Introduction
Reactive oxygen species (ROS) are a class of free
radical including hydrogen peroxide (H2O2),
superoxide anions (O2•-) and hydroxyl radicals (•OH)
(1). Compared with molecular oxygen,
ROS exhibit a high reaction activity and may induce the oxidative
damage of important biological molecules, including DNA, proteins
and lipids. As byproducts of cellular metabolism, ROS are tightly
controlled by endogenous scavenging systems that involve catalase,
superoxide dismutase (SOD) and glutathione (GSH) in living cells.
Therefore, under normal circumstances, ROS exist in a physiological
balance with biochemical antioxidants. However, when the levels of
ROS exceed the capacity of the scavenging system, a disturbance of
redox homeostasis occurs, resulting in oxidative damage and
potentially, the induction of apoptosis (2,3).
Oxidative damage caused by ROS has been demonstrated
to be associated with a number of diseases, including malignant
tumors (4). Elevated ROS levels in
cells cause genomic instability, and thereby promote the activation
of oncogenes and inactivation of tumor suppressor genes (5). ROS may also affect the invasion and
metastasis of cancer cells through the regulation of a number of
important signal transduction pathways and molecules, including
mitogen-activate protein kinases, phosphoinositide 3-kinase,
phosphatase and tensin homolog, redox factor-1, nuclear factor
(erythroid-derived 2)-like 2, SHC-transforming protein 1, ataxia
telangiectasia mutated and protein tyrosine phosphatases,
suggesting that ROS serve an important role in various stages of
tumorigenesis (6–8).
The spermatogenesis-associated gene 12
(SPATA12), located on chromosome 3p14, was identified in our
previous study by digital differential display assay (9). It was then demonstrated as a stage- and
cell-type-specific gene that may be involved in the development of
testicular maturation, and may negatively regulate β-catenin
signaling during spermatogenesis (10). Another previous study identified that
SPATA12 may be an inhibitor of testicular tumorigenesis
(11). Through a yeast two-hybrid
screening system, fluorescence microscopy and subcellular
co-localization assays, an interaction between SPATA12 and
chromodomain helicase DNA binding protein 2 (CHD2) in the nucleus
was demonstrated. CHD2 is a chromatin-remodeling factor
required for the maintenance of genomic stability, and is involved
in the later stage of the DNA damage response pathway by affecting
the transcriptional activity of p53 (12). Therefore, we hypothesized and verified
that SPATA12 expression may be induced under ultraviolet
(UV) C stress, and demonstrated that SPATA12 expression was
associated with the inhibition of cellular proliferation subsequent
to UVC-irradiated DNA damage (13).
These data suggest that SPATA12 may serve an important role
in maintaining genomic integrity. UV radiation exposure may induce
ROS formation, potentially leading to cell death, genomic
instability or malignant transformation (14). Therefore, it is important to
understand whether and how SPATA12 responds to oxidative
damage. The present study will provide a perspective for
understanding the biological function of the SPATA12 gene in
DNA damage induced by oxidative stress.
Materials and methods
Cell culture, cell treatment and
transient transfection
The human cancer HeLa cell line [strain, CCL-2;
American Type Culture Collection (ATCC), Manassas, VA, USA] and
MCF-7 (strain, HTB22; ATCC) were cultured in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% bovine calf serum and 100 µg/ml penicillin and streptomycin.
All cell lines were maintained in 5% CO2 and 95%
humidity at 37°C.
HeLa or MCF-7 cells were treated with 0, 30, 50 or
70 µM H2O2 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 8 h, and then allowed to recover for 4 h.
For the resveratrol (Xi'an XiaoCao Botanical Development Co., Ltd.,
China) treatment, the cells were treated with 20 µM dissolved in
dimethyl sulfoxide (DMSO) for 12 h. DMSO alone served as a control.
For the transfection of empty pRevTRE (Promega Corporation,
Madison, WI, USA) or pRevTRE-SPATA12 plasmids synthesized in
the laboratory of the College of Biology, Hunan University
(Changsha, China), cells were seeded in 6-well plates 24 h prior to
transfection, and then treated with TurboFect™ in
vitro Transfection Reagent (Fermentas; Thermo Fisher
Scientific, Inc.) and the plasmids, according to the manufacturer's
protocol. Subsequent to transfection, the cells were harvested,
washed in PBS (137 mM NaCl, 2.7 mM KCl, 8.1 mM
Na2HPO4, 1.5 mM KH2PO4,
pH 7.4) and lysed in lysis buffer; cell pellets were used for
further analyses.
Cell viability assay
An MTT assay was used to assess the viability of
cells following treatment with 0, 30, 50 or 70 µM
H2O2. HeLa or MCF-7 cells were plated at a
density of 1×104 cells/100 µl in 96-well plates.
Subsequent to H2O2 treatment, cells were
treated with 10 µl MTT solution (final concentration, 0.5 mg/ml),
and the plates were incubated for 4 h in a humidified incubator at
37°C to allow the MTT to be metabolized. The formazan crystals
formed in the cells were solubilized with 20% sodium dodecyl
sulfate in 50% aqueous N,N-dimethylformamide, and absorbance at 570
nm was measured with a microplate reader.
Determination of oxidative stress
HeLa or MCF-7 cells were exposed to 0, 30, 50 or 70
µM H2O2 for 8 h. Oxidative stress and levels
of damage in the cells were assessed according to SOD activity and
the GSH and malondialdehyde (MDA) content. All of these were
determined, respectively, according to the manufacturer's protocols
of an MDA assay kit (cat. no., A003-1), a SOD assay kit (cat. no.,
A001-1-1) and a reduced GSH assay kit (cat. no., A006-1) (all
purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). The total protein concentration of the cells was determined
with a BCA Protein Assay kit (Beijing Dingguo Changsheng
Biotechnology Co., Ltd., Beijing, China).
RNA isolation
Total RNA was isolated by TRIzol® reagent
(Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol, digested by RNase-free DNase, and stored at −80°C until
use. For quality control, RNA purity and integrity were evaluated
by agarose gel electrophoresis and the optical density
(OD)260/OD280 ratio, respectively.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Single stranded cDNA was synthesized using the
first-strand PrimeScript™ RT Reagent kit with gDNA
Eraser (Takara Bio, Inc.) according to the manufacturer's protocol.
cDNA was subjected to qPCR using SYBR-Green PCR Master Mix (Tiangen
Biotech, Co., Ltd., Beijing, China) and an MX3000 instrument
(Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA).
