Introduction
For early-stage non-small cell lung cancer (NSCLC),
surgery is still the first choice for treatment (1,2). However,
not all early-stage NSCLC patients are suited for surgery because
of advanced age, patient refusal, or other reasons (3,4).
Stereotactic body radiotherapy (SBRT) has been widely used as an
effective alternative treatment for primary lung tumors, and
reports excellent 3-year progression-free survival (PFS) of up to
90%, which is considerably better than past data with conventional
radiotherapy (5,6). In addition, several studies on SBRT in
patients deemed operable have shown that SBRT is also an option for
these patients (5–7). Furthermore, with the development of
anticancer drugs and immunotherapy, long-term survival has been
obtained even in cases with distant metastases. As several papers
report the usefulness of SBRT for ‘oligo-metastasis or
oligo-recurrence,’ SBRT is expected to play a role as radical local
therapy for pulmonary oligo-recurrence (8–12).
In SBRT, good local control is obtained by
increasing the dose per fraction, which extends the irradiation
time per fraction; It makes the treatment stressful for elderly
patients suffering from cardiopulmonary comorbidities, and
ultimately increase the risk of intra-fraction motion (13–15). We
have performed SBRT using a kind of intensity modulated radiation
therapy (IMRT) method called single-arc volumetric modulated arc
therapy (VMAT) created by SmartArc (Pinnacle3; Philips
Medical Systems B.V., Eindhoven, The Netherlands). VMAT allows
reduction of SBRT treatment times for each fraction, but is still
limited by the maximum dose rate of a conventional linear
accelerator (linac) with a flattened beam (16).
Expecting to further shorten treatment time, we
adopted the flattening filter-free (FFF) technique to our VMAT-SBRT
system in 2014. Compensation of the forward-peak bremsstrahlung by
a flattening-filter decreases the maximal dose output of a linac,
increasing the treatment time (17).
By removing the flattening filter (so called
flattening-filter-free), it is possible to increase the dose rate
and dramatically shorten the treatment time. Although the FFF
technique may improve clinical safety and efficacy by reducing
treatment time, few clinical data to support this are available. We
evaluated the safety and availability of VMAT-SBRT using the FFF
technique in treatment of primary and metastatic lung tumors.
Patients and methods
Patients and tumor
characteristics
From November 2013 to October 2015, 77 consecutive
patients with 79 pulmonary lesions received single-arc VMAT-SBRT at
the University of Tokyo. Of 77 cases, 10 were excluded due to lack
of imaging examination after treatment, and the remaining 67 cases
were included. Cases of obvious idiopathic pulmonary fibrosis were
excluded. Of these 67 patients, 35 patients (35 cases) were treated
between November 2013 and October 2014 with the flattening filter
(FF) technique, and 30 patients (32 lesions) between November 2014
and October 2015 were treated with the FFF technique. Among 54
primary lung tumors, tissue diagnosis had been obtained in 23
patients (43%), including 5 squamous cell carcinomas and 18
adenocarcinomas. Stage classification of primary cases (8th edition
of the UICC TNM classification) was ≤1A1, 11; 1A2, 22; 1Ac, 19; 2A,
3; 2B, 3; ≥3, 4. On computerized tomography (CT), a total of 85% of
the primary tumors measured ≤3 cm. A total of 54 cases (83%) were
solid tumors, 4 cases (6%) were pure ground glass opacity (GGO),
and 11 (%) were mixed. The study was approved by the ethics
committee of University of Tokyo Hospital (3372-(3)/2016) and written informed consent was
obtained from all patients.
Treatment planning
Planned dose was 55 Gy in four fractions
(biologically effective dose using the LQ model with a The
alpha/beta=10 Gy: BED10=105.8 Gy) for 55 peripheral
lesions, or 56 Gy in seven fractions (BED10=100.8 Gy)
for 14 central lesions to cover 95% of the PTV (D95%).
