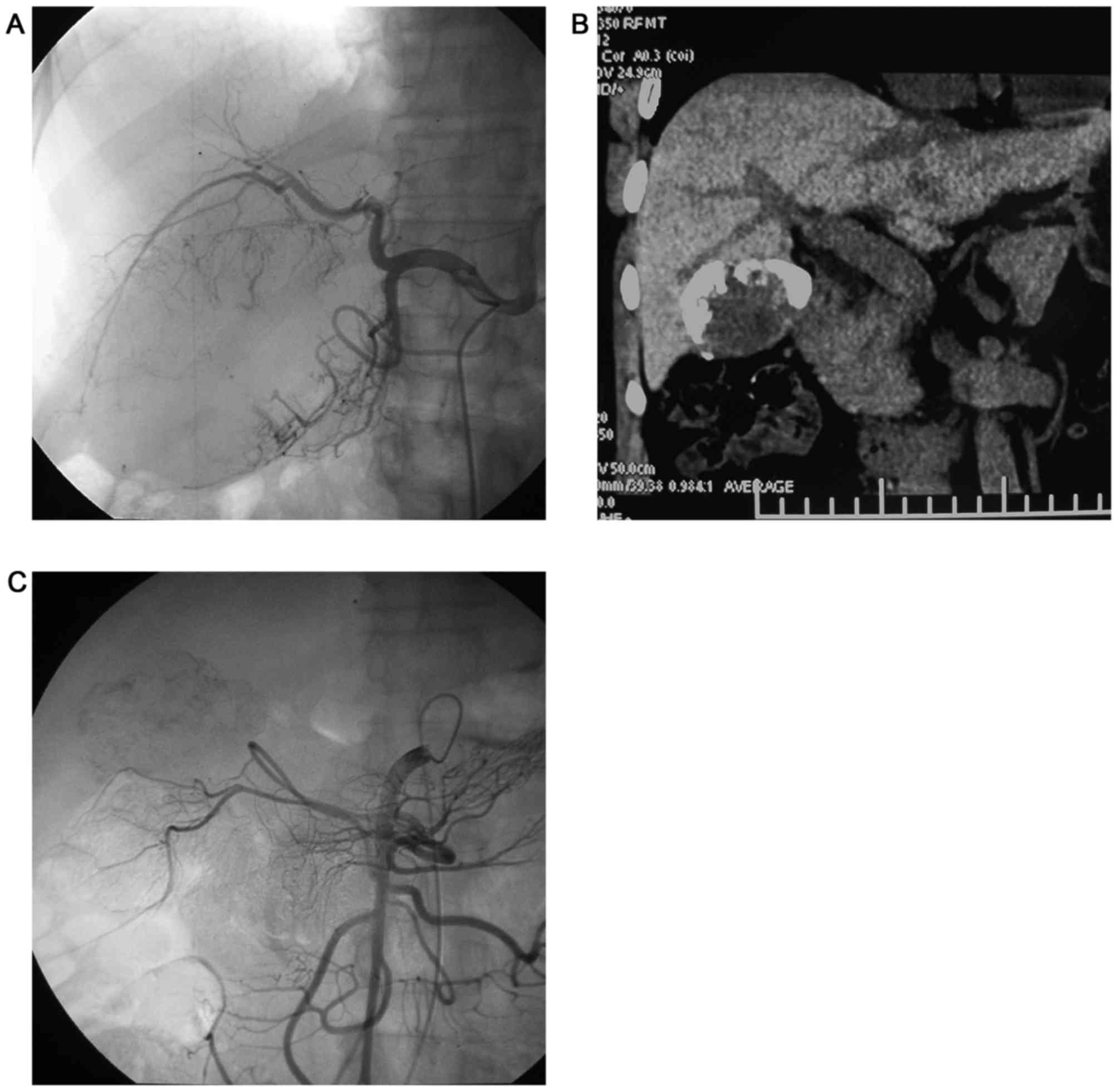
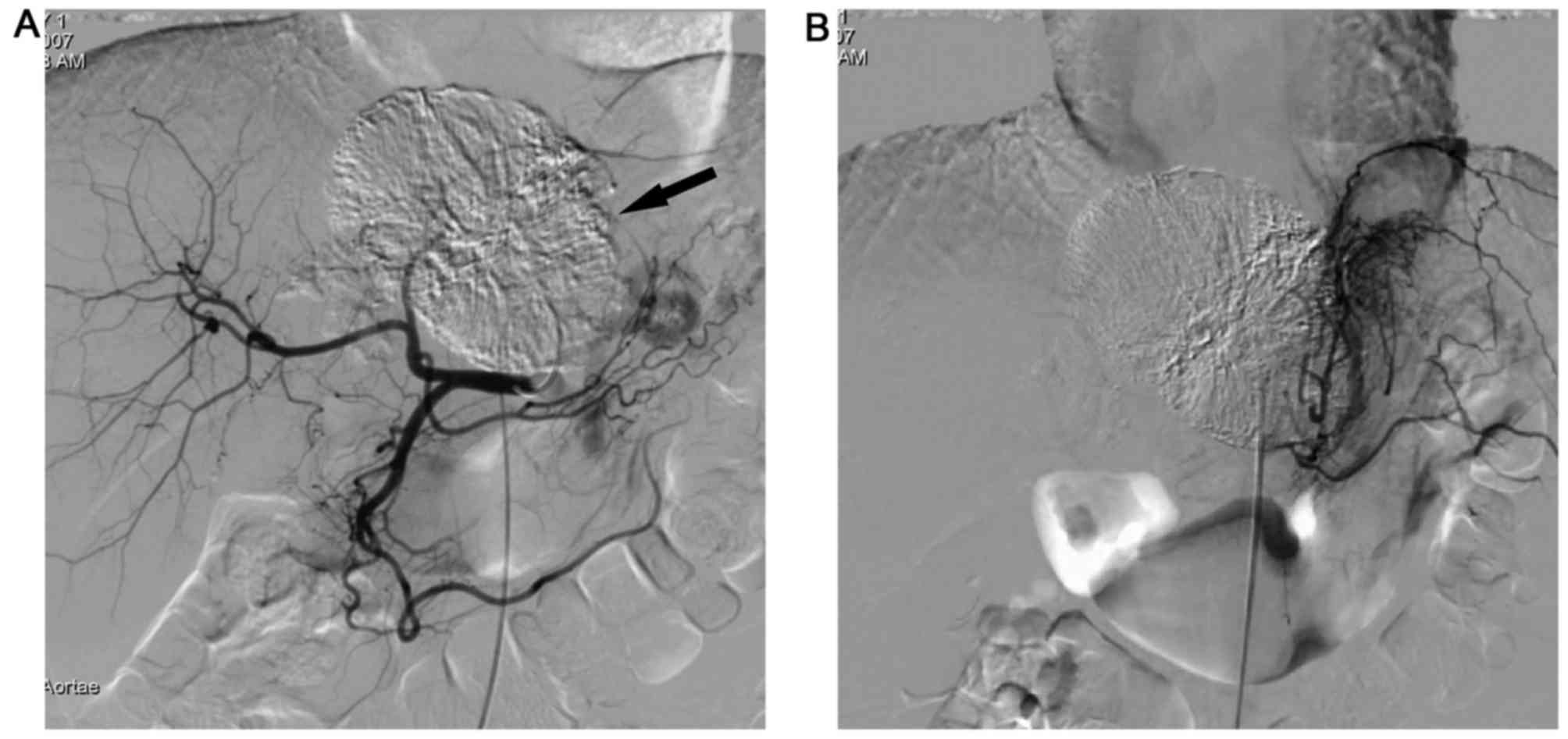
extrahepatic collateral arteries) (Figs.
1–4). All intrahepatic arteries
served as the main blood supply for the P-HCC in all patients.
Extrahepatic collateral vessels originated from the gastroduodenal
arteries (11/31), left gastric arteries (6/31), superior mesenteric
arteries (5/31), pancreaticoduodenal arteries (4/31), inferior
phrenic artery (3/31) and right adrenal arteries (2/31) (Table II). Extrahepatic collateral blood
supply to the P-HCC were significantly associated with a larger
tumor size (χ2=164.000, P<0.001); however, no
significant association was identified with regards to the tumor
location (χ2=7.358, P=0.061) (Table I), which demonstrated that the larger
the P-HCC tumor size, the greater the number of extrahepatic
collateral supplies.
 | Table II.Angiographic demonstration in 39
patients with pedunculated hepatocellular carcinoma prior to and
following TACE. |
Table II.
