Introduction
The femur is the longest bone in the human body and
the most commonly affected by metastatic tumors (1), which usually occur at the proximal and
distal ends; lesions confined to the diaphysis are unusual
(1,2).
Pathological fractures will develop in 25% of people with femoral
diaphyseal metastases (2), and is
characterized by severe pain, a reduced quality of life, and a
shorter lifespan (3–5). Non-surgical treatment of these fractures
will lead to a painful and functionless extremity in 77% of
patients with pathological fracture due to a metastatic tumor
(3). Although the plate fixation of
non-pathological fractures is useful, the bone quality adjacent to
permeative metastatic lesions is generally insufficient to achieve
rigid fixation (3).
Segmental resection of diaphyseal metastatic tumors
is more suitable for pain control and pathological fracture
(6–23), and there were many advantages,
including preservation of the juxta-articular bone and joint,
reduced long-term mechanical problems, and epiphysis preservation
in children (10–28). However, the optimal reconstruction
method after resection of malignant tumors involving the diaphysis
of long bones remains undefined (6,8,9,11).
Currently, various types of implants are in use, including
autogenous grafts (6), massive
allografts (7), extracorporeally
irradiated autogenous bone (8),
distraction osteogenesis (9) and
intercalary prostheses (IPs) (11–23).
These reconstruction methods have their particular
benefits and risks. Autologous grafts are ideal and particularly
useful in the replacement of short segments (6,24,25). Their disadvantages include limited
sources, difficulty in matching graft and defects in shape and
size, and morbidity at the donor site (24,25).
Additionally, it may take several years before the graft allows
full weight bearing (23). Allografts
provide an acceptable alternative in reconstructing tumor
resections, and allow ligament reconstruction, and accurate
matching of graft and defects (26).
Their disadvantages include the potential for disease transfer from
the donor to the patient, long period of time required for bone
union, possibility of fractures, high incidence rates of infection
and graft failure (9).
Extra-corporeally irradiated autogenous bone can be used as an
alternative to allografts (8);
however, it is brittle and takes a long time to revascularize and
incorporate into the surrounding bone (11,22).
Distraction osteogenesis and bone transport may provide adequate
biomechanical strength (27,28), but is time-consuming (1 mm/day) and
not suitable for large defects (<15 cm) (28). This potentially results in the
formation of new bone that lacks sufficient mechanical strength to
withstand physiological loading (18).
However, to the best of our knowledge, no
comparative study of the various available reconstruction methods
has been conducted, due to the paucity of relevant data. Therefore,
the purpose of the present study was to compare the clinical
outcomes and complications associated with the use of either
segmental allograft (SA) or IP for reconstruction of defects
following the intercalary resection of malignant bone tumors.
Patients and methods
Patients
All patients provided written informed consent for
their conclusion in the present study. The Institutional Review
Board/Ethics Committee of the Department of Bone Oncology, Tianjin
Hospital (Tianjin, China) approved the study.
The characteristics of 34 patients who had undergone
intercalary resection for a metastatic tumor with pathological
fracture in the femoral diaphysis between March 2011 and September
2015 at Tianjin Hospital were reviewed. Of these, 18 had received
SA, and 16 IP. There were 11 males and 24 females, with a mean age
of 64.5±11.3 years. The most common sites of metastasis were the
lung (26.5%), breast (17.6%) and liver (14.7%; Table I). All patients presented with severe
pain that prevented hip action or knee motion. Survival time was
calculated as the time from surgery until the event of mortality or
the most recent examination for the purpose of the study.
 | Table I.Comparative data variables between
the IP and SA groups. |
Table I.
Comparative data variables between
the IP and SA groups.
| Categories | IP | SA | P-value |
|---|
| Total | 16 | 18 |
|
| No. of females,
% | 62.5 | 72.2 | 0.717 |
| Clinical
characteristics, mean ± SD |
|
|
|
| Age,
years | 64.5±11.4 | 64.1±11.5 | 0.922 |
| Defect
size, mm | 101.9±26.1 | 100.0±31.12 | 0.851 |
|
Operative time, min | 105.3±24.6 | 114.7±25.3 | 0.281 |
| Blood
loss, ml | 715.6±342.4 |
1,319.4±1,700.5 | 0.064 |
|
Hospitalization time,
days | 8.9±3.8 | 12.3±6.7 | 0.002 |
|
Survival time, months | 6.9±3.7 | 7.4±3.0 | 0.763 |
| Time to
full-weight bearing, months | 79.0±12.0 | 103.4±24.2 | 0.003 |
|
Follow-up time, months | 9.0±6.8 | 12.4±6.7 | 0.086 |
| Complications, n
(%) |
|
|
|
|
Total | 3 (18.8) | 12 (66.7) | 0.007 |
|
Looseninga | 1 (6.3) | – | – |
|
Fracture | 0 (0) | 3 (16.7) | 0.230 |
|
Peri-prosthetic
fracturea | 1 (6.3) | – | – |
|
Infection | 1 (6.3) | 5 (27.8) | 0.180 |
| Local
recurrence | 1 (6.3) | 1 (5.6) | – |
|
Nonuniona | – | 7 (38.9) | – |
|
Implant-related | 2 (12.5) | 10 (55.6) | 0.013 |
|
Reoperation rate | 2 (12.5) | 7 (38.9) | 0.125 |
| MSTS score, mean ±
SD |
|
|
|
|
Postoperative (1 month) | 26.7±1.6 | 20.3±1.5 | <0.001 |
|
Postoperative (last
follow-up) | 27.1±1.7 | 26.9±1.6 | 0.986 |
| Visual analog scale
score, mean ± SD |
|
|
|
|
Preoperative | 8.7±0.7 | 8.6±0.8 | 0.642 |
|
Postoperative (1 day) | 2.2±0.9 | 2.4±1.0 | 0.527 |
|
Postoperative (last
follow-up)a | – | – | – |
The inclusion criteria were: i) Patients with
segmental bone loss from pathological fracture due to metastatic
tumor; ii) sparing of the joint above and below. Exclusion criteria
were: i) Patients with life expectancies of less than one month;
ii) inability to tolerate surgery; iii) involvement of the femoral
head or condyles.
Surgical technique
Tumor resection was performed according to the
principles defined by Enneking et al (29) with the aim of achieving wide excision
without violating the tumor. Proximal and distal imprints were
produced and the excised specimen was sent for histological
examination.