Central tumors were defined as such using RTOG 0236 criteria
(18,19).
All patients underwent CT based SBRT planning for
VMAT. Four-dimensional (4D) CT images for treatment planning were
acquired with 2-mm-thick sections using a 16-detector scanner
(Aquilion LB®; Toshiba Medical Systems, Otawara, Japan).
The patients were treated in the supine position while wearing an
abdominal compressor. A stereotactic body frame was also used to
minimize breathing artifacts for treatment planning CT. Scans were
performed using an external respiratory monitoring system (AZ-733
V®; Anzai Medical, Tokyo, Japan). In our institution,
4D-CT for planning divides the respiratory cycle into 10 sections.
Respiratory phase data were transferred to a treatment planning
system (TPS) (Pinnacle3®, version 9.10; Philips Medical
Systems B.V.). Gross tumor volume (GTV) was delineated in each
respiratory phase using the lung window (window, 1,600 HU; level,
−300 HU). These 10 GTVs were fused to form the internal target
volume (ITV) and then a uniform 5 mm margin was added to create the
planning target volume (PTV) (20–22).
Dose to targets and OARs
Planning target coverage aimed to cover PTV with 95%
of the prescribed dose. The main organs at risk (OARs) were healthy
lung, spinal cord, heart, and esophagus. Treatment plans were
required to meet explicit objectives as follows: V20 <10% (less
than 10% of the volume receiving 20 Gy) and V5 <25% for the
ipsilateral lung, V20 <0% and V5 <15% for the contralateral
lung, V15=0% for spinal cord, V30=0% for heart and liver, and
V50=0% for body (23).
SBRT procedure
All patients were treated using VMAT-SBRT with 6 or
10 MV FF or FFF beams. The maximum dose rate for FFF beams was 1500
monitor units (MU)/min for 6 MV and 2,400 MU/min for 10 MV
(18). VMAT plans were designed using