Following initial denaturation for 10 min at 95°C, 40–45 cycles of
PCR were performed. Each cycle consisted of a denaturing period of
30 sec at 95°C, and annealing and extension periods for 60 sec at
60°C. The transcript amount for target genes were normalized to the
human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to control
the variability in expression levels and analyzed using the 2-ΔΔCq
method (15). The primer sequences
used for qPCR are listed in Table
I.
 | Table I.Primers used for reverse
transcription quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription quantitative polymerase chain reaction analysis.
| Gene | Gene ID | Primer sequence
(5′-3′) | Product length,
bp |
|---|
| Sirtuin 1 | XM_006717737 | F:
CAGTGAGAAAATGCTGGCCT | 199 |
|
|
| R:
AAACTTGGACTCTGGCATGT |
|
| 8-oxoguanine DNA
glycosylase | NM_002542 | F:
TACCGAGGAGACAAGAGCC | 281 |
|
|
| R:
ATGAGCCGAGGTCCAAAA |
|
|
Spermatogenesis-associated gene 12 | NM_181727 | F:
TCACCTTCCCCTCATCTCCC | 170 |
|
|
| R:
TTTCACGCTTGTCCACTTTC |
|
| GAPDH | NM_001289746 | F:
GTCTCCTCTGACTTCAACAGCG | 131 |
|
|
| R:
ACCACCCTGTTGCTGTAGCCAA |
|
Dual-luciferase reporter assay
All recombinant reporter plasmids of the
SPATA12 promoter were constructed by our group in a previous
study (16). Firefly and
Renilla luciferase-containing plasmids (Promega Corporation)
were co-transfected into cells using TurboFect™ in
vitro Transfection Reagent, as previously. A total of 24 h
after transfection, cells were treated as previously and harvested,
and firefly and Renilla luciferase activities were
determined using a Dual-Luciferase Reporter Assay system (Promega
Corporation) on a Modulus™ luminometer (Turner
BioSystems; Thermo Fisher Scientific, Inc.). All reporter assays
were performed in triplicate. Non-treated cells were used as the
control group.
Hoechst staining
Apoptosis was assessed by a Hoechst 33258 staining
kit (Beyotime Institute of Biotechnology, Haimen, China). Following
H2O2 treatment, the cells were stained
according to the manufacturer's protocol. Then, the stained cells
were observed with a fluorescence microscope (Eclipse TE300; Nikon
Corporation, Tokyo, Japan).
Measurement of ROS
Intracellular ROS was measured by the oxidative
conversion of the probe 2′,7′-dichlorofluorescin diacetate
(DCFH-DA) into the fluorescent compound DCF using a ROS assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. In brief, cells treated with or without
H2O2 were collected by centrifugation and
washed twice with 50 mM PBS. The cells from each well were then
incubated with 10 µM DCFH-DA for 20 min at 37°C. Following two
washes with PBS, the fluorescence of the cells was determined using
a fluorescence spectrophotometer (model no. F-2500; Hitachi Ltd.,
Tokyo, Japan), containing FL solution software at excitation 488
nm/emission 529 nm, and fluorescence microscopy. Protein
concentrations of the cells were determined with the BCA Protein
Assay kit.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Experimental data analyses were performed by one-way
analysis of variance with post hoc analysis using a Dunnett's test
to determine the significant differences among groups. Statistical
analyses were performed using SPSS software, version 17.0 (SPSS
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment of cellular oxidative
damage model by H2O2
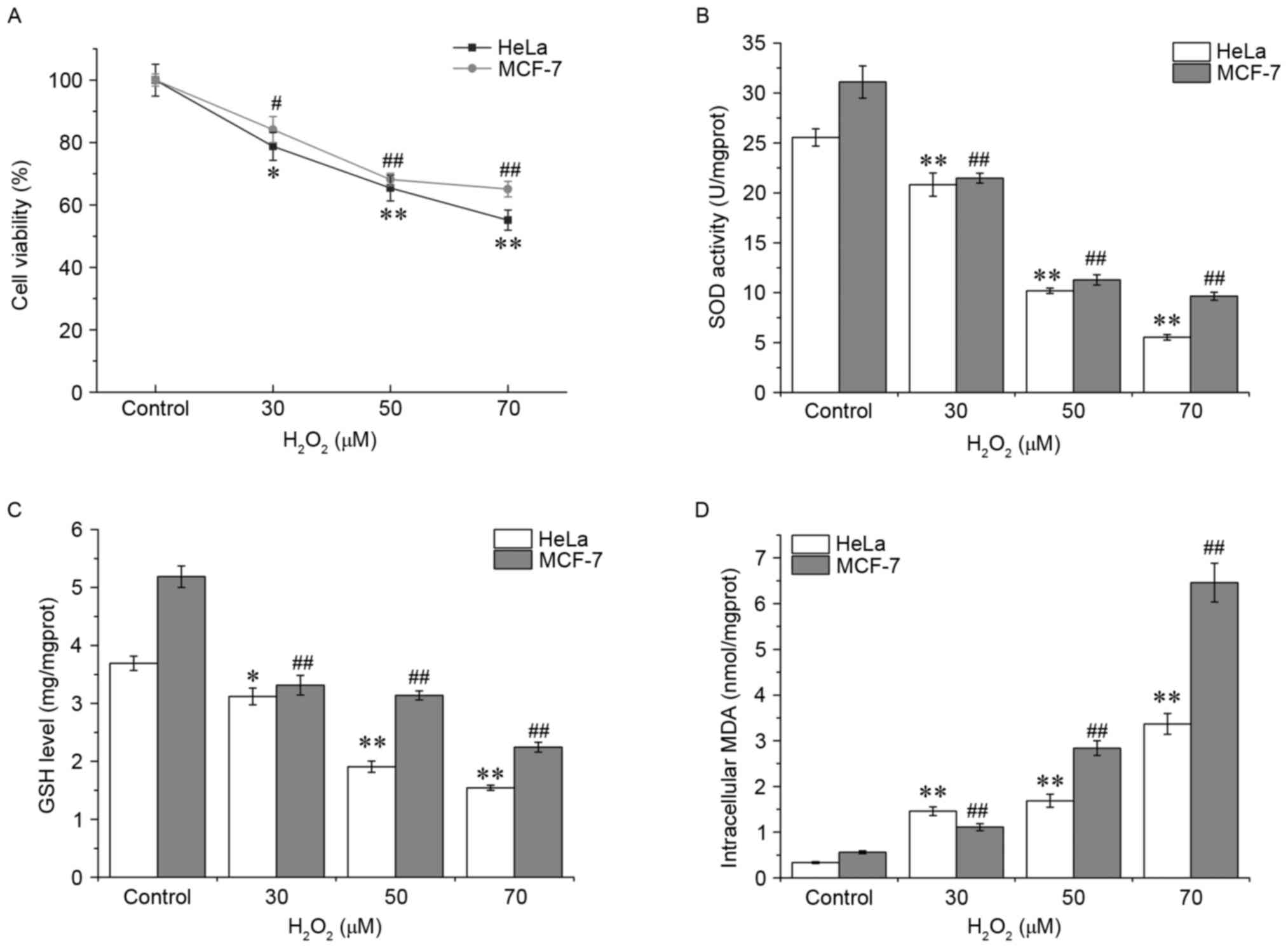
Oxidative stress was first assessed by measuring
cell viability, and the data of the MTT assay demonstrated that
H2O2 treatment decreased cell survival in
HeLa and MCF-7 cells in a dose-dependent manner (Fig. 1A). HeLa cells appeared to be more
vulnerable compared with the MCF-7 cells to
H2O2. Subsequently, SOD activities and GSH
and MDA levels were detected in the HeLa and MCF-7 cells. Compared
with 0 µM H2O2 control group, the SOD
activity and GSH content in HeLa cells with
H2O2 exposure were decreased significantly in
a concentration-dependent manner (P<0.01), whereas the MDA level
was markedly increased, reaching a peak at 70 µM (P<0.01,
Fig. 1B-D), indicating an increase in
the levels of oxidative stress experienced by the cells. Similar
patterns were observed in MCF-7 cells. These results suggested that
the antioxidant defense system is damaged in cells following
H2O2 exposure.