Fresh-frozen allografts were obtained and stored
according to a previously described technique (7). The selection of an allograft was
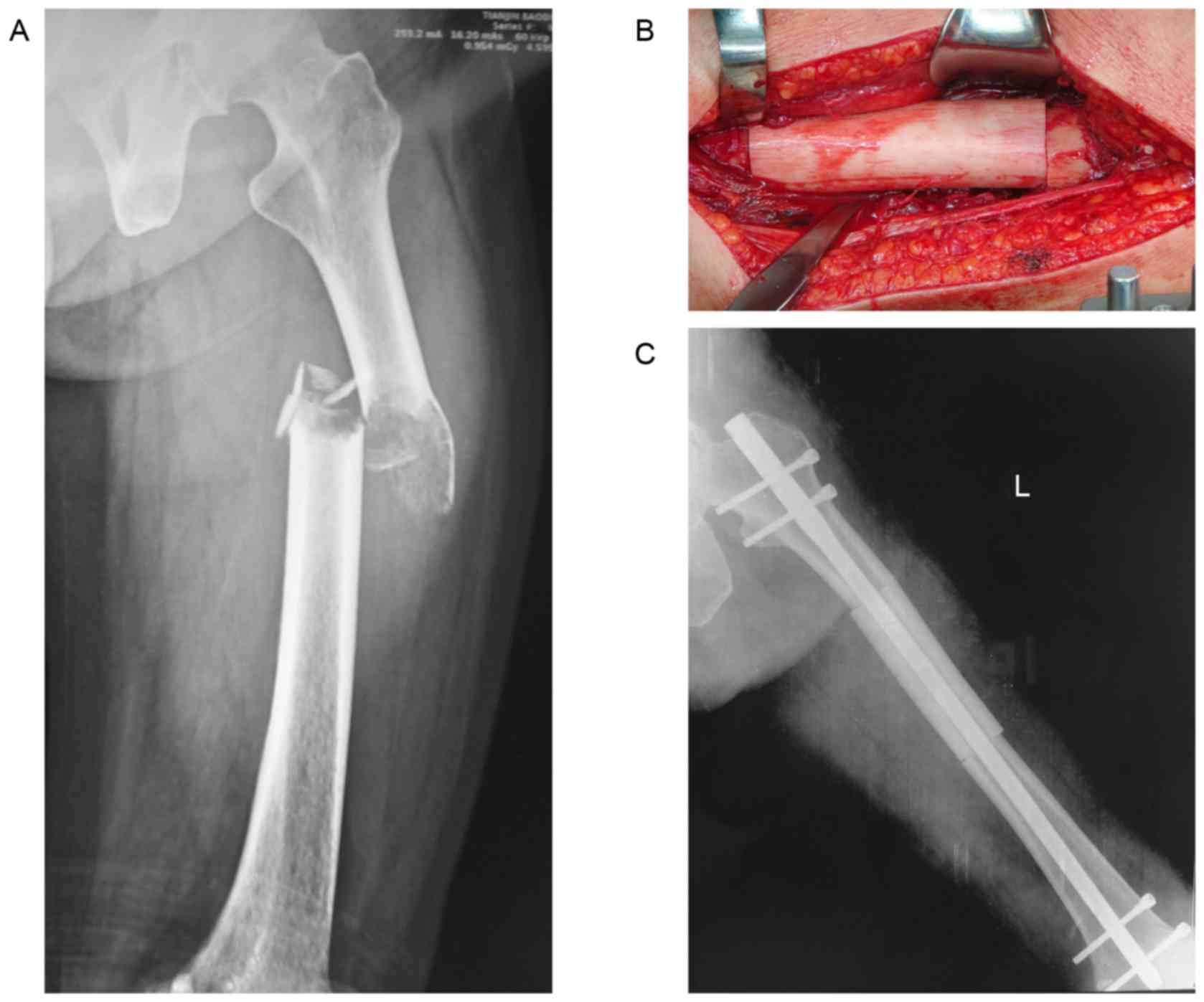
performed on the basis of a comparison of the patient's radiographs
(Fig. 1A) with those of the donor, in
order to achieve the closest possible anatomical match. After the
donor bone was thawed in warm saline, it was cut to the correct
length. All allograft-host junctions were performed with a
transverse osteotomy (Fig. 1B).
Allografts were attached to host bones with intramedullary nails in
7 patients (Fig. 1C), and plates in 5
patients.
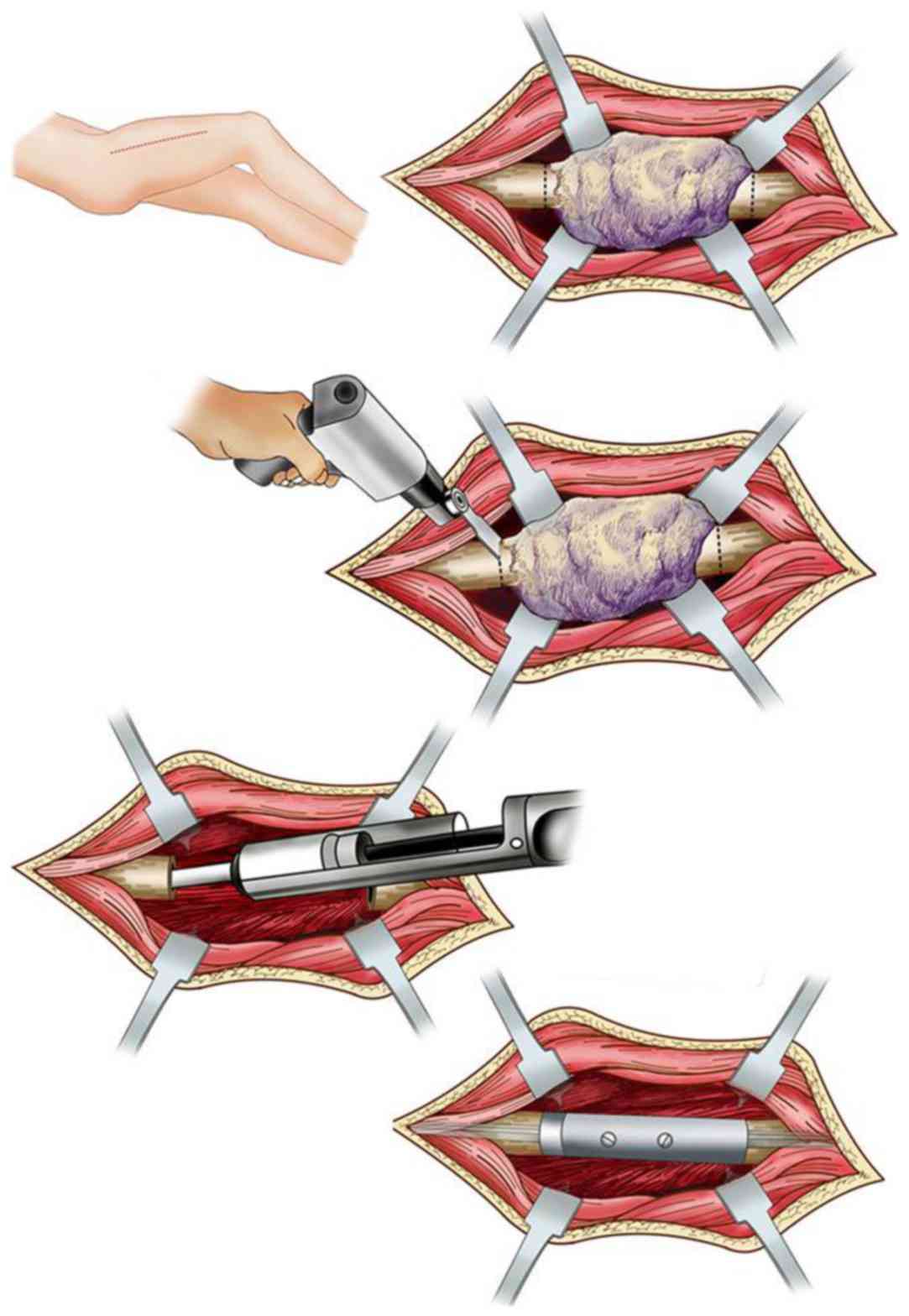
Each prosthetic was manufactured using
computer-aided design and manufacturing technologies after
determining the level of femoral transection with pre-operative
plain radiographs (Fig. 2A) and
computed tomography (CT)/magnetic resonance imaging (MRI) images.