a single partial arc with angle ranges of −40° to 180° (left lung)
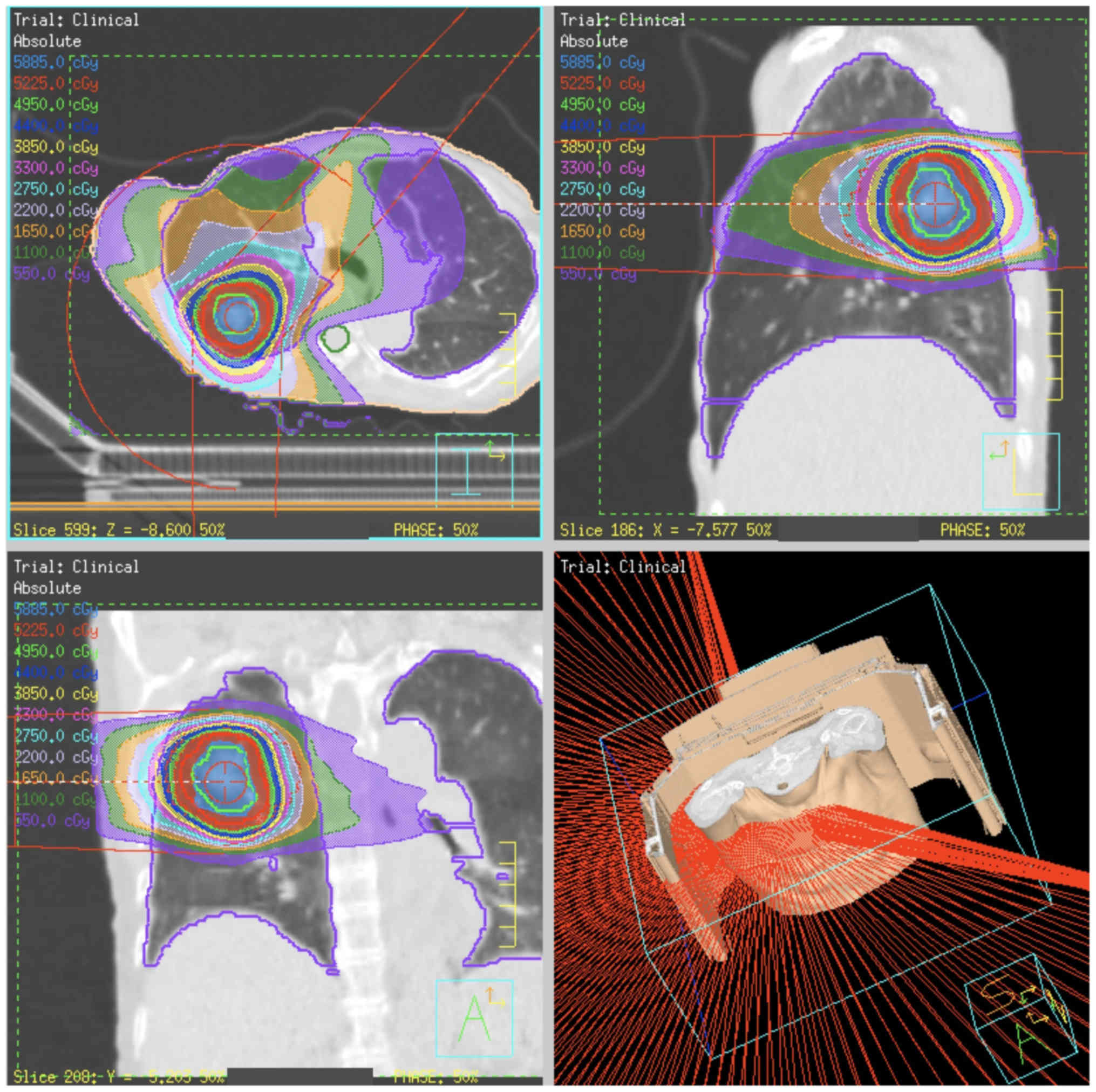
or −180° to 40° (right lung) (Fig.
1).
A conformal field shape was used to reduce the
interplay effect (24). In inverse
planning of VMAT, the conformal-like field shape can be created by
imposing a constraint on multi-leaf collimator (MLC) motion speed
(25); the MLC moves smoothly during
VMAT delivery, so that the constraint is intended to form field
shapes that do not hide the target. In this study, a constraint on
MLC motion of 0.2 cm/degree was applied.
Dosimetric planning and plan analysis were generated
with the TPS. The collapsed cone convolution method (which is
comparable to the superposition method) in the TPS was used for
heterogeneity correction for the lungs. All final calculations were
performed with a grid size of 2.0 mm. Dose distributions were
calculated using peak exhalation CT data.
Evaluation of dosimetric and technical
data
For each group of patients, technical parameters of
dose delivery were scored in terms of total number of MU, MU/Gy,
and beam-on times. Dosimetric quality of treatments was measured
from dose volume histogram (DVH) analysis. For the PTV and ITV, the
target coverage (mean, D2%, D50%,
D98%), homogeneity, and conformity were reported. The
homogeneity index (HI) was described as
(D2%-D98%)/D50%, and the
conformity index as the ratio of the volume receiving 95% of the
prescribed dose and the PTV/ITV volume (19,26). For
OARs, the mean dose, maximum dose (Dmax) and appropriate volumes of
Vx (volume receiving at least X Gy) were scored.
Evaluation of clinical data
The patients were usually examined two months after
SBRT and every six months thereafter. Chest CT or
18F-fluorodeoxyglucose positron emission
tomography/computed tomography (FDG-PET/CT) was used for follow-up.