Angiographic demonstration in 39
patients with pedunculated hepatocellular carcinoma prior to and
following TACE.
| Parameter | Feeding arteries
prior to TACEa, n | Feeding arteries
following repeated TACEb, n | χ2 | P-value |
|---|
| Intrahepatic
arteries | 39 | 53 (with 14 new
intrahepatic collateral vessels) | 4.278 | 0.039 |
| Extrahepatic
collateral arteries |
|
|
|
|
|
Gastroduodenal artery | 11 | 23 |
|
|
| Left
gastric artery | 6 | 13 |
|
|
|
Inferior phrenic artery | 3 | 11 |
|
|
|
Superior mesenteric
artery | 5 | 10 |
|
|
|
Pancreaticoduodenal
artery | 4 | 8 |
|
|
| Right
adrenal arteries | 2 | 8 |
|
|
|
Other | 0 | 5c |
|
|
Angiographic results subsequent to
repeated TACE
Following repeated TACE (2–9 sessions), angiography
revealed a total of 131 tumor-feeding arteries in all patients,
with 54 (41.2%) intrahepatic arteries adding new intrahepatic
collateral vessels (14/54) (Fig. 3),
and 78 (58.8%) extrahepatic collateral vessels from 31 cases
(79.5%); these vessels arose from gastroduodenal arteries (23/78),
left gastric arteries (13/78), inferior phrenic artery (11/78),
superior mesenteric arteries (10/78), pancreaticoduodenal arteries
(8/78), right adrenal arteries (8/78), right gastric arteries
(2/78), lumbar arteries (2/78) and the intercostal artery (1/78).
Compared with angiographies at the initial TACE, 47 new
extrahepatic blood vessels were added. These results reveal that
the number of extrahepatic collateral vessels significantly
increased following TACE (χ2=4.278, P=0.039; Table II).
All angiographies revealed that the intrahepatic
arteries served as the main blood supply for P-HCC, whereas the
extrahepatic collateral arteries served complementary functions in
P-HCC, regardless of whether the patient was pre- or post-TACE
(Figs. 1–4). Additionally, P-HCC tumor lesions were
prone to acquire parasitic blood supplies from adjacent vessels
following repeated TACE (Figs. 2 and
5).
Discussion
P-HCC is characterized by a rich blood supply,
similar to HCC. All angiographies conducted in the present study
revealed that the intrahepatic arteries function as the main blood
supply, with the extrahepatic collateral arteries supplying a
complementary blood supply for P-HCC, regardless of whether the
patient is pre- or post-TACE. Extrahepatic collateral supplies to
P-HCC are rich, and are significantly associated with tumor size;
these blood supplies often arise from the feeding arteries of the
organs adjoining tumor lesion and are readily established following
repeated TACE (14). Extrahepatic
collateral supplies to P-HCC are established under various
conditions (15–17). These collateral supplies may develop
following the interruption of the hepatic artery by surgical
ligation, arterial injury induced by repeated TACE or the placement
of a catheter (15–17). Adhesions between tumors protruding
from the liver and adjacent organs can exaggerate the degree of
extrahepatic collateral blood supplies, although the hepatic
arterial supply remains intact (3,15,18–22). In
the present study, extrahepatic collateral blood supplies to P-HCC
commonly arose from gastroduodenal arteries, left gastric arteries,
phrenic arteries, superior mesenteric arteries, pancreaticoduodenal
arteries and right adrenal arteries. In patients with P-HCC,
various extrahepatic collateral vessels develop and supply the
tumor (3,18–29).
Compared with those observed via angiographies at the initial TACE,
up to 79.5% of the patients in the present study had extrahepatic
collateral supplies following subsequent TACE; the results also
revealed an increasing trend in the number of extrahepatic
collateral vessels as the number of TACE treatments increased.