The shaft was constructed from two parts, which were connected
during surgery with two bolts (Fig.
2B). Each end had an intramedullary stem, which was cemented
into the femoral canal (Fig. 2C and
D).
The proximal and distal regions were then reamed to
accommodate the prosthesis. Rigid intramedullary reamers were used
to prepare the intramedullary spaces proximally and distally,
beginning with a small opening and reaming to a diameter ≥2 mm
greater than the diameter of the stem to be implanted. The aim was
to achieve a 2-mm mantle of poly (methyl methacrylate) around the
stem. Trial implants were used to determine the appropriate
combination of stem lengths, diameters and body sizes. When the
final implant had been selected, a standard cement gun was used to
introduce cement into the prepared intramedullary canal. The two
stems were simultaneously cemented in situ in the proximal
and distal canals (Fig. 2C and D).
Half of the prosthesis was then placed onto each stem, and was
assembled and connected using two locking bolts. The surgical
procedure is illustrated in Fig.
3.
Intravenous antibiotics were administered for three
days postoperatively. Active physiotherapy commenced, subject to
pain level, following drainage removal.
Functional outcome
Functional outcome was evaluated using the
Musculoskeletal Tumor Society (MSTS) scoring system for the lower
extremity (30). This system includes
numerical values from 0 to 5 points assigned for each of the
following six categories: Pain, level of activity and restriction,
emotional acceptance, use of orthopedic supports, walking ability,
and gait. The MSTS is calculated as a percentage of the maximum
possible score, with a higher percentage indicating a better
functional outcome. The visual analog scale (VAS) (31) was used to measure changes in pain from
preoperative to postoperative levels.
Postoperative complications
Postoperative complications were recorded, and
oncological follow-up was performed. Disease progression alone was
not considered a complication unless it led to prosthetic or graft
fracture. The loosening of each stem was evaluated based on
radiolucency and changes in position. Circumferential radiolucency
at either the bone-cement or the prosthetic-cement interface was
considered conclusive evidence of loosening. Allograft union was
defined as bridging bone across three of the four cortices
evaluated at each junction in the biplane radiographs (32). Union was assessed with CT if
conventional radiographs were inconclusive, as previously described
(33). Allografts or prostheses that
were removed or replaced because of nonunion (allograft), loosening
(prosthesis) or fracture (allograft and prosthesis) were defined as
failures. Implant survival was analyzed using the failure of the
allografts or prosthesis as an endpoint. The time period to failure
was defined as the period from the original surgery to when further
revision was required.
Follow-up
The patients were reviewed at intervals of one month
for the initial three months, followed by three-month intervals for
the remainder of the first year after surgery, with a subsequent
follow-up every six months. Clinical and radiological assessments
were performed at each visit to determine if there was local or
distant recurrence, function, or issues with the prosthesis.
Statistical analysis
Statistical analysis was conducted using SPSS v22.0
(IBM Corp., Armonk, NY, USA). Kaplan-Meier survival curves for the
implant and the patient were used to compare the rates of survival.
Fisher's exact test was used to evaluate the effect of categorical
variables, including sex and complications. Student's t-test was
used to evaluate continuous variables, including age, defect size
and operative time. Rank-sum test was used to compare other
continuous variables, including blood loss, hospital stay, survival
time, time to full-weight bearing, follow-up duration, MSTS and VAS
scores. P<0.05 was considered to indicate a statistically
significant difference.
Results
The mean follow-up duration was 9.6±6.6 (range,
2–28) months for all patients, with 10.2±6.5 (range, 2–27) months
for the SA group and 8.8±6.8 (range, 2–25) months for the IP group.
At the last follow-up, 28 patients were deceased, with a mean
survival time of 7.1±3.3 months, including 6.9±3.7 months for the
IP group and 7.4±3.0 months for the SA group. There were no
statistically significant differences in the follow-up duration
(P=0.086), patient survival time (P=0.763), operative time
(P=0.281) or blood loss (P=0.064) between the two groups.
Furthermore, there were no significant differences between the two
groups in age (P=0.922), sex (P=0.717) or length of resection
(P=0.851; Table I).
Cumulative prosthesis survival was 82.1% [95%
confidence interval (CI), 70.4–93.8)] at both one and two years,
and allograft survival was 54.1% (95% CI, 41.1–67.7) at both one
and two years (Fig. 4A). Cumulative
patient survival was 37.5% (95% CI, 25 to 49.6) at one year and
12.5% (95% CI, 4.2 to 20.8) at two years for the IP group, and
44.4% (95% CI, 32.7 to 56.1) at one year and 20.8% (95% CI, 12.9 to
30.7) at two years for the SA group (Fig.