Local tumor recurrences or distant relapses were evaluated
following the Response Evaluation Criteria In Solid Tumors (RECIST)
criteria (version 1.1). In case RECIST criteria were not useful
depending on the post-irradiation changes of normal tissue, an
FDG-PET/CT was performed to differentiate between tumor recurrence
and lung fibrosis. The 1-year local control rate (LCR), overall
survival (OS), and relapse-free survival (RFS) were evaluated and
compared between FF and FFF cases. Acute and late toxicities were
assessed according to the Common Terminology Criteria for Adverse
Events Version 4.0 (CTCAE v4.0).
Statistical analysis
The OS, LCR and RFS were defined over the period
from the time of the first day of SBRT until death, recurrence or
the last patient contact. Local failure was defined as progressive
and increasing CT scan abnormalities with a high maximum
2-(fluorine-18)-fluoro-2-deoxy-D-glucose (FDG) uptake of >3
standardized uptake value (SUV), with or without biopsy. They were
calculated using Kaplan-Meier curves, and the curves were then
compared using the log-rank test. The statistical analyses were
performed using R software, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of each treatment
group
Table I compares the
characteristics of the FF and FFF groups. There was no significant
difference in the age distribution of subjects in the groups, and
>50% patients in both groups were >80 years old. The mean PTV
of the FF and FFF groups was 50.4 cm3 (range,
14.1–225.63) and 40.1 cm3 (range, 9.0–135.2), ITV volume
was 17.1 cm3 (range, 2.3–103.3) and 14.8 cm3
(range, 1.4–67.2), respectively. There were no significant
differences between PTV and ITV (P=0.32, 0.64), respectively.
Regarding tumor location, in the second half period using FFF, the
proportion of central lesions had a tendency being larger (11 to
31%, P=0.069). Of the 35 patients in the FF group, 30 of 35 lesions
(86%, including 25 primary cases) were NSCLC, while in the FFF
group, 30 lesions (94%, including 29 primary cases) in 32 patients
were NSCLC, the rest being pulmonary oligo-recurrence.
 | Table I.Patient background and comparison
between the FF and FFF groups. |
Table I.
Patient background and comparison
between the FF and FFF groups.
|
| Total mean
(range) | FF | FFF | P-value | Test |
|---|
| Number |
|
|
|
|
|
|
Patients-lesions | 65–67 | 35–35 | 30–32 |
|
|
| Sex |
|
|
|
|
|
|
Male | 40 | 22 | 18 | 0.81 | χ2 |
|
Female | 25 | 13 | 12 |
|
|
| Mean-age
(range) |
| 77 (56–89) | 76 (46–86) | 0.85 | Unpaired t |
|
Age-classification |
|
|
|
|
|
| ≧80
years | 32 | 17 | 15 | 0.91 | χ2 |
| <80
years | 33 | 18 | 15 |
|
|
| General
condition |
|
|
|
|
|
|
Karnofsky index |
| 90 (70–100) | 90 (80–100) | 0.69 | Unpaired t |
| Smoking status |
|
|
|
|
|
|
Active | 6 | 3 | 3 |
|
|
|
Former | 22 | 10 | 12 |
|
|
|
Never | 23 | 10 | 13 |
|
|
| Not
known | 14 | 12 | 2 |
|
|
|
Brickman index |
| 450 (0–2,000) | 630 (0–3,000) | 1 | unpaired t |
| Pulmonary
lesions |
|
|
|
|
|
| Primary
NSCLC | 54 | 25 | 29 |
|
|
|
Recurrent/residual NSCLC | 6 | 5 | 1 |
|
|
|
Metastatic pulmonary
lesions | 7 | 5 | 2 |
|
|
| Tumor location |
|
|
|
|
|
|
Peripheral | 51 | 33 | 22 | 0.05 | χ2 |
|
Central | 14 | 4 | 10 |
|
|
| Involved lobe |
|
|
|
|
|
| Right
upper | 20 | 10 | 10 |
|
|
| Right
middle/lower | 21 | 13 | 8 |
|
|
| Left
upper | 8 | 5 | 3 |
|
|
| Left
lower | 15 | 9 | 6 |
|
|
|
Other | 5 | 0 | 5 |
|
|
| PTV |
|
|
|
|
|
|
cm3 | 45.6
(9.0–225.6) | 50.4
(14.1–225.6) | 40.1
(9.0–135.2) | 0.32 | Unpaired t |
| ITV |
|
|
|
|
|
|
cm3 | 16.0
(1.4–103.3) | 17.1
(2.3–103.3) | 14.8
(1.4–67.2) | 0.64 | unpaired t |
Table II summarizes
the tumor features, focusing on primary NSCLC in both groups. The
pathological diagnosis of NSCLC was confirmed in 42%, of which
adenocarcinoma: squamous cell carcinoma ratios were 8:2 and 9:3,
respectively. In the FF and FFF groups, solid lesions accounted for
76 and 81% of each, respectively.
