Discoidin domain receptors (DDRs) are receptor
tyrosine kinases (RTKs) characterized by an ~155 amino acid
extracellular discoidin homology domain that binds to and is
activated by collagens in their native triple-helical form. There
are two types of DDR kinases: DDR1 and DDR2. DDR1 is activated by
collagens type I–VI and VIII, whereas DDR2 is activated by
fibrillar collagens type I and III (1–3).
Alternative splicing generates five DDR1 isoforms: DDR1a, DDR1b,
DDR1c, DDR1d and DDR1e. DDR1a, DDR1b and DDR1c are full-length
functional receptors, whereas DDR1d and DDR1e are truncated and
kinase-inactive receptors (4). DDR1
signaling is required for differentiation, immune response, normal
skeletal development, mammary gland branching morphogenesis,
migration and wound healing (5). The
expression of DDR1 in several different types of human cancer,
including breast cancer, renal clear cell carcinoma, non-small cell
lung carcinoma, esophageal cancer, astrocytoma, prostate cancer,
hepatocellular carcinoma and Hodgkin's lymphoma, suggests a
function in tumor progression (6–14).
Breast carcinoma is the most common type of
malignancy in women, with 1.7 million new cases diagnosed worldwide
in 2012 (15). Collagens are a major
component of the extracellular matrix (ECM); increasing evidence
has demonstrated that collagen performs a critical role in the
development and progression of breast carcinoma (13,14). In
normal breast tissues, if fibrillar collagen deposition is
increased, high mammographic density will be detected; it leads to
a 2-fold increased risk of breast carcinoma development (16,17).
Therefore, DDR1 may serve an important role in breast carcinoma. In
renal clear cell carcinoma and a number of other types of cancer,
DDR1 was significantly overexpressed in high-grade and
advanced-stage tumors (6,12), suggesting that it may be suitable for
use as a prognostic marker or therapeutic target. However, in
breast carcinoma, the expression of DDR1 and the stage of the
cancer do not appear to be associated (18). Different expression levels of DDR1 may
be a reflection of its complex effects in breast carcinoma. This
review summarizes the current knowledge regarding DDR1 and
discusses its complex effects in breast carcinoma.
DDRs are unique among RTKs as they are activated by
collagen, an ECM protein. Collagens are major components of the
ECM, accounting for ~30% of the total protein mass in the human
body (19). Evidence has demonstrated
that high mammographic density, which is partly due to increased
fibrillar collagen deposition, is associated with a 2-fold
increased risk of breast cancer development (16,17). The
native triple-helical collagen serves as a ligand of DDR1; DDR1
cannot be activated by heat-denatured collagen (20,21). DDR1
is activated by specific types of collagens, as aforementioned. The
DDR collagen-binding sites are entirely contained within the
discoidin 1 domains (1–3). Leitinger et al (21) demonstrated that the isolated discoidin
1 domains of DDR1 and DDR2 bind directly to collagen with high
affinity and that binding requires these domains to be dimerized.
Initial mutagenesis experiments mapped the collagen-binding sites
to three spatially adjacent surface-exposed loops that are highly
conserved between the DDRs (21,22). DDR1
and DDR2 bind the GVMGVO (O, hydroxyproline) motif within fibrillar
collagens I–III (23–25). Analysis revealed that the activation
of DDR1 by collagen results in the binding of CD9 to Tyr513,
SH2-domain containing protein to Tyr703, 796 and 740, and the p85
subunit of phosphoinositide 3-kinase to Tyr881 (20,26–28). These
interactions were confirmed and additional binding proteins,
including Ras GTPase activating protein, SH-2 domain-containing
inositol 5′ polyphosphatase (SHIP)1, SHIP2, signal transducer and
activator of transcription and SRC family kinases, were identified
using proteomics approaches (29).
Therefore, following binding to collagen, DDR1 becomes
phosphorylated at tyrosine residues and can activate various
downstream signaling pathways.
Studies with large sets of clinical follow-up data
and patients have been performed to verify the DDR1 expression
profiles of different histological types of breast carcinoma
(Table I). Invasive ductal and
lobular carcinomas are the most common histological types of breast
carcinoma (30,31). DDR1 was identified to be
differentially expressed between lobular and ductal carcinomas by a
pairwise comparison analysis (32).