4B). No significant difference was observed in patient survival
at one year (P=0.508) and two years (P=0.778), or implant survival
at one month (P=0.370) and two years (P=0.127), between the
groups.
Time to full weight bearing
The median time to full weight bearing was 92±22.8
days for 30 patients. The time to full weight bearing was
significantly shorter for the IP group compared with the SA group
(IP, 79.0±12.0 vs. SA, 103.4±24.2 days; P=0.003). The median
duration of post-operative hospitalization was significantly lower
for IP compared with SA (IP, 8.9±3.8 vs. SA, 12.3±6.7 days;
P=0.002). The median duration of post-operative hospitalization for
all patients was 10.7±4.1 days.
Complications
At one year after surgery, the overall complication
rate was significantly lower in the IP group than the SA group
(18.8 vs. 66.7%, P=0.007). The complications included infection,
nonunion, fracture (prosthetic or peri-prosthetic), local
recurrence and loosening (Tables
I–III). There was 1 case of
local recurrence and 1 of infection in the IP group, and 1 case of
local recurrence and 5 of infection in the SA group. The rate of
local recurrence was similar in the patients with prostheses and
those with allografts [1 in 16 (6.3%) vs. 1 in 18 (5.6%)]. The 2
patients with local recurrence at 7 months refused secondary
surgery, and succumbed to disease at 8 and 10 months
post-operation, respectively. The infection rate was lower in the
SA group [1 in 16 (6.3%) vs. 5 in 18 (27.8%)] than in the IP group,
which may be associated with the small number of cases. All
infected patients required reoperation.
 | Table III.Characteristics of the patients in
the segmental allograft group. |
Table III.
Characteristics of the patients in
the segmental allograft group.
|
|
|
|
|
|
|
|
|
|
|
|
| Visual analog scale
score | MSTS score |
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| No. | Age, years | Sex | Diagnosis | Resection length,
mm | Follow-up,
months | Status | FW, weeks | HT, days | Blood loss, ml | Operative time,
min | Complications | Preoperative | 1 day | Last follow-up | 1 month | Last follow-up |
|---|
| 1 | 48 | F | TC | 95 | 20 | A | 120 | 14 | 1,200 | 140 |
| 10 | 3 | 1 | 21 | 25 |
| 2 | 67 | F | CC | 85 | 3 | D | – | 10 | 900 | 100 |
| 8 | 2 | – | 20 | 26 |
| 3 | 71 | M | LIC | 105 | 7 | D | 100 | 16 | 1,150 | 85 | NU (4 months) | 9 | 3 | – | 19 | 26 |
| 4 | 42 | F | LC | 120 | 21 | A | 120 | 18 | 1,000 | 110 | NU, IF (4
months) | 10 | 4 | 1 | 19 | 22 |
| 5 | 81 | F | RC | 160 | 28 | A | 110 | 20 | 8,000 | 110 | NU (6 months) | 8 | 1 | 1 | 19 | 27 |
| 6 | 72 | M | LC | 90 | 10 | D | 89 | 9 | 700 | 120 | RE (7 months) | 9 | 2 | – | 20 | 26 |
| 7 | 67 | F | CC | 70 | 6 | D | 76 | 10 | 600 | 120 |
| 7 | 3 | – | 21 | 25 |
| 8 | 77 | F | LC | 100 | 7 | D | 92 | 11 | 350 | 130 | IF (1 month) | 9 | 3 | – | 18 | 26 |
| 9 | 56 | F | BC | 80 | 14 | D | 67 | 12 | 1,550 | 130 |
| 9 | 1 | – | 20 | 25 |
| 10 | 67 | M | RC | 125 | 12 | A | 88 | 10 | 900 | 90 | FC (8 months) | 8 | 1 | 1 | 20 | 25 |
| 11 | 65 | F | BC | 40 | 6 | D | 89 | 18 | 550 | 95 | FC, IF (1
months) | 9 | 3 | – | 21 | 25 |
| 12 | 79 | F | LC | 70 | 7 | D | 82 | 14 | 600 | 100 | NU, IF (4
months) | 8 | 2 | – | 22 | 25 |
| 13 | 57 | F | LC | 170 | 9 | D | 97 | 9 | 1,000 | 110 | IF (10 days) | 9 | 4 | – | 23 | 25 |
| 14 | 61 | M | PC | 85 | 10 | D | 167 | 10 | 950 | 160 | NU, FC (7
months) | 8 | 3 | – | 21 | 25 |
| 15 | 47 | F | LIC | 95 | 7 | D | 118 | 7 | 1,000 | 150 | NU (6 months) | 9 | 2 | – | 18 | 26 |
| 16 | 62 | M | PC | 90 | 6 | D | 122 | 14 | 1,700 | 155 | NU (5 months) | 8 | 3 | – | 20 | 25 |
| 17 | 79 | F | BC | 120 | 10 | D | 117 | 9 | 750 | 75 |
| 8 | 2 | – | 22 | 28 |
| 18 | 56 | F | GC | 100 | 2 | D | – | 11 | 850 | 85 |
| 9 | 1 | – | 23 | 29 |
The implant-related complication rate, including
loosening or fracture (prosthetic or peri-prosthetic) for the IP
group and fracture or nonunion for the SA group, was significantly
lower for the IP group compared with the SA group (12.5 vs. 55.6%;
P=0.013). The reoperation rate of the SA group was higher than the
IP group (12.5 vs. 38.9%), however there was no statistically
significant difference between the two groups (P=0.125). In the SA
group, 7 patients underwent further surgery. Of these, 4 patients
were revised to a combination of an autologous fibular graft with a
new allograft, 2 required an above-knee amputation, and 1 was
revised to a new custom-made knee tumor prosthesis replacement. A
total of 2 patients in the IP group underwent subsequent surgery.
Of these, 1 patient, who obtained a peri-prosthetic fracture after
a fall at 4 months, was revised to a new proximal part with an
extra-cortical plate, and is alive at 25 months after surgery,
whereas 1 patient with infection in IP underwent a two-stage
revision and was given intravenous antibiotics for 10 days
following surgery; the infection was subsequently eradicated,
according to biochemical tests. Aseptic loosening due to disease
progression around the proximal stem occurred in 1 patient after 7
months; however, this was mild and did not require revision.
Since the majority of patients died within the first
year subsequent to surgery, and there was missing data for 82.4% of
the relevant patients, the complications 1 year following the
operation between the two groups were not compared.