H2O2 exposure
upregulates the expression of SPATA12 mRNA
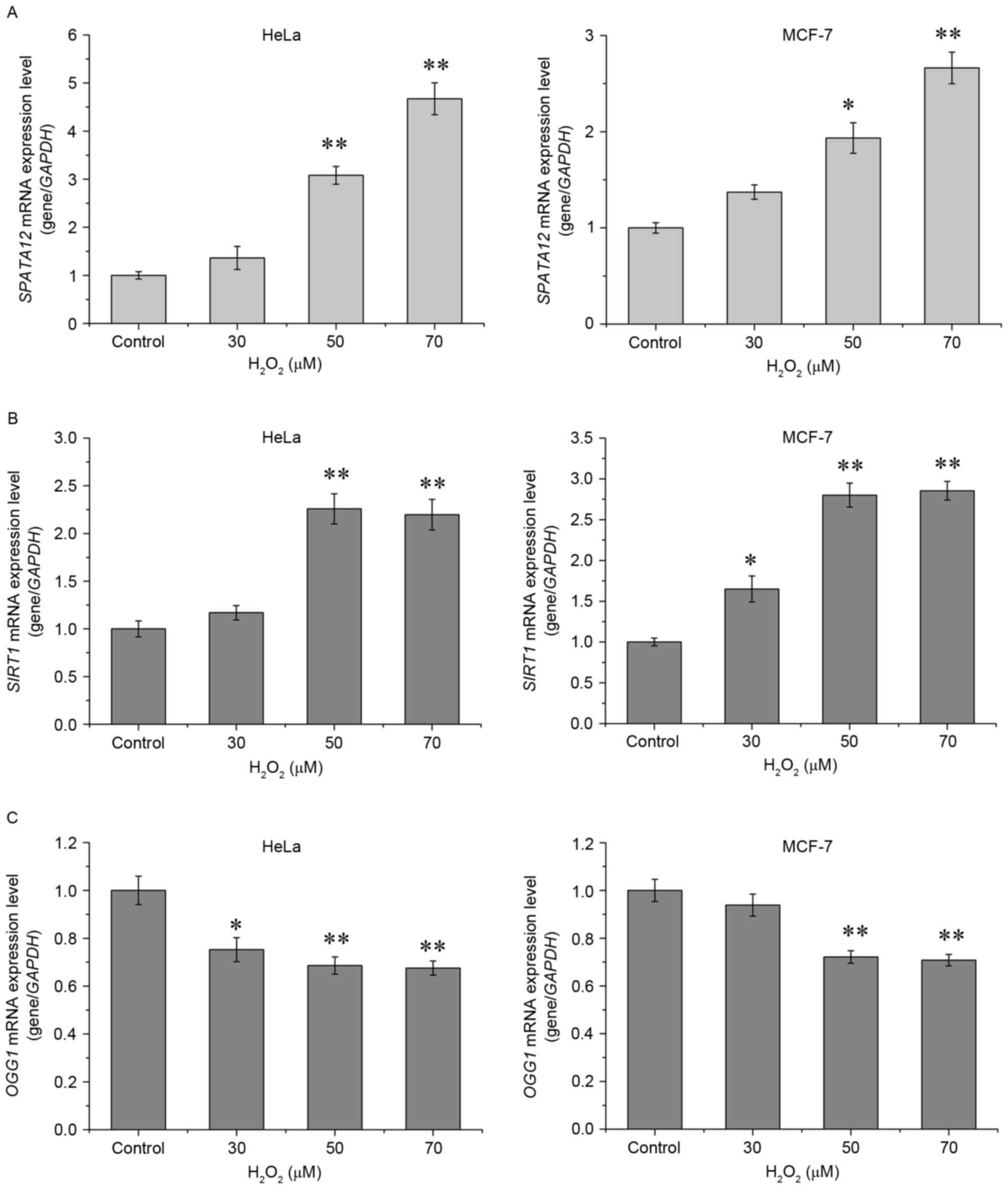
The expression of SPATA12 mRNA was detected
by qPCR in order to determine whether it was active in the cellular
response to oxidative stress. As demonstrated in Fig. 2A, H2O2
significantly increased the mRNA expression of SPATA12 in
the HeLa (P<0.01 at 50 and 70 µM) and in the MCF-7 cells
(P<0.05 at 50 µM, P<0.01 at 70 µM). As a control, the
expression levels of two antioxidant genes, Sirtuin 1
(SIRT1) and 8-oxoguanine DNA glycosylase 1 (OGG1),
were also examined. SIRT1 and OGG1 are generally used
as biomarkers to evaluate the level of oxidative stress in cells
(17–19). Consistent with the previous studies,
H2O2 at high concentrations (50 or 70 µM)
markedly increased the level of SIRT1 mRNA (P<0.01,
Fig. 2B), while oxidative stress
suppressed the levels of OGG1 mRNA (P<0.01, Fig. 2C) (17,18). These
results indicated that SPATA12 may be upregulated by
H2O2 stimulation.