 | Table II.The tumor features, focusing on
primary NSCLC in both groups. |
Table II.
The tumor features, focusing on
primary NSCLC in both groups.
| Primary NSCLC | FF | FFF | P-value | Test |
|---|
| Number of
lesions | 25 | 27 |
|
|
| Pathology |
|
|
|
|
|
Adenocarcinoma | 8 | 9 | 0.91 | χ2 |
|
Squamous cell | 2 | 3 |
|
|
|
Others | 0 | 0 |
|
|
|
Unknown | 15 | 15 |
|
|
| Lesion texture |
|
|
|
|
|
GGO | 3 | 1 | 0.53 | χ2 |
|
Part-solid | 3 | 4 |
|
|
|
Solid | 19 | 22 |
|
|
| Tumor diameter |
|
|
|
|
| Mean
(range) | 22.9 (0–51) | 24.0 (4–60) | 0.68 | Unpaired t |
| T-Stage |
|
|
|
|
| T0 | 3 | 1 | 0.65 | χ2 |
|
T1A1 | 1 | 1 |
|
|
|
T1A2 | 12 | 12 |
|
|
|
T1A3 | 6 | 8 |
|
|
|
T1B | 1 | 1 |
|
|
|
T2A | 1 | 2 |
|
|
|
T2B | 1 | 2 |
|
|
There was no significant difference in the
distribution of tumor sizes (solid part) between the groups
(P=0.15). Tumors were classified based on the T classification of
the UICC eighth edition as shown in Table II.
Dosimetric and technical data
Technical features for the FF and FFF groups are
summarized in Table III. Between FF
and FFF, no statistical difference was observed for PTV/ITV
coverage in terms of D98% and D50%. There was
no significant difference between the two groups even at
D2%, rather as an indicator of HI. The FFF plans were
not inferior, with a mean conformity index of 1.32 (range
1.04–3.11), while the FF plans showed 1.51 (range 0.82–2.21) for
PTV (P=0.95). The same was true for ITV. The difference in the HI
of the two techniques was also not significant (P=0.20). Comparing
the mean MU values, no significant differences were found between
the two groups too (P=0.63).
 | Table III.Technical features for FF and FFF
techniques. |
Table III.
Technical features for FF and FFF
techniques.