MSTS
At one month after surgery, the MSTS scores were
significantly higher in the IP group compared with the SA group
(IP, 26.7±1.6 vs. SA, 20.3±1.5; P<0.001). A total of 12 patients
with IP reconstruction recovered normal function with a walking
brace, compared with only 4 patients with SA reconstruction, with
the remaining 14 patients predominantly confined to bed.
At the last follow-up, the patients in the IP group
had a mean MSTS score of 27.1±1.7, vs. 26.9±1.6 in the SA group;
the mean MSTS score was 91.7% in the IP and 89.7% in the SA groups.
No significant difference was observed between the two groups at
the final follow-up. In addition, the surviving patients were able
to participate in social activities with friends and family. One of
the four patients with SA required a walking aid for long distances
and all three surviving patients with IP achieved full weight
bearing. All surviving patients were satisfied with the outcome,
and would have been willing to undergo the same procedure again
under similar circumstances.
Other clinicopathological variables, including age
(P=0.311), sex (P=0.237), primary tumor (P=0.507) and resection
size (P=0.097) did not affect the MSTS score.
Bone defect size
There were no significant differences in bone loss
and distance from articular surface between the two groups. The
mean bone defect was 10.2±2.6 cm in the IP group and 10.0±3.1 cm in
the SA group. The mean distance from the proximal articular surface
to the proximal end of the defect was 7.7±1.6 cm in the IP group,
and 6.1±1.3 cm in the SA group (P=0.912). The mean distance from
the distal articular surface to the distal end of the defect was
6.9±2 cm in the IP group, and 5.7±1.7 cm in the SA group (P=0.722).
Although there was no significant difference in the length of the
residual bone, the distance from the distal and proximal joints was
shorter in the allograft group.
VAS
There were no significant differences between the
two groups in the preoperative and postoperative VAS pain score.
The mean VAS scores improved significantly from the baseline in
both groups (Fig. 3A, P<0.05) at 1
day after surgery. The mean preoperative VAS score was 8.7±0.7,
which improved to a mean of 2.3±0.9 points at 1 day post-surgery
for all patients and 0.8±0.4 for 5 patients at final follow-up.
There was a significant improvement in pain at 1 day post-operation
(P<0.05) when compared with the preoperative status. There was
no significant difference between the VAS score at day 1
post-operation and at the last follow-up. The relief of pain on the
first day after operation was observed in 88% of patients.
Discussion
Reconstruction of bone defects after femoral
diaphyseal tumor resection is challenging. Ideally, a
reconstruction would provide stability, preservation and early
motion of adjacent joints and survive for the lifespan of the
patients (10–14,16–19,22).
However, patients with a femoral diaphyseal pathological fracture
due to a metastatic tumor, in contrast to those with a primary
tumor with or without fracture and those with a metastatic tumor
without fracture, experience greater pain, a poorer physical
condition and a shorter life expectancy. Therefore, the goals of
surgical intervention are focused on decreasing early complications
and restoring early function in the limited lifespan of the patient
(34). Currently, the reconstructive
methods use biological and non-biological materials, with varying
benefits and complications. The present study is the first to
compare two common surgical techniques for the reconstruction of
bone defects following femoral diaphyseal tumor resection.
Segmental prosthesis was described by Chin et
al (35), who identified that it
conferred a significantly higher strength against torsional load
than fixation with Dynamic Compression plate or a Rush rod, and
first reported the clinical application of segmental prosthesis in
4 patients with diaphyseal metastatic tumors and pathological
fractures. Subsequently, IPs to reconstruct a segmental defect
became increasingly popular for metastatic tumors, due to their
ability to facilitate the early return of function and immediate
pain relief. However, the prostheses are liable to undergo
loosening, wear and breakage (10–14,16–19,22).
Published reports have shown complication rates ranging from 14 to
50%, including mechanical failure of the prosthesis and aseptic
loosening of the proximal or distal stem (10–23). Of
these, aseptic loosening is a major problem and often occurs in the
late postoperative period. Hanna et al (17) identified that aseptic loosening
occurred in 1 patient at 89 months after surgery and the shaft of
the endoprosthesis fractured in 2 patients at 33 and 106 months.
McGrath et al (36) identified
that 4 patients developed postoperative aseptic loosening, at a
mean time of 29 months. Sewell et al (18) reported that aseptic loosening occurred
in 4 patients after a mean of 23.5 months. These reports indicate
that prosthesis-related complications predominantly occurred at ~2
years after surgery.
As with the IP, SA also provides an acceptable
alternative in reconstructing tumor resections and allows the
reconstruction of ligaments, and accurate matching of the graft to
the defect (7,37). However, significant complications are
associated with the use of segmental bone allografts, particularly
when they are inserted into a systemically compromised host or the
operative site is depleted of soft tissues, or has been subjected
to radiation (8,33,38,39). The
most commonly encountered complications are fracture, nonunion and
infection. The rate of fracture has been reported to be between 14
and >50%, and infection rates in other studies of allograft
reconstruction range from 6 to 30% (12,19,22). The
rate of nonunion has been reported to be 15–71% (4,7,9,33,37,40–43). Of
these, nonunion of the host-allograft junction is a common problem,
occurring in the early postoperative period. Abudu et al
(11) summarized that the
complication rate of SA is ~50% within the first 2 years of
surgery, and 70% of patients require additional procedures to
achieve adequate reconstruction.
In the present study, all complications in the two
groups also occurred within 1 year, yet the overall complication
rate was significantly lower for IP compared with SA, and the
implant-related complications rate, including loosening or fracture
(prosthetic or peri-prosthetic) for the IP group and fracture or
nonunion for the SA group, was also significantly lower in the IP
group compared with the SA group. Thus, the complication rate,
independent of disease progression, was just 12.5% for the IP group
compared with 68.8% for the SA group.
In general, the survival time of patients with
fractures induced by metastatic tumors is shorter than those with a
primary tumor with or without fracture and those with metastatic
tumors without fracture. Based on previous reports, the time to
prosthesis-related complications is typically equivalent to or
longer than the survival time of the patients, which explains the
fewer complications in the present series. Spencer et al
(44) reported that the mean
postoperative survival time was 7.1 months for patients with
humeral metastases. Ofluoglu et al (34) reported that the mean postoperative
survival time was 11.4 months for patients with cancer metastasis.
Schürmann et al (45) stated
that the average survival time was 14.7 months for patients with
the pathological fracture of humeral shaft. Benevenia et al
(23) reported that the average time
from surgery to the development of a complication was 6 months
(range, 1–9 months) in cases of metastatic disease.