DDR1 was overexpressed in ductal carcinomas, as confirmed by
immunohistochemistry, in which DDR1 was positive in 96.2% of ductal
carcinomas compared with only 13.8% of lobular carcinomas (33). Considering this, DDR1 may represent a
novel tissue marker in the differentiation of ductal and lobular
breast carcinoma as an addition to the well-established marker
E-cadherin (32–34). In triple-negative breast carcinoma, a
DDR1low/DDR2high subtype has been identified
that may be more invasive and associated with a worse prognosis
(13). In human breast cancer stem
cells with the CD44highCD24low phenotype,
DDR1 expression was reduced (35–37). In
other histological types of breast carcinoma, the expression level
of DDR1 is lower in the more mesenchymal and invasive Basal B type
cell lines, a subtype with enhanced invasive properties (38). Overall, the DDR1 expression profiles
of different histological types of breast carcinoma may vary, as
summarized in Table I.
The epithelial-to-mesenchymal transition (EMT)
program promotes cell motility, invasion and metastasis (39–41). EMT
is characterized by an increase in cell motility, invasiveness and
stem cell-like properties. Tumor cells that undergo EMT express
fewer epithelial markers, including E-cadherin and cytokeratins,
but express more mesenchymal markers, including vimentin and
N-cadherin, with a possible switch in DDR expression from DDR1
(epithelial) to DDR2 (mesenchymal) (42,43). DDR1,
as opposed to DDR2, is downregulated to induce the expression of
the EMT transcription factors Twist and Snail in breast epithelial
cells, suggesting a differential regulation of DDRs during the
development of EMT (2,44,45). In
breast carcinoma cells, DDR1 negatively regulates EMT. DDR1 is
expressed predominantly in regions of cell-cell contact, where it
interacts with and stabilizes E-cadherin in normal epithelial cells
(46–49). Studies on tissue samples of patients
with breast cancer also demonstrate a negative correlation between
DDR1 and zinc finger E-box-binding homeobox 1 (ZEB1) expression.
ZEB1 is a key regulator of the EMT program in human breast cancer
cells and can directly suppress the transcription of E-cadherin to
promote EMT (33). Therefore, when
the expression level of DDR1 is high, it may inhibit ZEB1 and the
EMT program. Furthermore, the overexpression of DDR1a or DDR1b
reduces the invasive phenotype and regulates the F-actin
cytoskeletal organization of breast cancer cells (46). Therefore, in breast cancer cells, DDR1
serves a negative function in the EMT program.
Studies have demonstrated that DDR1 functions in the
regulation of cell adhesion and migration in tumors (50–53).
Neuhaus et al (54) used
chemokine-driven transwell migration assays to assess the migration
of small interfering RNA (siRNA)-transfected cells and detected a
marked reduction of cell migration following the knockdown of DDR1
in T47D and MDA-MB-468 breast cancer cell lines; T47D cell
migration was reduced by 23% and MDA-MB-468 migration by 57%. It
was concluded that when DDR1 is downregulated, the migration
ability is also decreased. A study has demonstrated that DDR1 can
mediate cell migration by means of regulating the migration
suppressor Syk kinase (55),
providing further evidence for a pro-migratory role of DDR1.
Castro-Sanchez et al (56)
demonstrated that in MDA-MB-231 breast cancer cells, DDR1 mediates
matrix metalloproteinase (MMP)-2 and-9 secretion and invasion
induced by native type IV collagen. In NIH3T3 fibroblasts and MCF7
breast cancer cells, DDR1 was demonstrated to inhibit cell
spreading, but to promote migration, via interaction with
non-muscle myosin heavy chain-IIA, a contractile protein associated
with cell spreading (57).