SPATA12 functions as an
antioxidant
The levels of the aforementioned oxidative
parameters (SOD activity, and GSH and MDA levels) were examined
following SPATA12 gene transfection into HeLa or MCF-7 cells
to understand its role in oxidative damage. Fig. 3A demonstrates that SPATA12
expression did not alter SOD activity or GSH content following
oxidative damage to cells. However, it was observed that
SPATA12 expression significantly reduced the induction of
MDA by H2O2 (P<0.05 in HeLa cells,
P<0.01 in MCF-7 cells). Subsequently, intracellular ROS
production was assessed by measuring the oxidation of DCFH-DA, to
investigate the potential protective action of SPATA12
against oxidative stress. DCFH-DA can cross cell membranes and is
hydrolyzed enzymatically by intracellular esterases to form
non-fluorescent DCFH. Intracellular ROS may oxidize DCFH into the
fluorescent DCF; therefore, the intensity of DCF fluorescence is
directly proportional to the level of intracellular ROS (20). Compared with the control group,
SPATA12 attenuated the levels of
H2O2-induced DCF green fluorescence
(P<0.01 vs. control group, P<0.05 vs. pRevTRE group),
indicating a reduction in the levels of oxidative stress
experienced by the cells (Fig. 3B).
Additionally, using a Hoechst staining experiment, a decrease in
evident chromatin condensation was observed in the superposition
field of view following SPATA12 gene transfection, which
demonstrated that apoptosis induced by H2O2
(70 µM) was suppressed by SPATA12 expression (Fig. 3C). These data suggested that the
SPATA12 gene functions as an antioxidant through attenuating
the level of MDA and ROS, and inhibiting
H2O2-induced apoptosis.
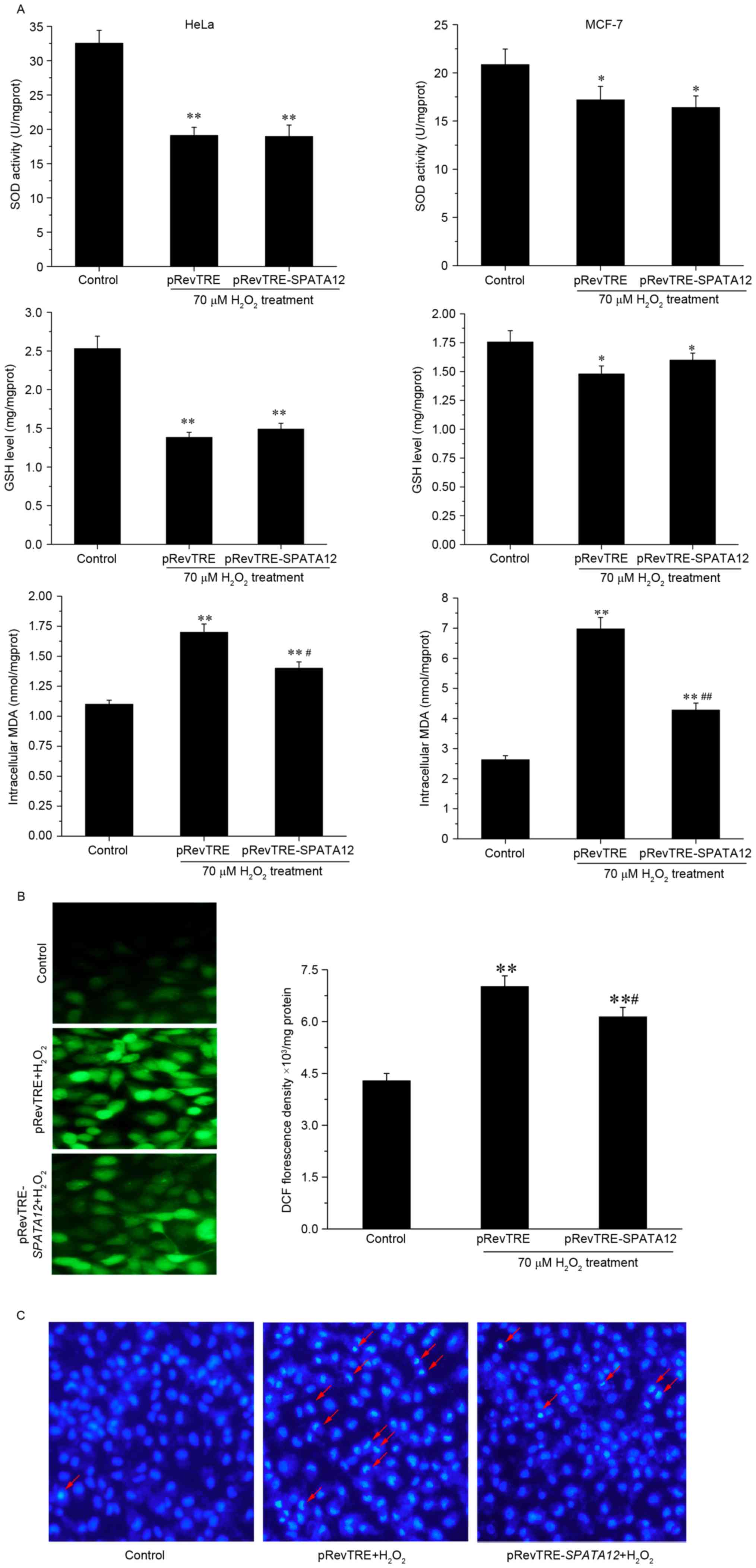 | Figure 3.Role of SPATA12 expression in
oxidative damage. (A) The effect of SPATA12 on intracellular
SOD activity, and GSH and MDA content in HeLa and MCF-7 cells
following H2O2 treatment. (B) The effect of
SPATA12 on ROS production induced by
H2O2 was observed by fluorescence microscopy
(left panel; magnification, ×400) and spectrophotometry (right).
(C) SPATA12 inhibited the apoptosis induced by
H2O2 in HeLa cells. The apoptosis was
detected by Hoechst staining. Crescent-shaped blue fluorescence
staining was observed in cells treated by
H2O2; the red arrow indicates the apoptotic
cells with visible chromatin condensation (magnification, ×100).
The data are presented as mean ± standard deviation (n=3).
*P<0.05, **P<0.01 vs. the control group (0 µM
H2O2 and no plasmid transfection),
#P<0.05, ##P<0.01 vs. pRevTRE group.