In the present study, the mean survival time
following surgery for non-surviving patients was 7.1 months, with
no prosthesis-related complications besides disease progression,
with the exception of 1 patient with peri-prosthetic fracture
subsequent to a fall at 4 months. However, 55.6% of patients in the
SA group developed allograft-related complications within the
limited lifetime of the patient. Therefore, the low incidence of
early complications makes segmental prosthesis a reasonable
alternative for patients with a shorter life expectancy (22).
The early return of function is an important goal of
various reconstruction techniques for patients with limited life
expectancy. Aldlyami et al (13) suggested that the early return of
function is important for patients who will not survive their
tumors, with a median survival time of 23 months, and recommended
to restore the patient's ability for full weight bearing to enable
a relatively normal life during this time.
In general, biological reconstruction is considered
to be more time-consuming than endoprosthetic replacement, with a
prolonged period of immobilization following surgery (17). Bus et al (33) reported clinical results of 87 patients
with intercalary allograft reconstruction following the resection
of primary bone tumors and reported that the median time to full
weight bearing was 9 months. Deijkers et al (40) revealed that the mean consolidation
time was 17 months in diaphyseal junctions, and 13.4 months in
metaphyseal junctions. San Julian Aranguren et al (46), identified that the mean consolidation
time for the diaphyseal allograft reconstruction was 16 months, and
systemic chemotherapy or external radiotherapy delayed
consolidation. Brunet et al (47) reported that the cumulative probability
of union was only 46% (95% CI, 0–99%) at 1 year. Although the
implantation of a SA is a biological option and allograft bone
formation by creeping substitution occurs at the allograft-host
junction, providing a biological means of reconstruction (41), histological examination has shown that
this does not exceed 2 cm at the allograft-host osteotomy, or >3
mm at the ends of the graft (42).
Therefore, even in cases of early bone healing, the biological
stability is poor. To avoid implant fracture, the patient must
either completely avoid weight bearing, or only partially bear
their weight for the initial few months following surgery (13).
For diaphyseal prosthesis, weight bearing as
tolerated commences within 48 h of surgery (32), and patients recover mobility quickly,
obtaining full function by 12 weeks (11). Hamada et al (4) reported that 4 patients with SA were
mobilized from bed to chair within the first 48 h post-operation.
Of these, 2 patients required no ambulatory supports and 2 required
canes. Ahlmann et al (14)
reported that 35 patients with segmental prosthetic reconstruction
were discharged 4–6 days after surgery, with full weight bearing.
In the present study, 12 patients with IP reconstruction started
early ambulation on crutches at 1 month after surgery, in contrast
to only 4 patients with SA reconstruction. The remaining 14
patients in the SA group were predominantly confined to bed. A
longer bed rest period was identified in the present study than
previously reported, which may be associated with physician
practices and patient care; however, it was significantly shorter
for IP compared with SA. Additionally, there was a longer hospital
stay, with a median duration of 10.7±4.1 days for all patients, but
it was significantly lower for IP compared with SA. Therefore, the
early return of function makes segmental prosthesis a more
reasonable alternative for these patients.
In this study, no statistically significant
differences were found in bone loss and distance from the articular
surface between the two groups. However, previous reports showed
that the length of bone resection and the thickness of the
remaining bone are the most critical factors for IP and SA.
Benevenia et al (23)
recommended IP as the only reconstruction method in patients with
skeletal defects ≥5 cm in the femur. Ruggieri et al
(19) reported that loosening was the
most common in reconstructions for a >10-cm length of bone
resection. Abudu et al (11)
suggested that the shortest length of bone suitable for fixation of
the prosthesis is 5 cm, but that this short segment fixation is
risky due to the possibility of early loosening. Sewell et
al (18) recommended that
fixation maybe further enhanced by using extracortical plates when
the short-segment intramedullary fixation is <4 cm.
For allograft reconstruction, an SA can be used
following resection of a tumor that extends into the epiphysis if
≥1 cm of the juxta-articular bone is spared (40). Aponte-Tinao et al (43), hypothesized that the distance between
the articular joint cartilage and the tumor should be ≥2 cm, to
obtain a bone width margin of 1 cm and a remaining residual
epiphysis of 1 cm to allow the fixation of the osteotomy junction.
Frisoni et al (48) reported
that an osteotomy line >5 cm from the joint line increased the
incidence of delayed union and a resection gap >17 cm was not an
indication for allograft. To reduce the number of failures, Bus
et al (33) recommended
reconsidering the use of allografts for reconstructions of defects
≥15 cm, particularly for older patients, and applying bridging
osteosynthesis using plate fixation. Muscolo et al (7) suggested that a residual epiphysis ≥1 cm
should be obtained in order to allow fixation of the osteotomy
junction. In the present study, although there were no significant
differences in the bone defect and the length of the residual bone,
the distance from the distal and proximal joints was shorter in the
SA group than the IP group.
In the present study, MSTS scores were significantly
higher for the IP group compared with the SA group (IP, 26.7±1.6
vs. SA, 20.3±1.5) at 1 month after surgery, with no significant
difference between the two groups at the final follow-up. The
patients in the IP and SA groups had an initial mean MSTS score of
27.1±1.7 and 26.9±1.6, respectively, and the mean MSTS score was
91.7% in the IP group and 89.7% in the SA group at the final
follow-up. The postoperative function was excellent in all cases,
even in patients with complications, since function of the adjacent
joints was preserved. These results are similar to those of
previous reports (10–14,16–19,22).
Hanna et al (17) reported
that the mean MSTS score for the patients retaining their
diaphyseal endoprosthesis for 10 years after surgery was 87%
(range, 67–93%). Ruggieri et al (19) reported that the mean MSTS score for
the upper extremity was 90% (range, 87–95%) and the MSTS score for
the lower extremity was 86% (range, 70–95%) indicating excellent
results for both upper and lower extremities. In the present study,
all patients had returned to their normal life at the final
follow-up.
The present study had specific limitations: Firstly,
the follow-up duration of the implant was short, evidently limited
by the patients' short life expectancy. It is difficult to obtain
long-term follow-up results in patients with primary tumors without
a longer lifespan. Secondly, the sample size was small due to
infrequent indications, which these patients have little desire to
seek surgical intervention in China. Finally, the tumor stage and
number of metastases at the time of treatment, coupled with the
heterogeneity in diagnoses and adjuvant treatments, made any type
of statistical comparison difficult.