Furthermore, native type IV collagen induces a transient increase
of CD9-cell surface levels and cell migration through a DDR1 and
CD9-dependent pathway in MDA-MB-231 breast cancer cells (58). Therefore, numerous studies demonstrate
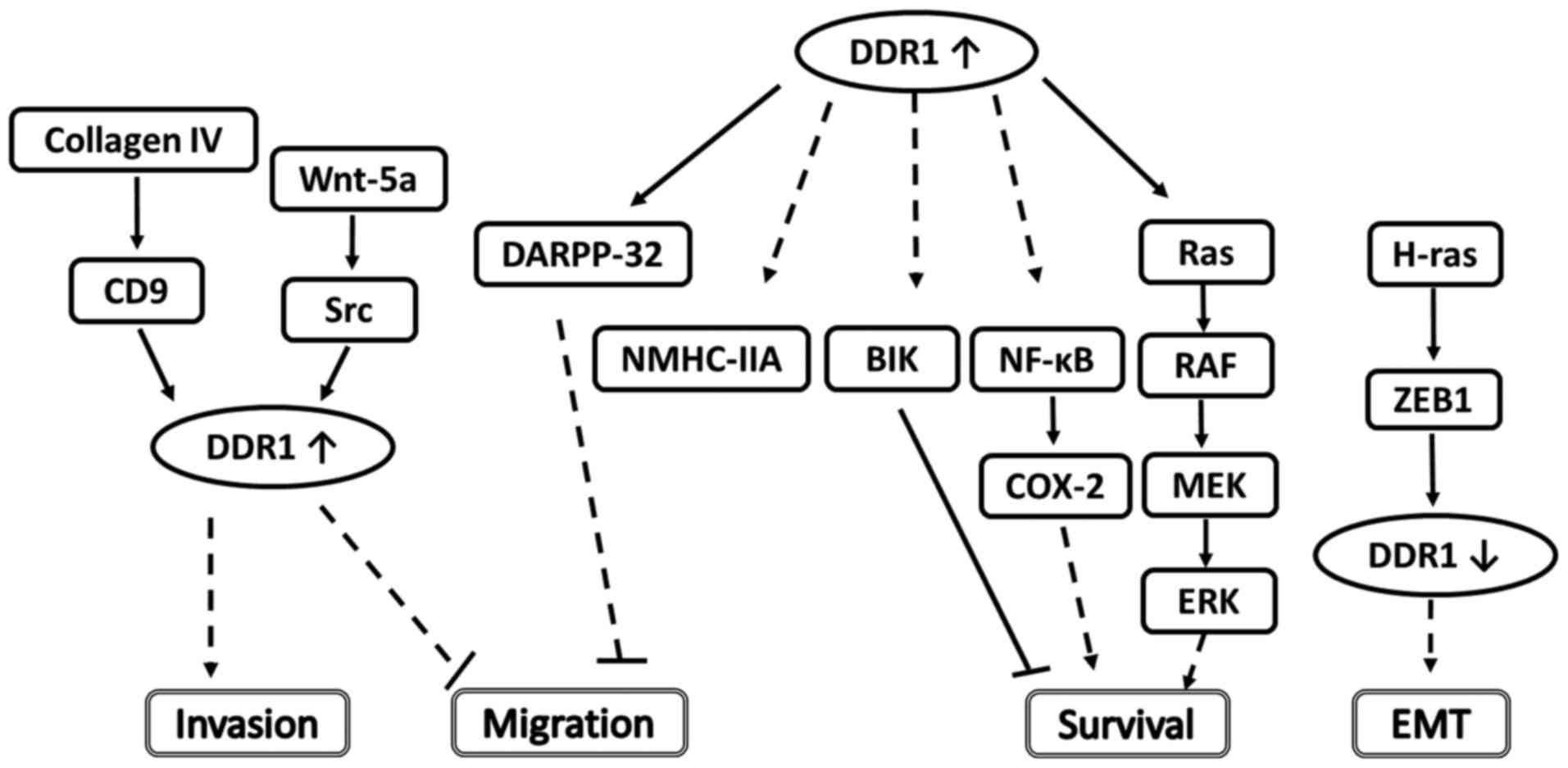
that DDR1 performs a pro-migratory function (Fig. 1).
Regarding the role of DDR1 in invasion, accumulating
evidence produced with matrigel invasion assays indicates that DDR1
can promote invasion in a number of types of human cancer cell
line, including breast (56), lung
(8), prostate (9), pituitary adenoma (67), hepatocellular carcinoma (14) and glioma (68,69). The
pro-invasive function of DDR1 may be mediated by the upregulation
of the expression of MMPs, particularly MMP-2 and MMP-9. The
elevated expression of MMPs contributes to the degradation of
extracellular matrix components, facilitating cancer cell invasion
(56). Products that can suppress the
pro-invasion function of DDR1 have been developed; Santa Cruz
Biotechnology have developed two novel antibodies that can inhibit
DDR1a-mediated Matrigel invasion (68) and DDR1 activation in human MDA-MB-231
cells (58). Therefore, previous
studies predominantly support that DDR1 promotes invasion in breast
cancer.
Varying DDR1 expression levels have been identified
in a variety of types of human cancer. Assent et al
(70) identified that in breast
cancer, DDR1 performed a role in monitoring the cellular
microenvironment and triggering apoptosis via the induction of
Bcl-2-interacting killer. To confirm that apoptosis could be
induced by DDR1, they used siRNA to reduce the DDR1 expression
level in MCF-7 cells and incubated them with collagen gels. They
determined that the downregulation of DDR1 inhibited apoptosis by
~60% in breast cancer cells. A further study identified that the
catalytic activity of membrane type-1-MMP (MT1-MMP) impaired this
DDR1-initiated apoptotic program, although the mechanisms by which
MT1-MMP may interfere with DDR1-initiated signaling remain unclear
(70).