SOD, superoxide dismutase; GSH, glutathione; MDA, malondialdehyde;
TRE, TPA-responsive element; SPATA12, spermatogenesis-associated
gene 12. |
Activator protein-1 (AP-1) may be
involved in the transcriptional upregulation of SPATA12 in response
to H2O2
AP-1 is a transcription factor that is sensitive to
oxidative stress (21). Our previous
study demonstrated that the SPATA12 core promoter is located
at 77–302 bp, and the AP-1 transcription factor binding site in
this core region (Fig. 4A) is
essential to the promoter activity and involved in the
transcriptional upregulation of SPATA12 in response to UVC
radiation (13,16). In order to investigate the change in
SPATA12 promoter activity in response to
H2O2 and the potential role of the AP-1
binding site during this oxidative damage process, the effect of
H2O2 on the activity of a series of
SPATA12 gene promoters, including the full-length promoter
pGL3-958/302, and the truncated promoter fragments pGL3-946/90 and
pGL3-136/302 that were constructed in our previous study (16), were analyzed using a dual luciferase
reporter gene assay. The pGL3-136/302 and pGL3-958/302 fragments,
with the exception of pGL3-946/90, contain the core promoter
sequence. Fig. 4A indicates that the
luciferase activity level of the pGL3-946/90 promoter fragment was
unchanged, while the activity levels of pGL3-958/302 and
pGL3-136/302 were increased significantly following
H2O2 treatment (P<0.01), indicating that
the SPATA12 gene was upregulated by
H2O2 at the mRNA level. This result was
concordant with the data obtained in the qPCR assay.
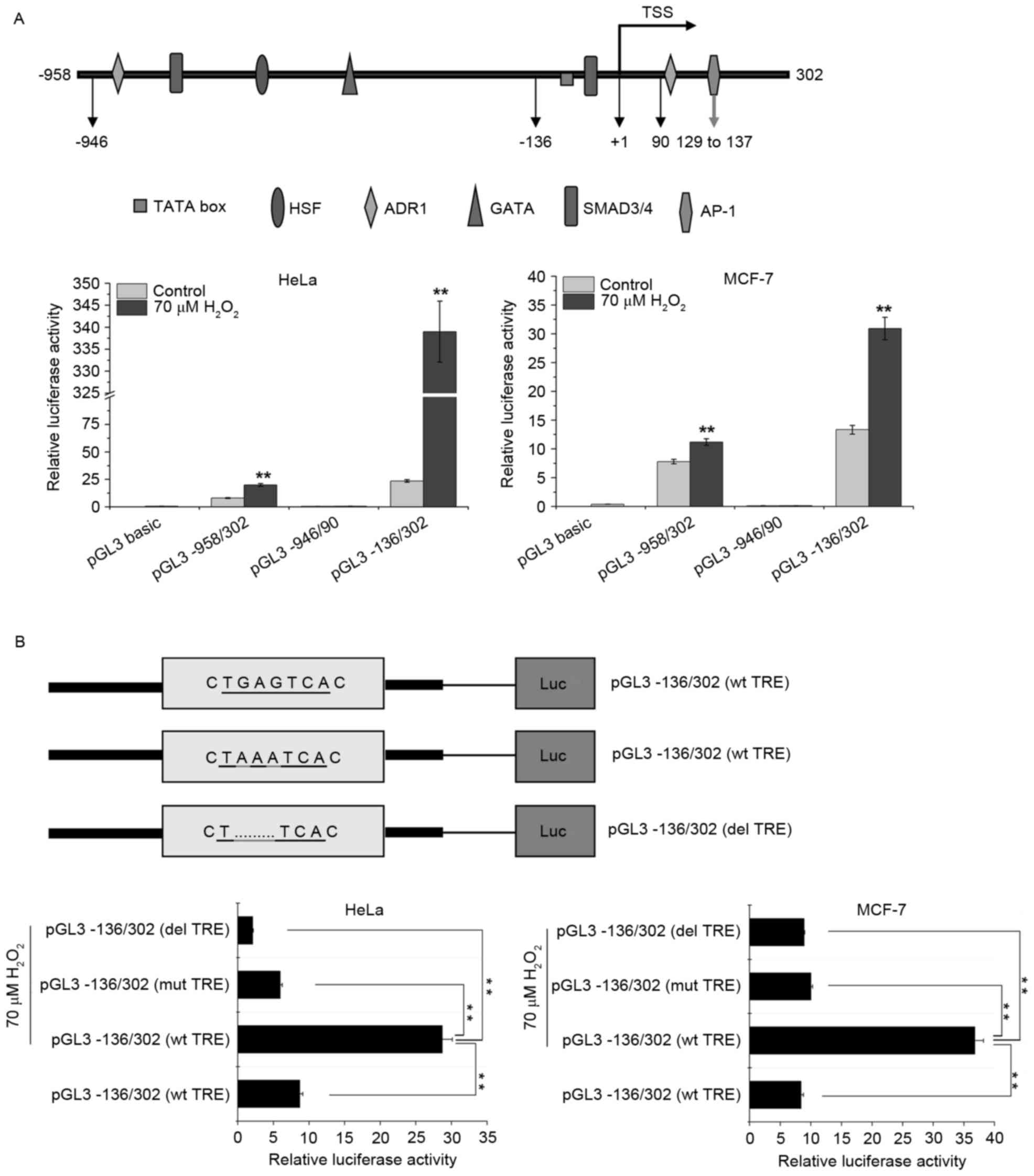 | Figure 4.AP-1 may be involved in the
transcriptional upregulation of SPATA12 in response to the
induction of oxidative stress by H2O2. (A)
Top panel: Schematic representation of the upstream and
5′-untranslated regions of the SPATA12 gene. Bottom panel:
Effects of H2O2 on the activity level of full
length and truncated constructs of the SPATA12 promoter in
the HeLa and MCF-7 cells. **P<0.01 vs. 0 µM
H2O2 control. (B) Top panel: Schematic
illustration of the luciferase reporter constructs within the
SPATA12 core promoter pGL3-136/302 (wt, mut, del). The
sequence in the rectangular box indicates the AP-1 binding motif,
and the section underlined is the TRE element of the AP-1 binding
motif. Bottom panel: The relative luciferase activity of the
SPATA12 core promoter pGL3-136/302 in cells with or without
H2O2 treatment. All SPATA12 promoter
fragments, including the wt, mut and del versions of the TRE
element constructs were linked with the firefly luciferase gene in
the pGL3-basic vector and the recombinant plasmids were transiently
co-transfected with a Renilla plasmid into cells prior to
H2O2 treatment. The pGL3-basic vector was
used as a negative control. Renilla luciferase was used as
an internal control. **P<0.01. Data are presented as the mean ±
SD (n=3). AP-1, activator protein-1; SPATA12,
spermatogenesis-associated gene 12; wt, wild type; mut, point
mutation; del, insertion deletion; TRE, TPA-responsive element; SD,
standard deviation; TSS, transcription start site; HSF, heat shock
factor; ADR1, activated disease resistance 1; SMAD3/4, mothers
against decapentaplegic homologs 3/4; Luc, luciferase. |
The AP-1 binding site within the SPATA12 core
promoter (77–302 bp) encompasses the sequence 5′-TGAGTCA-3′, a core
sequence in the AP-1 motif also known as the TPA responsive element
(TRE) (22), is demonstrated in
Fig. 4B. The point mutation construct
pGL3-136/302 (mut 132–134) and the deletion construct pGL3-136/302
(del 132–134) were generated in our previous study (16). As demonstrated by Fig. 4B, the relative luciferase activity of
the promoter in the cells with H2O2
stimulation was reduced when the TRE was mutated or deleted
(P<0.01). This result revealed that TRE within the
SPATA12 promoter is of major importance for the
responsiveness of this transcription unit to
H2O2 treatment, and that AP-1 may be involved
in the H2O2-induced transcriptional
upregulation of SPATA12.