The use of a segmental prosthesis to reconstruct an
intercalary defect is attractive for the following reasons. First,
the surgery is relatively straightforward, and the duration of
hospitalization is comparatively short. Second, IP can compensate
for significantly greater compressive loads, and provide
significantly greater immediate stability (3,49). Third,
the reconstruction of segmental prostheses is not affected by
adjuvant chemotherapy and radiotherapy to control tumors (18), whereas chemotherapy and radiotherapy
can negatively influence the allograft-host junction (15,48).
Fourth, a low incidence of early complications and the early return
of function evidently improve the quality of life of the
patients.
In conclusion, SA and IP can provide satisfactory
functional outcomes for large skeletal defects created by wide
intercalary excisions. Segmental prosthesis is a more reasonable
alternative for patients with metastatic tumors and pathological
fracture due to their shorter lifespans, while SA is suitable for
patients with shorter remaining epiphysis (1–3 mm) or a longer life
expectancy.
References
|
1
|
Clain A: Secondary malignant disease of
bone. Br J Cancer. 19:15–29. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moon B, Lin P, Satcher R, Bird J and Lewis
V: Intramedullary nailing of femoral diaphyseal metastases: Is it
necessary to protect the femoral neck? Clin Orthop Relat Res.
473:1499–1502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douglass HO Jr, Shukla SK and Mindell E:
Treatment of pathological fractures of long bones excluding those
due to breast cancer. J Bone Joint Surg Am. 58:1055–1061. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamada K, Naka N, Tamiya H, Ozaki R,
Outani H, Fujimoto T, Hashimoto N, Yoshikawa H and Araki N:
Intercalary endoprosthetic reconstruction for impending
pathological fractures in patients with femoral diaphyseal bone
metastases. Eur J Orthop Surg Traumatol. 19:547–551. 2009.
View Article : Google Scholar
|
|
5
|
Park DH, Jaiswal PK, Al-Hakim W, Aston WJ,
Pollock RC, Skinner JA, Cannon SR and Briggs TW: The use of massive
endoprostheses for the treatment of bone metastases. Sarcoma.
2007:621512007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi DW, Wang P, Ye ZM, Yu XC, Hu YC, Zhang
GC, Yan XB, Zheng K, Zhao LM and Zhang HL: Clinical and
radiographic results of reconstruction with fibular autograft for
distal radius giant cell tumor. Orthop Surg. 8:196–204. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muscolo DL, Ayerza MA, Aponte-Tinao LA and
Ranalletta M: Partial epiphyseal preservation and intercalary
allograft reconstruction in high-grade metaphyseal osteosarcoma of
the knee. J Bone Joint Surg Am. 86-A:1–2693. 2004.PubMed/NCBI
|
|
8
|
Nakamura T, Abudu A, Grimer RJ, Carter SR,
Jeys L and Tillman RM: The clinical outcomes of extracorporeal
irradiated and re-implanted cemented autologous bone graft of
femoral diaphysis after tumour resection. Int Orthop. 37:647–651.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dormans JP, Ofluoglu O, Erol B, Moroz L
and Davidson RS: Case report: Reconstruction of an intercalary
defect with bone transport after resection of Ewing's sarcoma. Clin
Orthop Relat Res. 1–264. 2005.
|
|
10
|
Damron TA, Sim FH, Shives TC, An KN, Rock
MG and Pritchard DJ: Intercalary spacers in the treatment of
segmentally destructive diaphyseal humeral lesions in disseminated
malignancies. Clin Orthop Relat Res. 1–243. 1996.PubMed/NCBI
|
|
11
|
Abudu A, Carter SR and Grimer RJ: The
outcome and functional results of diaphyseal endoprostheses after
tumour excision. J Bone Joint Surg Br. 78:652–657. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henry JC, Damron TA, Weiner MM, Higgins
ME, Werner FW and Sim FH: Biomechanical analysis of humeral
diaphyseal segmental defect fixation. Clin Orthop Relat Res. 1–239.
2002.
|
|
13
|
Aldlyami E, Abudu A, Grimer RJ, Carter SR
and Tillman RM: Endoprosthetic replacement of diaphyseal bone
defects. Long-term results. Int Orthop. 29:25–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahlmann ER and Menendez LR: Intercalary
endoprosthetic reconstruction for diaphyseal bone tumours. J Bone
Joint Surg Br. 88:1487–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Damron TA, Leerapun T, Hugate RR, Shives
TC and Sim FH: Does the second-generation intercalary humeral
spacer improve on the first? Clin Orthop Relat Res. 466:1309–1317.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mavrogenis AF, Sakellariou VI, Tsibidakis
H and Papagelopoulos PJ: Adamantinoma of the tibia treated with a
new intramedullary diaphyseal segmental defect implant. J Int Med
Res. 37:1238–1245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanna SA, Sewell MD, Aston WJ, Pollock RC,
Skinner JA, Cannon SR and Briggs TW: Femoral diaphyseal
endoprosthetic reconstruction after segmental resection of primary
bone tumours. J Bone Joint Surg Br. 92:867–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sewell MD, Hanna SA, McGrath A, Aston WJ,
Blunn GW, Pollock RC, Skinner JA, Cannon SR and Briggs TW:
Intercalary diaphyseal endoprosthetic reconstruction for malignant
tibial bone tumours. J Bone Joint Surg Br. 93:1111–1117. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruggieri P, Mavrogenis AF, Bianchi G,
Sakellariou VI, Mercuri M and Papagelopoulos PJ: Outcome of the
intramedullary diaphyseal segmental defect fixation system for bone
tumors. J Surg Oncol. 104:83–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hamada K, Naka N, Omori S, Outani H,
Oshima K, Joyama S, Araki N and Yoshikawa H: Intercalary
endoprosthesis for salvage of failed intraoperative extracorporeal
autogeneous irradiated bone grafting (IORBG) reconstruction. J Surg
Case Rep. 2014(pii): rju0142014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu YC: Surgical technique for
reconstruction of diaphyseal defect with endoprosthesis following
intercalary resection in femoral shaft. Orthop Surg. 6:329–331.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao SC, Zhang CQ and Zhang CL:
Custom-made intercalary endoprosthetic reconstruction for a
parosteal osteosarcoma of the femoral diaphysis: A case report.
Oncol Lett. 10:3279–3285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benevenia J, Kirchner R, Patterson F,
Beebe K, Wirtz DC, Rivero S, Palma M and Friedrich MJ: Outcomes of
a modular intercalary endoprosthesis as treatment for segmental
defects of the femur, Tibia and Humerus. Clin Orthop Relat Res.