Previous studies have demonstrated that DDR1 is a
direct transcriptional target of p53. In wild-type p53-containing
cells exposed to genotoxic drugs, DDR1 can function as a survival
effector (78). During genotoxic
stress, the inhibition of DDR1 function led to the markedly
increased apoptosis of wild-type p53-containing cells via a
caspase-dependent pathway (78). Das
(80) reported that DDR1 induced
cyclooxygenase-2 (COX-2) expression, resulting in enhanced
chemoresistance in MDA-MB-435 and T47D breast cancer cells.
Subsequent to using short hairpin RNA against DDR1 to eliminate
DDR1-mediated COX-2 induction, they identified that the
chemosensitivity of the breast cancer cells was increased. They
also demonstrated that DDR1 activated the nuclear factor-κB (NF-κB)
pathway under genotoxic stress. When they inhibited the activation
of the NF-κB pathway, the level of DDR1-induced COX-2 was reduced,
leading to enhanced breast cancer cell chemosensitivity. Therefore,
DDR1-mediated COX-2 induction was NF-κB-dependent (80). However, the effect of DDR1 on
genotoxic drug resistance requires further study.
The present review described the complex functions
of DDR1 in regulating EMT, migration, invasion, apoptosis, survival
and chemoresistance to genotoxic drugs in breast carcinoma, as well
as illustrating the identified up/downstream signaling molecules
that mediate these effects (Table
II, Fig. 1). The effects of DDR1
expression in breast carcinoma may depend on the histological type,
grade and hormone receptor status of the tumor (Table I). Considering the critical role of
collagen-induced DDR1 in the migration, invasion, apoptosis and
chemoresistance of breast carcinoma cells, the associated molecular
mechanisms require further investigation.
In summary, regulation via DDR1 may be critical for
breast tumor suppression or promotion and therefore, the
development of small-molecule drugs targeting DDR1 may be a novel
strategy for anticancer therapy according to the histological type,
grade and hormone receptor status of the breast tumor. To the
present day, studies have identified imatinib, nilotinib and
dasatinib as DDR1 inhibitors (81,82).
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301806), Jiangsu
Planned Projects for Postdoctoral Research Funds (grant no.
1501060A), Jiangsu Overseas Research & Training Program for
University Prominent Young & Middle-aged Teachers and
Presidents and the Jiangsu Province Graduate Innovation Project
(grant no. SJLX15_0727).
|
1
|
Vogel WF, Abdulhussein R and Ford CE:
Sensing extracellular matrix: An update on discoidin domain
receptor function. Cell Signal. 18:1108–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valiathan RR, Marco M, Leitinger B, Kleer
CG and Fridman R: Discoidin domain receptor tyrosine kinases: New
players in cancer progression. Cancer Metastasis Rev. 31:295–321.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shrivastava A, Radziejewski C, Campbell E,
Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke
G and Yancopoulos GD: An orphan receptor tyrosine kinase family
whose members serve as nonintegrin collagen receptors. Mol Cell.
1:25–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Playford MP, Butler RJ, Wang XC, Katso RM,
Cooke IE and Ganesan TS: The genomic structure of discoidin
receptor tyrosine kinase. Genome Res. 6:620–627. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hou G, Vogel W and Bendeck MP: The
discoidin domain receptor tyrosine kinase DDR1 in arterial wound
repair. J Clin Invest. 107:727–735. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quan J, Yahata T, Adachi S, Yoshihara K
and Tanaka K: Identification of receptor tyrosine kinase, discoidin
domain receptor 1 (DDR1), as a potential biomarker for serous
ovarian cancer. Int J Mol Sci. 12:971–982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakada M, Kita D, Teng L, Pyko IV,
Watanabe T, Hayashi Y and Hamada J: Receptor tyrosine kinases:
Principles and functions in glioma invasion. Adv Exp Med Biol.