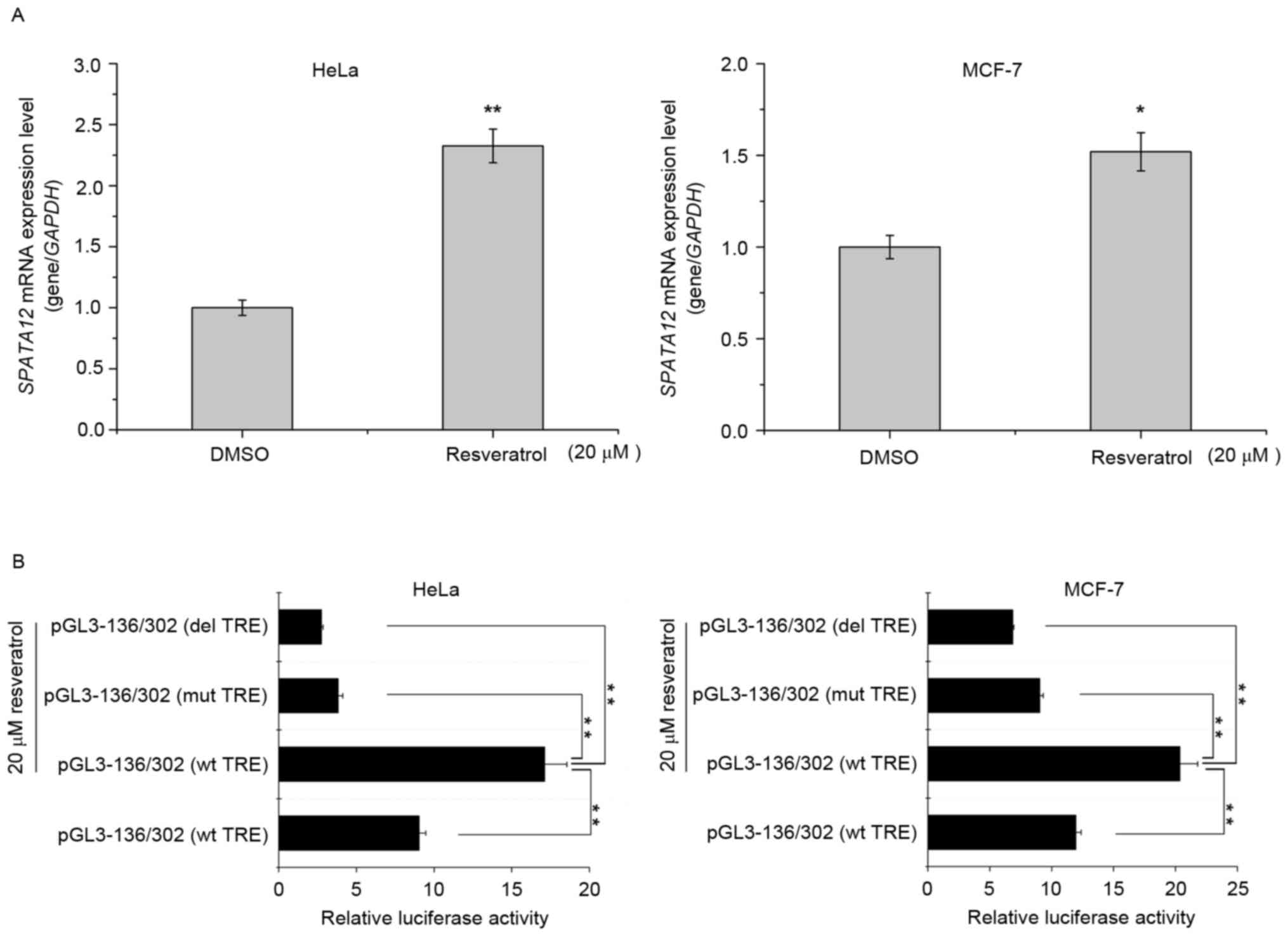
Resveratrol upregulates the expression
of SPATA12 via the AP-1 binding site
It has been suggested that resveratrol may improve
the expression of AP-1 targeted genes by enhancing the activity of
AP-1 (23). We hypothesized that
resveratrol may increase the expression of SPATA12 by
inducing AP-1. With an MTT assay (data not shown), the cytotoxicity
of resveratrol at different concentrations was examined, and the
nontoxic treatment of 20 µΜ was selected for use on cells for 12 h.
As demonstrated in Fig. 5A,
resveratrol treatment increased the expression of SPATA12
mRNA in HeLa and MCF-7 cells (P<0.01 in HeLa cells, P<0.05 in
MCF-7 cells). Then, a dual luciferase reporter assay was used to
detect the effect of resveratrol on the activity of the
SPATA12 core promoter with the AP-1 binding site. As
indicated in Fig. 5B, the luciferase
activity of the pGL3-136/302 promoter (wild-type TRE) was increased
following resveratrol treatment (P<0.01), but decreased markedly
when TRE was mutated or deleted (P<0.01). These results
indicated that resveratrol may upregulate the transcription level
of SPATA12 via the AP-1 binding site, and it may be used as
an activator of SPATA12.