474:539–548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mottard S, Grimer RJ, Abudu A, Carter SR,
Tillman RM, Jeys L and Spooner D: Biological reconstruction after
excision, irradiation and reimplantation of diaphyseal tibial
tumours using an ipsilateral vascularised fibular graft. J Bone
Joint Surg Br. 94:1282–1287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schuh R, Panotopoulos J, Puchner SE,
Willegger M, Hobusch GM, Windhager R and Funovics PT: Vascularised
or non-vascularised autologous fibular grafting for the
reconstruction of a diaphyseal bone defect after resection of a
musculoskeletal tumour. Bone Joint J. 96-B:1–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mankin HJ, Gebhardt MC, Jennings LC,
Springfield DS and Tomford WW: Long-term results of allograft
replacement in the management of bone tumors. Clin Orthop Relat
Res. 1–97. 1996.PubMed/NCBI
|
|
27
|
He X, Zhang HL and Hu YC: Limb salvage by
distraction osteogenesis for distal tibial osteosarcoma in a young
child: A case report. Orthop Surg. 8:253–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuchiya H, Tomita K, Minematsu K, Mori Y,
Asada N and Kitano S: Limb salvage using distraction osteogenesis.
A classification of the technique. J Bone Joint Surg Br.
79:403–411. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 1–120. 1980.
|
|
30
|
Enneking WF, Dunham W, Gebhardt MC,
Malawar M and Pritchard DJ: A system for the functional evaluation
of reconstructive procedures after surgical treatment of tumors of
the muscu-loskeletal system. Clin Orthop Relat Res. 1–246.
1993.PubMed/NCBI
|
|
31
|
Reed MD and Van Nostran W: Assessing pain
intensity with the visual analog scale: A plea for uniformity. J
Clin Pharmacol. 54:241–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agarwal M, Puri A, Gulia A and Reddy K:
Joint-sparing or physeal-sparing diaphyseal resections: The
challenge of holding small fragments. Clin Orthop Relat Res.
468:2924–2932. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bus MP, Dijkstra PD, van de Sande MA,
Taminiau AH, Schreuder HW, Jutte PC, van der Geest IC, Schaap GR
and Bramer JA: Intercalary allograft reconstructions following
resection of primary bone tumors: A nationwide multicenter study. J
Bone Joint Surg Am. 96:e262014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ofluoglu O, Erol B, Ozgen Z and Yildiz M:
Minimally invasive treatment of pathological fractures of the
humeral shaft. Int Orthop. 33:707–712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chin HC, Frassica FJ, Hein TJ, Shives TC,
Pritchard DJ, Sim FH and Chao EY: Metastatic diaphyseal fractures
of the shaft of the humerus. The structural strength evaluation of
a new method of treatment with a segmental defect prosthesis. Clin
Orthop Relat Res. 1–239. 1989.
|
|
36
|
McGrath A, Sewell MD, Hanna SA, Pollock
RC, Skinner JA, Cannon SR and Briggs TW: Custom endoprosthetic
reconstruction for malignant bone disease in the humeral diaphysis.
Acta Orthop Belg. 77:171–179. 2011.PubMed/NCBI
|
|
37
|
Aponte-Tinao L, Farfalli GL, Ritacco LE,
Ayerza MA and Muscolo DL: Intercalary femur allografts are an
acceptable alternative after tumor resection. Clin Orthop Relat
Res. 470:728–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dick HM, Malinin TI and Mnaymneh WA:
Massive allograft implantation following radical resection of
high-grade tumors requiring adjuvant chemotherapy treatment. Clin
Orthop Relat Res. 88–95. 1985.PubMed/NCBI
|
|
39
|
Gharedaghi M, Peivandi MT, Mazloomi M,
Shoorin HR, Hasani M, Seyf P and Khazaee F: Evaluation of clinical
results and complications of structural allograft reconstruction
after bone tumor surgery. Arch Bone Jt Surg. 4:236–242.
2016.PubMed/NCBI
|
|
40
|
Deijkers RL, Bloem RM, Kroon HM, Van Lent
JB, Brand R and Taminiau AH: Epidiaphyseal versus other intercalary
allografts for tumors of the lower limb. Clin Orthop Relat Res.
439:151–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Farfalli GL, Aponte-Tinao L, Lopez-Millán
L, Ayerza MA and Muscolo DL: Clinical and functional outcomes of
tibial intercalary allografts after tumor resection. Orthopedics.
35:e391–e396. 2012.PubMed/NCBI
|
|
42
|
Enneking WF and Mindell ER: Observations
on massive retrieved allografts. J Bone Joint Surg Am.
73:1123–1142. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aponte-Tinao L, Ayerza MA, Muscolo DL and
Farfalli GL: Survival, recurrence, and function after epiphyseal
preservation and allograft reconstruction in osteosarcoma of the
knee. Clin Orthop Relat Res. 473:1789–1796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Spencer SJ, Holt G, Clarke JV, Mohammed A,
Leach WJ and Roberts JL: Locked intramedullary nailing of
symptomatic metastases in the humerus. J Bone Joint Surg Br.
92:142–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schürmann M, Gradl G, Andress HJ, Kauschke
T, Hertlein H and Lob G: Metastatic lesions of the humerus treated
with the isoelastic diaphysis prosthesis. Clin Orthop Relat Res.
1–214. 2000.
|
|
46
|
San Julian, Aranguren M, Leyes M, Mora G
and Cañadell J: Consolidation of massive bone allografts in
limb-preserving operations for bone tumours. Int Orthop.
19:377–382. 1995.PubMed/NCBI
|
|
47
|
Brunet O, Anract P, Bouabid S, Babinet A,
Dumaine V, Toméno B and Biau D: Intercalary defects reconstruction
of the femur and tibia after primary malignant bone tumour
resection. A series of 13 cases. Orthop Traumatol Surg Res.
97:512–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Frisoni T, Cevolani L, Giorgini A, Dozza B
and Donati DM: Factors affecting outcome of massive intercalary
bone allografts in the treatment of tumours of the femur. J Bone
Joint Surg Br. 94:836–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sakellariou VI, Mavrogenis AF, Babis GC,
Soucacos PN, Magnissalis EA and Papagelopoulos PJ: Comparison of
four reconstructive methods for diaphyseal defects of the humerus
after tumor resection. J Appl Biomech. 28:568–578. 2012. View Article : Google Scholar : PubMed/NCBI
|