986:143–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang SH, Baek HA, Lee HJ, Park HS, Jang
KY, Kang MJ, Lee DG, Lee YC, Moon WS and Chung MJ: Discoidin domain
receptor 1 is associated with poor prognosis of non-small cell lung
carcinomas. Oncol Rep. 24:311–319. 2010.PubMed/NCBI
|
|
9
|
Shimada K, Nakamura M, Ishida E, Higuchi
T, Yamamoto H, Tsujikawa K and Konishi N: Prostate cancer antigen-1
contributes to cell survival and invasion though discoidin receptor
1 in human prostate cancer. Cancer Sci. 99:39–45. 2008.PubMed/NCBI
|
|
10
|
Nemoto T, Ohashi K, Akashi T, Johnson JD
and Hirokawa K: Overexpression of protein tyrosine kinases in human
esophageal cancer. Pathobiology. 65:195–203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Willenbrock K, Kuppers R, Renne C, Brune
V, Eckerle S, Weidmann E, Bräuninger A and Hansmann ML: Common
features and differences in the transcriptome of large cell
anaplastic lymphoma and classical Hodgkin's lymphoma.
Haematologica. 91:596–604. 2006.PubMed/NCBI
|
|
12
|
Song J, Chen X, Bai J, Liu Q, Li H, Xie J,
Jing H and Zheng J: Discoidin domain receptor 1 (DDR1), a promising
biomarker, induces epithelial to mesenchymal transition in renal
cancer cells. Tumour Biol. 37:11509–11521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toy KA, Valiathan RR, Núñez F, Kidwell KM,
Gonzalez ME, Fridman R and Kleer CG: Tyrosine kinase discoidin
domain receptors DDR1 and DDR2 are coordinately deregulated in
triple-negative breast cancer. Breast Cancer Res Treat. 150:9–18.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen Q, Cicinnati VR, Zhang X, Iacob S,
Weber F, Sotiropoulos GC, Radtke A, Lu M, Paul A, Gerken G and
Beckebaum S: Role of microRNA-199a-5p and discoidin domain receptor
1 in human hepatocellular carcinoma invasion. Mol Cancer.
9:2272010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maskarinec G, Pagano IS, Little MA, Conroy
SM, Park SY and Kolonel LN: Mammographic density as a predictor of
breast cancer survival: The Multiethnic Cohort. Breast Cancer Res.
15:R72013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tice JA, O'Meara ES, Weaver DL, Vachon C,
Ballard-Barbash R and Kerlikowske K: Benign breast disease,
mammographic breast density and the risk of breast cancer. J Natl
Cancer Inst. 105:1043–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ameli F, Rose IM and Masir N: Expression
of DDR1 and DVL1 in invasive ductal and lobular breast carcinoma
does not correlate with histological type, grade and hormone
receptor status. Asian Pac J Cancer Prev. 16:2385–2390. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Egeblad M, Rasch MG and Weaver VM: Dynamic
interplay between the collagen scaffold and tumor evolution.
Current Opin Cell Biol. 22:697–706. 2010. View Article : Google Scholar
|
|
20
|
Vogel W, Gish GD, Alves F and Pawson T:
The discoidin domain receptor tyrosine kinases are activated by
collagen. Mol Cell. 1:13–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leitinger B: Molecular analysis of
collagen binding by the human discoidin domain receptors, DDR1 and
DDR2. Identification of collagen binding sites in DDR2. J Biol
Chem. 278:16761–16769. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abdulhussein R, McFadden C, Fuentes-Prior
P and Vogel WF: Exploring the collagen-binding site of the DDR1
tyrosine kinase receptor. J Biol Chem. 279:31462–31470. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Konitsiotis AD, Raynal N, Bihan D,
Hohenester E, Farndale RW and Leitinger B: Characterization of high
affinity binding motifs for the discoidin domain receptor DDR2 in
collagen. J Biol Chem. 283:6861–6868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu H, Raynal N, Stathopoulos S, Myllyharju
J, Farndale RW and Leitinger B: Collagen binding specificity of the
discoidin domain receptors: Binding sites on collagens II and III
and molecular determinants for collagen IV recognition by DDR1.
Matrix Biol. 30:16–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leitinger B: Transmembrane collagen
receptors. Annu Rev Cell Dev Biol. 27:265–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
L'Hote CG, Thomas PH and Ganesan TS:
Functional analysis of discoidin domain receptor 1: Effect of
adhesion on DDR1 phosphorylation. FASEB J. 16:234–236. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koo DH, McFadden C, Huang Y, Abdulhussein
R, Friese-Hamim M and Vogel WF: Pinpointing
phosphotyrosine-dependent interactions downstream of the collagen
receptor DDR1. FEBS Lett. 580:15–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang CZ, Su HW, Hsu YC, Shen MR and Tang
MJ: A discoidin domain receptor 1/SHP-2 signaling complex inhibits
alpha2beta1-integrin-mediated signal transducers and activators of
transcription 1/3 activation and cell migration. Mol Biol Cell.