Discussion
Cellular antioxidant defenses are complex, and act
to minimize the levels of ROS while simultaneously allowing ROS to
serve their useful functions in cell signaling and redox regulation
(24). It has been demonstrated that
a number of essential maintenance repair systems become deficient
in tumor cells, resulting in the accumulation of cellular damage
(25).
Based on our previous studies, we hypothesized that
SPATA12 may exhibit antioxidant properties. As
H2O2 is relatively stable and easy to use, it
is an important tool for the study of various types of oxidative
damage (26). In the present study,
exogenous H2O2 was employed to induce
oxidative stress and establish a model of cellular oxidative
damage. As demonstrated in Fig. 1,
compared with the control group, the SOD activity and the GSH level
in the HeLa and MCF7 cells exposed to H2O2
were decreased significantly in a dose-dependent manner, whereas
the MDA level was increased, indicating that oxidative stress was
generated following H2O2 treatment. In
addition, H2O2 may increase and decrease the
expression levels of SIRT1 and OGG1, respectively, at
the mRNA level, which is consistent with previous studies, and
demonstrates oxidative damage in these cells (17,27). Using
this model for cellular oxidative damage, an increase in
SPATA12 expression was identified, suggesting that
SPATA12 responded to oxidative stress.
Subsequently, the potential role of SPATA12
in the process of oxidative damage was considered. Fig. 3 demonstrated that the expression of
SPATA12 reduced the MDA content, but not the SOD activity or
GSH content. Under the same experimental conditions, SPATA12 was
observed to reduce the production of ROS caused by
H2O2. In order to further confirm the
antioxidant function of the SPATA12 gene, Hoechst staining was
performed, and the results indicated that SPATA12 inhibited
H2O2-induced apoptosis. Taken together, these
results implied that the SPATA12 gene may have an
antioxidant role, and that this function may be achieved by
decreasing the ROS and MDA content of cells.
In an attempt to obtain more insight into the
underlying mechanism of the SPATA12 gene in oxidative
damage, the changes in SPATA12 promoter activity in response
to H2O2 stimulation and the possible roles of
the transcription factor binding sites during this process were
discussed. Following H2O2 treatment, the
activity of the full-length promoter (pGL3-958/302) and the core
promoter (pGL3-136/302) were increased, which indicated that
SPATA12 expression may be induced by
H2O2 at the mRNA level. This result was
consistent with the data from the qPCR assay. Conversely, the
activity of pGL3-946/90 was very low with or without
H2O2 treatment.
We hypothesized that there may be a negative
regulatory element located at the −946 to 90 bp region that may
affect the transcriptional activity of the SPATA12 promoter;
this requires further consideration and study in the future. Our
previous study also demonstrated that the AP-1 binding site in the
SPATA12 core promoter region is essential for the activity
of SPATA12 promoter (16).
AP-1 is a basic leucine zipper transcription factor, which
regulates specific gene expression during the process of cell
growth, development, differentiation and apoptosis. Through the
leucine zipper, AP-1 identifies the TRE in the target genes'
promoter regions. AP-1 is also an important oxidative
stress-sensitive transcription factor. During oxidation, the
activation of AP-1 is primarily mediated by the phosphorylation
pathway, through c-Jun N-terminal kinase (JNK). Generally, JNK is
activated by ROS in cells; the activated JNK consequently activates
the c-Jun and c-Fos proteins, inducing the transcriptional activity
of AP-1, which promotes the expression of target genes. AP-1
binding sites exist on the c-Jun gene promoter, and the activation
of AP-1 may further induce the transcription of c-Jun by combining
with this site, forming a positive feedback loop that induces a
cascade (28). In the present study,
the data from the dual luciferase reporter gene assay indicated
that AP-1 mediated the response of SPATA12 to
H2O2 stimulation, and the TRE element of the
AP-1 binding site served a key role during this process. This
suggests that SPATA12 may respond to oxidative damage via
AP-1, and may have the ability to withstand cellular oxidative
damage.
Due to the toxicity of synthetic antioxidants,
including butylhydroxyanisole and butylhydroxytoluene, previous
studies have attempted to identify natural active ingredients with
antioxidant functions from plants and herbs for application as
clinical chemotherapeutics or daily health care products (29). The efficacy and safety of an
increasing number of Chinese herbal monomer components have been
confirmed (30). Traditional Chinese
Medicine features a variety of antioxidants, including agents that
act as stimulating factors to activate the antioxidant cell
signaling pathways, regulate the expression of downstream target
genes and serve unique roles in the defense against oxidative
damage (31). Thiel and Rössler
(23), identified that resveratrol
may activate the transcriptional expression of AP-1-targeted genes.
Therefore, we hypothesized that resveratrol may also regulate the
expression of SPATA12. The results of the qPCR assay
performed in the present study confirmed this hypothesis, and the
dual luciferase reporter gene assay revealed that AP-1 may have
mediated the improvement of SPATA12 transcriptional activity
by resveratrol.
In conclusion, the data of the present study suggest
that the antioxidant properties of SPATA12 are associated
with its ability to decrease the levels of ROS and MDA in tumor
cells. Under oxidative stress, SPATA12 was able to inhibit
oxidative damage and apoptosis induced by
H2O2, to a certain extent. The regulation of
AP-1 may be one mechanism to induce the antioxidant activity of
SPATA12 during the process of oxidative DNA damage. In
addition, resveratrol may activate the expression of SPATA12
via AP-1, which may be considered a potential activator of the
SPATA12 gene.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270735).
References
|
1
|
Han YH, Moon HJ, You BR, Kim SZ, Kim SH
and Park WH: The effects of buthionine sulfoximine,
diethyldithiocarbamate or 3-amino-1,2,4-triazole on propyl
gallate-treated HeLa cells in relation to cell growth, reactive
oxygen species and glutathione. Int J Mol Med. 24:261–268.
2009.
|
|
2
|
Ding B, Chi SG, Kim SH, Kang S, Cho JH,
Kim DS and Cho NH: Role of p53 in antioxidant defense of
HPV-positive cervical carcinoma cells following H2O2 exposure. J
Cell Sci. 120:2284–2294. 2007. View Article : Google Scholar
|
|
3
|
Pallepati P and Averill-Bates DA:
Activation of ER stress and apoptosis by hydrogen peroxide in HeLa
cells: Protective role of mild heat preconditioning at 40°C.