Therefore, it was hypothesized that the main cause of the
development of extrahepatic collaterals was attenuation of the
hepatic arterial circulation by TACE (18–28).
Technically, angiographies of blood supplies to the
liver, including the celiac, common hepatic and superior mesenteric
arteries, should be initially performed during TACE in all patients
with P-HCC, as the intrahepatic arteries manifest as the main blood
supply to P-HCC (14). An arteriogram
of the inferior phrenic artery, which is a major source of
diaphragmatic blood supply to the liver (12,19,22–26,29),
is routinely performed in patients who have an interrupted hepatic
arterial circulation owing to previous treatment, or in patients
with tumors located near the diaphragm, which may be identified in
the initial angiography.
Extrahepatic collateral blood supplies are sought
when a tumor stain that corresponds to P-HCC, identified using
imaging modalities in terms of the location and size of the tumor,
is not present on the angiograms of these arteries. Extrahepatic
collateral blood supplies are obtained through nearby blood vessels
attributed to P-HCC protruding from the liver, exaggerating the
degree of extrahepatic collateral blood supply sourced from
adjacent organs (18–22). In practice, the catheter must be
placed at the arterial orifice to avoid omitting the origin of the
extrahepatic collateral blood supply during the selective
catheterization. Use of a microcatheter is required for highly
selective catheterization when tumor-feeding branches that arise
from the extrahepatic collaterals are difficult to catheterize,
owing to their branching phenotype (23). These branches are usually of small
caliber and branch at acute angles, giving a twisted
appearance.
P-HCC is primarily treated by surgical resection, as
a wider resection margin may be obtained, and patients have a
higher percentage of capsule formation around the tumor, resulting
in less vascular invasion than in conventional HCC (5). No symptoms present themselves in
patients with early-stage P-HCC; however, once diagnosed, rapid
tumor growth ensues, accompanied by intrahepatic metastasis and
invasion of neighboring visceral organs (30). Therefore, up to 39.4% of patients with
P-HCC cannot undergo surgical resection (7). If the patients are able to tolerate the
procedure, TACE is the first option for the treatment of
unresectable HCC, including for patients with intermediate- and
advanced-stage disease (8–11,31). The
results of the present study demonstrated that the supply of blood
to P-HCC is complicated and arises from hepatic arteries and
extrahepatic collateral supplies (3).
Extrahepatic collateral blood supplies may prohibit effective
treatment by TACE. For the transcatheter management of P-HCC to be
effective, these collateral blood supplies must be adequately
embolized (18–28).
TACE treatment has clear limitations for P-HCC tumor
control. On one hand, the anatomical features of the extrahepatic
collateral vessels, vessels that are often tiny and twisted, make
highly selective catheterization and embolization of every feeding
artery practically impossible, even when using a microcatheter
(23). Additionally, P-HCC tumor
lesions adjoin neighboring organs and share feeding arteries (i.e.,
the origin of extrahepatic collateral supply), which limit arterial
injection with chemotherapeutic agents lipiodol emulsion (CALE) and
embolization, resulting in poor or no CALE deposition in the tumor
(14). Furthermore, a tumor fed by
extrahepatic collateral blood supplies may have multiple feeding
arteries, as the extrahepatic collateral vessel connects with the
hepatic artery and other extrahepatic collateral blood supplies
(12,18). Finally, not all extrahepatic
collateral supplies are present on the angiographies and so will
not receive the chemoembolization. Therefore, TACE alone cannot
result in complete tumor necrosis in patients with P-HCC, and a
combination of other therapies, including local ablation and oral
sorafenib therapy, should be subsequently utilized (32).
The present study had a number of limitations worth
noting. Firstly, there were no patients with conventional HCC
enrolled as a control for comparison in this retrospective study.
Secondly, a microcatheter was not used in highly selective
catheterization, particularly in earlier cases, resulting in the
omission of a number of extrahepatic collateral supplies. Thirdly,
the data only consisted of intermediate or advanced P-HCC, which
cannot be treated surgically, unlike early-stage P-HCC. These
unresectable cases of P-HCC may form adhesions between the tumor
and adjacent organs more readily, which could exaggerate the degree
of extrahepatic collateral blood supplies.