17:2839–2852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lemeer S, Bluwstein A, Wu Z, Leberfinger
J, Müller K, Kramer K and Kuster B: Phosphotyrosine mediated
protein interactions of the discoidin domain receptor 1. J
Proteomics. 75:3465–3477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nascimento AF, Raut CP and Fletcher CD:
Primary angiosarcoma of the breast: Clinicopathologic analysis of
49 cases, suggesting that grade is not prognostic. Am J Surg
Pathol. 32:1896–1904. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Petridis C, Brook MN, Shah V, Kohut K,
Gorman P, Caneppele M, Levi D, Papouli E, Orr N, Cox A, et al:
Genetic predisposition to ductal carcinoma in situ of the breast.
Breast Cancer Res. 18:222016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Turashvili G, Bouchal J, Baumforth K, Wei
W, Dziechciarkova M, Ehrmann J, Klein J, Fridman E, Skarda J,
Srovnal J, et al: Novel markers for differentiation of lobular and
ductal invasive breast carcinomas by laser microdissection and
microarray analysis. BMC Cancer. 7:552007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Spaderna S, Schmalhofer O, Wahlbuhl M,
Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A,
et al: The transcriptional repressor ZEB1 promotes metastasis and
loss of cell polarity in cancer. Cancer Res. 68:537–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dejmek J, Dib K, Jonsson M and Andersson
T: Wnt-5a and G-protein signaling are required for collagen-induced
DDR1 receptor activation and normal mammary cell adhesion. Int J
Cancer. 103:344–351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Islam N, Kwon SC, Senie R and Kathuria N:
Breast and cervical cancer screening among South Asian women in New
York city. J Immigr Minor Health. 8:211–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shipitsin M, Campbell LL, Argani P,
Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T,
Serebryiskaya T, Beroukhim R, Hu M, et al: Molecular definition of
breast tumor heterogeneity. Cancer Cell. 11:259–273. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blick T, Hugo H, Widodo E, Waltham M,
Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME and Thompson EW:
Epithelial mesenchymal transition traits in human breast cancer
cell lines parallel the CD44 (hi/)CD24 (lo/-) stem cell phenotype
in human breast cancer. J Mammary Gland Biol Neoplasia. 15:235–252.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sheen YY, Kim MJ, Park SA, Park SY and Nam
JS: Targeting the transforming growth factor-β signaling in cancer
therapy. Biomol Ther (Seoul). 21:323–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Savagner P: Leaving the neighborhood:
molecular mechanisms involved during epithelial-mesenchymal
transition. Bioessays. 23:912–923. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hay ED: An overview of
epithelio-mesenchymal transformation. Acta Anat (Basel). 154:8–20.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Savagner P, Boyer B, Valles AM, Jouanneau
J and Thiery JP: Modulations of the epithelial phenotype during
embryogenesis and cancer progression. Cancer Treat Res. 71:229–249.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maeyama M, Koga H, Selvendiran K,
Yanagimoto C, Hanada S, Taniguchi E, Kawaguchi T, Harada M, Ueno T
and Sata M: Switching in discoid domain receptor expressions in
SLUG-induced epithelial-mesenchymal transition. Cancer.
113:2823–2831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Taube JH, Herschkowitz JI, Komurov K, Zhou
AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et
al: Core epithelial-to-mesenchymal transition interactome
gene-expression signature is associated with claudin-low and
metaplastic breast cancer subtypes. Proc Natl Acad Sci USA.