|
| FF mean
(range) | FFF mean
(range) | P-value |
|---|
| PTV |
|
|
|
|
D2% | 61.9
(59.4–76.4) | 62.5
(52.7–69.6) | 0.72 |
|
D98% | 54.1
(50.4–67.5) | 54.2
(49.0–54.9) | 0.85 |
|
D50% | 59.0
(50.4–67.5) | 59.1
(51.6–63.3) | 0.81 |
| CI | 1.51
(0.82–2.21) | 1.32
(1.04–3.11) | 0.95 |
| HI | 0.15
(0.04–0.36) | 0.13
(0.06–0.26) | 0.20 |
| ITV |
|
|
|
|
D2% | 62.7
(59.6–75.3) | 63.2
(52.7–70.4) | 0.71 |
|
D98% | 57.5
(54.0–61.0) | 57.6
(51.3–59.8) | 0.94 |
|
D50% | 60.6
(57.6–69.7) | 60.6
(51.9–64.2) | 0.52 |
| CI | 1.51
(0.82–2.21) | 1.32
(1.04–3.11) | 0.95 |
| HI | 0.15
(0.04–0.36) | 0.13
(0.06–0.26) | 0.20 |
| MU | 2,181
(1,203.5–2,414.2) | 2,076
(718.5–2,506.0) | 0.63 |
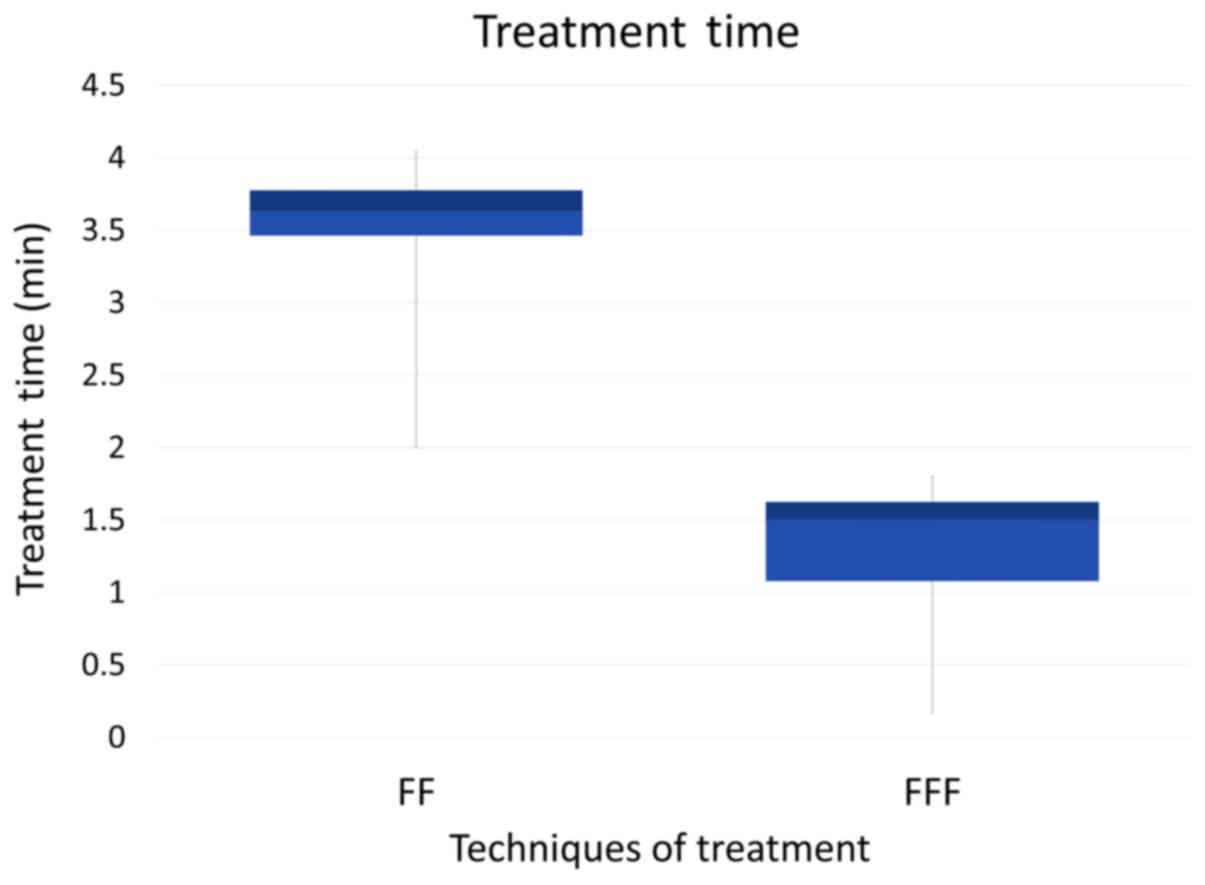
| TT (min) | 3.64
(2.00–4.02) | 1.48
(0.50–1.80) | 0.010 |
On the other hand, the beam-on time was
significantly shortened with use of the FFF plan (P<0.01)
(Fig. 2); the mean beam-on-time of
the FFF plan was less than half that of the FF plan. This is in
agreement with other studies (27–29).