The intrahepatic arteries serve as the main blood
supply for P-HCC, whereas the extrahepatic collateral arteries are
complementary, regardless of whether patients are pre- or
post-TACE. The extrahepatic collateral blood supplies to P-HCC that
arises from adjacent vessels are rich, closely associated with
tumor size, and are frequently newly established following repeated
TACE. As the present study reports treatment at a single
institution, the results may not necessarily be applicable to other
institutions. The retrospective design and small population size
may have resulted in unforeseen bias. Therefore, the results should
be validated in a larger prospective study in the future.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (Grant: 81471730).
References
|
1
|
Eggel H: Uber das primare Carcinom der
Leber. Beitr Pathol Anat Allg Pathol. 30:506–604. 1901.
|
|
2
|
Roux: Un cas de cancer primitif du foie
avec pericholecystite calculeuse, perforation intestinale:
Hemostase hepatique. Rev Med Suisse Romande. 17:114–119. 1897.
|
|
3
|
Horie Y, Katoh S, Yoshida H, Imaoka T,
Suou T and Hirayama C: Pedunculated hepatocellular carcinoma.
Report of three cases and review of the literature. Cancer.
51:746–751. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horie Y, Shigoku A, Tanaka H, Tomie Y,
Maeda N, Hoshino U, Koda M, Shiota G, Yamamoto T, Kato S, et al:
Prognosis for pedunculated hepatocellular carcinoma. Oncology.
57:23–28. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yeh CN, Lee WC, Jeng LB and Chen MF:
Pedunculated hepatocellular carcinoma: Clinicopathologic study of
18 surgically resected cases. World J Surg. 26:1133–1138. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moritz MW, Shoji M, Sicard GA, Shioda R
and DeSchryver K: Surgical therapy in two patients with
pedunculated hepatocellular carcinoma. Arch Surg. 123:772–774.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anthony PP and James K: Pedunculated
hepatocellular carcinoma. Is it an entity? Histopatholoy.
11:403–414. 1987.
|
|
8
|
Lopez PM, Villanueva A and Llovet JM:
Systematic review: Evidence-based management of hepatocellular
carcinoma-an updated analysis of randomized controlled trials.
Aliment Pharmacol Ther. 23:1535–1547. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM,
Poon RT, Fan ST and Wong J: Randomized controlled trial of
transarterial lipiodol chemoembolization for unresectable
hepatocellular carcinoma. Hepatology. 35:1164–1171. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Llovet JM, Real MI, Montana X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: A
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang P, Zeng ZC, Wang BL, Zhang JY, Fan J,
Zhou J and Hu Y: The degree of Lipiodol accumulation can be an
indicator of successful treatment for unresectable hepatocellular
carcinoma (HCC) patients-in the case of transcatheter arterial
chemoembolization (TACE) and external beam radiotherapy (EBRT). J
Cancer. 7:1413–1420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyayama S, Matsui O, Taki K, Minami T,
Ryu Y, Ito C, Nakamura K, Inoue D, Notsumata K, Toya D, et al:
Extrahepatic blood supply to hepatocellular carcinoma: Angiographic
demonstration and transcatheter arterial chemoembolization.
Cardiovasc Intervent Radiol. 29:39–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuminaga Y, Kam J and Louie-Johnsun M:
Multi-centre, prospective evaluation of the Seldinger technique for
difficult male urethral catheter insertions by non-urology trained
doctors. BJU Int. 120:(Suppl 3): S21–S27. 2017. View Article : Google Scholar
|
|
14
|
Huang D, Chen Y, Chen S, Zeng Q, Zhao J,
Wu R and Li Y: TACE plus percutaneous chemotherapy-lipiodol
treatment of unresectable pedunculated hepatocellular carcinoma.