107:15449–15454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Koh M, Woo Y, Valiathan RR, Jung HY, Park
SY, Kim YN, Kim HR, Fridman R and Moon A: Discoidin domain receptor
1 is a novel transcriptional target of ZEB1 in breast epithelial
cells undergoing H-Ras-induced epithelial to mesenchymal
transition. Int J Cancer. 136:E508–E520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yeh YC, Wu CC, Wang YK and Tang MJ: DDR1
triggers epithelial cell differentiation by promoting cell adhesion
through stabilization of E-cadherin. Mol Biol Cell. 22:940–953.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hidalgo-Carcedo C, Hooper S, Chaudhry SI,
Williamson P, Harrington K, Leitinger B and Sahai E: Collective
cell migration requires suppression of actomyosin at cell-cell
contacts mediated by DDR1 and the cell polarity regulators Par3 and
Par6. Nat Cell Biol. 13:49–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eswaramoorthy R, Wang CK, Chen WC, Tang
MJ, Ho ML, Hwang CC, Wang HM and Wang CZ: DDR1 regulates the
stabilization of cell surface E-cadherin and E-cadherin-mediated
cell aggregation. J Cell Physiol. 224:387–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang L, Devarajan E, He J, Reddy SP and
Dai JL: Transcription repressor activity of spleen tyrosine kinase
mediates breast tumor suppression. Cancer Res. 65:10289–10297.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kamohara H, Yamashiro S, Galligan C and
Yoshimura T: Discoidin domain receptor 1 isoform-a (DDR1alpha)
promotes migration of leukocytes in three-dimensional collagen
lattices. FASEB J. 15:2724–2726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jonsson M and Andersson T: Repression of
Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and
migration of mammary cells. J Sci. 114:2043–2053. 2001.
|
|
53
|
Hou G, Vogel WF and Bendeck MP: Tyrosine
kinase activity of discoidin domain receptor 1 is necessary for
smooth muscle cell migration and matrix metalloproteinase
expression. Circ Res. 90:1147–1149. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Neuhaus B, Bühren S, Böck B, Alves F,
Vogel WF and Kiefer F: Migration inhibition of mammary epithelial
cells by Syk is blocked in the presence of DDR1 receptors. Cell Mol
Life Sci. 68:3757–3770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dejmek J, Leandersson K, Manjer J,
Bjartell A, Emdin SO, Vogel WF, Landberg G and Andersson T:
Expression and signaling activity of Wnt-5a/discoidin domain
receptor-1 and Syk plays distinct but decisive roles in breast
cancer patient survival. Clin Cancer Res. 11:520–528.
2005.PubMed/NCBI
|
|
56
|
Castro-Sanchez L, Soto-Guzman A,
Guaderrama-Diaz M, Cortes-Reynosa P and Salazar EP: Role of DDR1 in
the gelatinases secretion induced by native type IV collagen in
MDA-MB-231 breast cancer cells. Clin Exp Metastasis. 28:463–477.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang Y, Arora P, McCulloch CA and Vogel
WF: The collagen receptor DDR1 regulates cell spreading and
motility by associating with myosin IIA. J Cell Sci. 122:1637–1646.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Castro-Sanchez L, Soto-Guzman A,
Navarro-Tito N, Martinez-Orozco R and Salazar EP: Native type IV
collagen induces cell migration through a CD9 and DDR1-dependent
pathway in MDA-MB-231 breast cancer cells. Eur J Cell Biol.
89:843–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hansen C, Greengard P, Nairn AC, Andersson
T and Vogel WF: Phosphorylation of DARPP-32 regulates breast cancer
cell migration downstream of the receptor tyrosine kinase DDR1. Exp
Cell Res. 312:4011–4018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dierick H and Bejsovec A: Cellular
mechanisms of wingless/Wnt signal transduction. Curr Top Dev Biol.
43:153–190. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pruitt MM, Letcher EJ, Chou HC, Bastin BR
and Schneider SQ: Expression of the wnt gene complement in a
spiral-cleaving embryo and trochophore larva. Int J Dev Biol.
58:563–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dale TC: Signal transduction by the Wnt
family of ligands. Biochem J. 329:209–223. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Brennan KR and Brown AM: Wnt proteins in
mammary development and cancer. J Mammary Gland Biol Neoplasia.
9:119–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Katoh M: WNT/PCP signaling pathway and
human cancer (Review). Oncol Rep. 14:1583–1588. 2005.PubMed/NCBI
|
|
65
|
Yang Y: Wnt signaling in development and
disease. Cell Biosci. 2:142012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Roarty K and Serra R: Wnt5a is required
for proper mammary gland development and TGF-beta-mediated
inhibition of ductal growth. Development. 134:3929–3939. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yoshida D and Teramoto A: Enhancement of
pituitary adenoma cell invasion and adhesion is mediated by
discoidin domain receptor-1. J Neurooncol. 82:29–40. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ram R, Lorente G, Nikolich K, Urfer R,
Foehr E and Nagavarapu U: Discoidin domain receptor-1a (DDR1a)
promotes glioma cell invasion and adhesion in association with
matrix metalloproteinase-2. J Neurooncol. 76:239–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yamanaka R, Arao T, Yajima N, Tsuchiya N,
Homma J, Tanaka R, Sano M, Oide A, Sekijima M and Nishio K:
Identification of expressed genes characterizing long-term survival
in malignant glioma patients. Oncogene. 25:5994–6002. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Assent D, Bourgot I, Hennuy B, Geurts P,
Noël A, Foidart JM and Maquoi E: A Membrane-Type-1 Matrix
Metalloproteinase (MT1-MMP)-discoidin domain receptor 1 axis
regulates collagen-induced apoptosis in breast cancer cells. PLoS
One. 10:e01160062015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Roberts ME, Magowan L, Hall IP and Johnson
SR: Discoidin domain receptor 1 regulates bronchial epithelial
repair and matrix metalloproteinase production. Eur Respir J.