Biochim Biophys Acta. 1813:1987–1999. 2011. View Article : Google Scholar
|
|
4
|
Thanan R, Oikawa S, Hiraku Y, Ohnishi S,
Ma N, Pinlaor S, Yongvanit P, Kawanishi S and Murata M: Oxidative
stress and its significant roles in neurodegenerative diseases and
cancer. Int J Mol Sci. 16:193–217. 2014. View Article : Google Scholar
|
|
5
|
Waris G and Ahsan H: Reactive oxygen
species: Role in the development of cancer and various chronic
conditions. J Carcinog. 5:142006. View Article : Google Scholar
|
|
6
|
Nishikawa M: Reactive oxygen species in
tumor metastasis. Cancer Lett. 266:53–59. 2008. View Article : Google Scholar
|
|
7
|
Wu WS: The signaling mechanism of ROS in
tumor progression. Cancer Metastasis Rev. 25:695–705. 2006.
View Article : Google Scholar
|
|
8
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar
|
|
9
|
Dan L, Lifang Y and Guangxiu L: Expression
and possible functions of a novel gene SPATA12 in human testis. J
Androl. 28:502–512. 2007. View Article : Google Scholar
|
|
10
|
Lin Y, Liu Z, Liu X, Zhang Y, Rong Z and
Li D: Microarray-based analysis of the gene expression profile in
GC-1 spg cells transfected with spermatogenesis associated gene 12.
Int J Mol Med. 31:459–466. 2013. View Article : Google Scholar
|
|
11
|
Liu Z, Lin Y, Liu X, Yu W, Zhang Y and Li
D: Experimental study of inhibition of tumor cell proliferation by
a novel gene SPATA12. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
37:222–227. 2012.(In Chinese).
|
|
12
|
Rajagopalan S: Functional analysis of
chromodomain helicase DNA binding protein 2(CHD2) mediated genomic
stability. PhD diss., Uni Tennessee. 2010.
|
|
13
|
Zhang Y, Yang L, Lin Y, Rong Z, Liu X and
Li D: SPATA12 and its possible role in DNA damage induced by
ultraviolet-C. PLoS One. 8:e782012013. View Article : Google Scholar
|
|
14
|
Nishigori C, Hattori Y and Toyokuni S:
Role of reactive oxygen species in skin carcinogenesis. Antioxid
Redox Signal. 6:561–570. 2004. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Li D, Lin Y, Liu Z, Zhang Y, Rong Z and
Liu X: Transcriptional regulation of human novel gene SPATA12
promoter by AP-1 and HSF. Gene. 511:18–25. 2012. View Article : Google Scholar
|
|
17
|
Hasegawa K, Wakino S, Yoshioka K,
Tatematsu S, Hara Y, Minakuchi H, Washida N, Tokuyama H, Hayashi K
and Itoh H: Sirt1 protects against oxidative stress-induced renal
tubular cell apoptosis by the bidirectional regulation of catalase
expression. Biochem Biophys Res Commun. 372:51–56. 2008. View Article : Google Scholar
|
|
18
|
Yang L, Wang Y, Lin Z, Zhou X, Chen T, He
H, Huang H, Yang T, Jiang Y, Xu W, et al: Mitochondrial OGG1
protects against PM2.5-induced oxidative DNA damage in BEAS-2B
cells. Exp Mol Pathol. 99:365–373. 2015. View Article : Google Scholar
|
|
19
|
Zheng T and Lu Y: SIRT1 protects human
lens epithelial cells against oxidative stress by inhibiting
p53-dependent apoptosis. Curr Eye Res. 41:1068–1075. 2016.
View Article : Google Scholar
|
|
20
|
Marimoutou M, Le Sage F, Smadja J,
Lefebvre d'Hellencourt C, Gonthier MP and Robert-Da Silva C:
Antioxidant polyphenol-rich extracts from the medicinal plants
Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana
protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS
inflammatory mediators by regulating the expression of superoxide
dismutase and NF-κB genes. J Inflamm (Lond). 12:102015. View Article : Google Scholar
|
|
21
|
Ma Q: Transcriptional responses to
oxidative stress: Pathological and toxicological implications.
Pharmacol Ther. 125:376–393. 2010. View Article : Google Scholar
|
|
22
|
Wang M, Zhu K, Zhang L, Li L and Zhao J:
Thioredoxin 1 protects astrocytes from oxidative stress by
maintaining peroxiredoxin activity. Mol Med Rep. 13:2864–2870.
2016. View Article : Google Scholar
|
|
23
|
Thiel G and Rössler OG: Resveratrol
stimulates AP-1-regulated gene transcription. Mol Nutr Food Res.
58:1402–1413. 2014. View Article : Google Scholar
|
|
24
|
Halliwell B: Reactive species and
antioxidants. Redox biology is a fundamental theme of aerobic life.
Plant Physiol. 141:312–322. 2006. View Article : Google Scholar
|
|
25
|
Poljsak B, Šuput D and Milisav I:
Achieving the balance between ROS and antioxidants: When to use the
synthetic antioxidants. Oxid Med Cell Longev. 2013:9567922013.
View Article : Google Scholar
|
|
26
|
Hu TJ, Shuai XH, Chen JR, Wei YY and Zheng
RL: Protective effect of a Potentilla anserine polysaccharide on
oxidative damages in mice. Int J Biol Macromol. 45:279–283. 2009.
View Article : Google Scholar
|
|
27
|
Kim KC, Lee IK, Kang KA, Kim HS, Kang SS
and Hyun JW: Baicalein (5,6,7-trihydroxyflavone) reduces oxidative
stress-induced DNA damage by upregulating the DNA repair system.
Cell Biol Toxicol. 28:421–433. 2012. View Article : Google Scholar
|
|
28
|
Liebermann DA, Gregory B and Hoffman B:
AP-1 (Fos/Jun) transcription factors in hematopoietic
differentiation and apoptosis. Int J Oncol. 12:685–700. 1998.
|
|
29
|
Kahl R and Kappus H: Toxicology of the
synthetic antioxidants BHA and BHT in comparison with the natural
antioxidant vitamin E. Z Lebensm Unters Forsch. 196:329–338.
1993.(In German). View Article : Google Scholar
|
|
30
|
Matkowski A, Jamiołkowska-Kozlowska W and
Nawrot I: Chinese medicinal herbs as source of antioxidant
compounds-where tradition meets the future. Curr Med Chem.
20:984–1004. 2013. View Article : Google Scholar
|
|
31
|
Xiong L, Xie J, Song C, Liu J, Zheng J,
Liu C, Zhang X, Li P and Wang F: The activation of Nrf2 and its
downstream regulated genes mediates the antioxidative activities of
xueshuan xinmaining tablet in human umbilical vein endothelial
cells. Evid Based Complement Alternat Med. 2015:1872652015.
View Article : Google Scholar
|