Medicine. 96:e76502017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Charnsangavej C, Chuang VP, Wallace S, Soo
CS and Bowers T: Angiographic classification of hepatic arterial
collaterals. Radiology. 144:485–494. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Michels NA: Collateral arterial pathways
to the liver after ligation of the hepatic artery and removal of
the celiac axis. Cancer. 6:708–724. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koehler RE, Korobkin M and Lewis F:
Arteriographic demonstration of collateral arterial supply to the
liver after hepatic artery ligation. Radiology. 117:49–54. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyayama S, Matsui O, Akakura Y, Yamamoto
T, Nishida H, Yoneda K, Kawai K and Nishijima H: Hepatocellular
carcinoma with blood supply from omental branches: Treatment with
transcatheter arterial embolization. J Vasc Interv Radiol.
12:1285–1290. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung JW, Park JH, Han JK, Choi BI, Kim TK
and Han MC: Transcatheter oily chemoembolization of the inferior
phrenic artery in hepatocellular carcinoma: The safety and
potential therapeutic role. J Vasc Interv Radiol. 9:495–500. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirota S, Matsumoto S, Fukuda T, Yoshikawa
T, Motohara T and Ichikawa S: Solitary hepatocellular carcinoma fed
by the cystic artery: Limitation of transcatheter arterial
embolization. Cardiovasc Intervent Radiol. 22:206–209. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanigawa N, Sawada S, Okuda Y, Shinzato S,
Mishima K, Asai T, Ohmura N and Kobayashi M: A case of small
hepatocellular carcinoma supplied by the cystic artery. AJR Am J
Roentgenol. 170:675–676. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SI, Lee DY, Won JY and Lee JT:
Extrahepatic collateral supply of hepatocellular carcinoma by the
intercostal arteries. J Vasc Interv Radiol. 14:461–468. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soo CS, Chuang VP, Wallace S,
Charnsangavej C and Carrasco H: Treatment of hepatic neoplasm
through extrahepatic collaterals. Radiology. 147:45–49. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JH, Chung JW, Han JK, Park JH, Choi BI
and Han MC: Transcatheter arterial embolization of the internal
mammary artery in hepatocellular carcinoma. J Vasc Interv Radiol.
6:71–77. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duprat G, Charnsangavej C, Wallace S and
Carrasco CH: Inferior phrenic artery embolization in the treatment
of hepatic neoplasms. Acta Radiol. 29:427–429. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakai M, Sato M, Kawai N, Minamiguchi H,
Masuda M, Tanihata H, Takeuchi T, Terada M and Kishi K:
Hepatocellular carcinoma: Involvement of the internal mammary
artery. Radiology. 219:147–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kodama Y, Shimizu T, Endo H, Hige S,
Kamishima T, Holland GA, Miyamoto N and Miyasaka K: Spontaneous
rupture of hepatocellular carcinoma supplied by the right renal
capsular artery treated by transcatheter arterial embolization.
Cardiovasc Intervent Radiol. 25:137–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyayama S, Matsui O, Nishida H, Yamamori
S, Minami T, Shinmura R, Kozaka K, Notsumata K, Toya D, Tanaka N,
et al: Transcatheter arterial chemoembolization for unresectable
hepatocellular carcinoma fed by the cystic artery. J Vasc Interv
Radiol. 14:1155–1161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyayama S, Matsui O, Taki K, Minami T,
Ito C, Shinmura R, Takamatsu S, Kobayashi M, Notsumata K, Toya D,
et al: Transcatheter arterial chemoembolization for hepatocellular
carcinoma fed by the reconstructed inferior phrenic artery:
Anatomical and technical analysis. J Vasc Interv Radiol.
15:815–823. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishizaki T, Matsumata T, Adachi E,
Hayashi H and Sugimachi K: Pedunculated hepatocellular carcinoma
and surgical treatment. Br J Cancer. 67:115–118. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chung GE, Lee JH, Kim HY, Hwang SY, Kim
JS, Chung JW, Yoon JH, Lee HS and Kim YJ: Transarterial
chemoembolization can be safely performed in patients with
hepatocellular carcinoma invading the main portal vein and may
improve the overall survival. Radiology. 258:627–634. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Becher G, Sozgen T, Olschewski M,
Laubenberger J, Blum HE and Allgaier HP: Combined TACE and PEI for
paliative treatment of unresectable hepatocellular carcinoma. World
J Gastroenterol. 11:6104–6109. 2005. View Article : Google Scholar : PubMed/NCBI
|