37:1482–1493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Dang N, Hu J, Liu X, Li X, Ji S, Zhang W,
Su J, Lu F, Yang A, Han H, et al: CD167 acts as a novel
costimulatory receptor in T-cell activation. J Immunother.
32:773–784. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Vogel WF, Aszódi A, Alves F and Pawson T:
Discoidin domain receptor 1 tyrosine kinase has an essential role
in mammary gland development. Mol Cell Biol. 21:2906–2917. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Curat CA and Vogel WF: Discoidin domain
receptor 1 controls growth and adhesion of mesangial cells. J Am
Soc Nephrol. 13:2648–2656. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Franco C, Ahmad PJ, Hou G, Wong E and
Bendeck MP: Increased cell and matrix accumulation during
atherogenesis in mice with vessel wall-specific deletion of
discoidin domain receptor 1. Circ Res. 106:1775–1783. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ambrogio C, Gomez-Lopez G, Falcone M,
Vidal A, Nadal E, Crosetto N, Blasco RB, Fernández-Marcos PJ,
Sánchez-Céspedes M, Ren X, et al: Combined inhibition of DDR1 and
Notch signaling is a therapeutic strategy for KRAS-driven lung
adenocarcinoma. Nat Med. 22:270–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Duncan JS, Whittle MC, Nakamura K, Abell
AN, Midland AA, Zawistowski JS, Johnson NL, Granger DA, Jordan NV,
Darr DB, et al: Dynamic reprogramming of the kinome in response to
targeted MEK inhibition in triple-negative breast cancer. Cell.
149:307–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ongusaha PP, Kim JI, Fang L, Wong TW,
Yancopoulos GD, Aaronson SA and Lee SW: p53 induction and
activation of DDR1 kinase counteract p53-mediated apoptosis and
influence p53 regulation through a positive feedback loop. EMBO J.
22:1289–1301. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fanale M: Activated DDR1 increases RS cell
survival. Blood. 122:4152–4154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Das S: Discoidin domain receptor 1
receptor tyrosine kinase induces cyclooxygenase-2 and promotes
chemoresistance through nuclear factor-B pathway activation. Cancer
Res. 66:8123–8130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Rix U, Hantschel O, Duernberger G, Rix
Remsing LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent
P, Colinge J, et al: Chemical proteomic profiles of the BCR-ABL
inhibitors imatinib, nilotinib and dasatinib, reveal novel kinase
and nonkinase targets. Blood. 110:4055–4063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bantscheff M, Eberhard D, Abraham Y,
Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau
C, et al: Quantitative chemical proteomics reveals mechanisms of
action of clinical ABL kinase inhibitors. Nat Biotechnol.
25:1035–1044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Barker KT, Martindale JE, Mitchell PJ,
Kamalati T, Page MJ, Phippard DJ, Dale TC, Gusterson BA and
Crompton MR: Expression patterns of the novel receptor-like
tyrosine kinase, Ddr, in human breast-tumors. Oncogene. 10:569–575.
1995.PubMed/NCBI
|
|
84
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Turashvilia G, Bouchala J, Ehrmanna J,
Kolara Z, Fridmanb E and Skardaa J: Novel immunohistochemical
markers for the differentiation of lobular and ductal invasive
breast carcinomas. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub. 151:59–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Maquoi E, Assent D, Detilleux J, Pequeux
C, Foidart JM and Noël A: MT1-MMP protects breast carcinoma cells
against type I collagen-induced apoptosis. Oncogene. 31:480–493.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Juin A, Di Martino J, Leitinger B, Henriet
E, Gary AS, Paysan L, Bomo J, Baffet G, Gauthier-Rouvière C,
Rosenbaum J, et al: Discoidin domain receptor 1 controls linear
invadosome formation via a Cdc42-Tuba pathway. J Cell Biol.
207:517–533. 2014. View Article : Google Scholar : PubMed/NCBI
|