Dose to the OARs
Table IV shows the
doses to the OARs with the FF and the FFF techniques. No
significant difference was observed between the two treatment plans
for the lung dose, in terms of the mean dose (D mean) or the V20,
V10, or V5. We evaluated the dose to other OARS, and found no
significant differences between the FF and the FFF plans.
 | Table IV.Summary of dose volume histogram
analysis for organs at risk. |
Table IV.
Summary of dose volume histogram
analysis for organs at risk.
| Organ | Dose volume | FF (n=35) mean
(range) | FFF (n=30) mean
(range) | P-value |
|---|
| Lung | V20 Gy (%) | 8.0 (1.8–16.9) | 7.9 (2.7–15.4) | 0.90 |
|
| V10 Gy (%) | 14.2
(2.5–36.7) | 14.5
(6.4–29.0) | 0.82 |
|
| V5 Gy (%) | 21.6
(4.9–49.2) | 21.8
(5.2–42.6) | 0.94 |
|
| Dmean (cGy) | 5.50
(0.2–11.5) | 5.5 (2.0–9.6) | 0.99 |
| Spinal cord | Dmax (cGy) | 13.8
(4.2–27.8) | 11.60
(0.2–25.7) | 0.16 |
| Heart | Dmean (cGy) | 4.57
(0.01–23.8) | 3.96
(0.1–10.5) | 0.75 |
Tumor control
The median follow-up period was 18 months (range
2–35) for all, and 24 months for the FF group (range 2–35) and 12
months for the FFF group (range 2–23). The median OS, PFS and LCR
times had not been reached at the time of data analysis, and no
significant differences were observed (P=0.164, 0.26, 0.847,
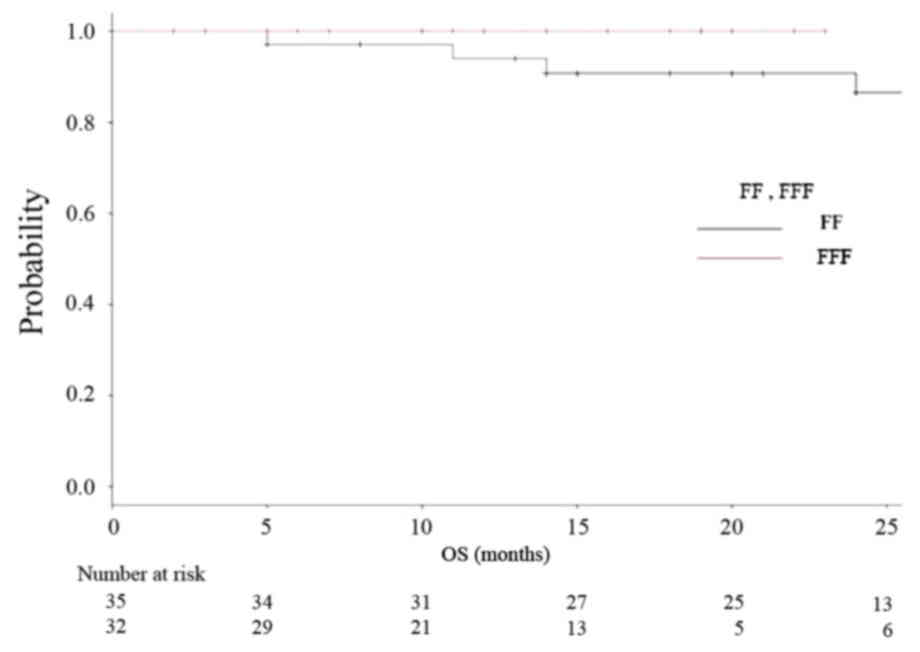
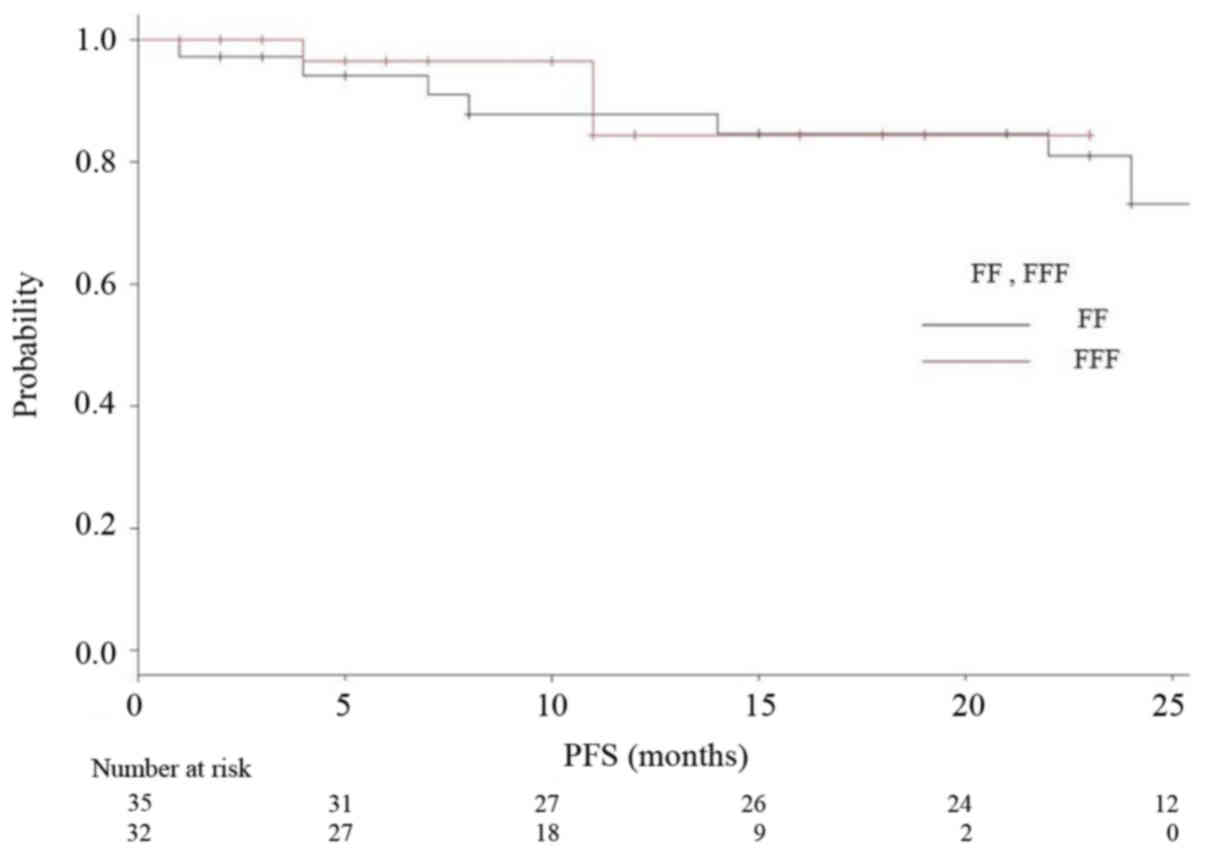
respectively). Kaplan-Meier analyses of OS and RFS in both groups
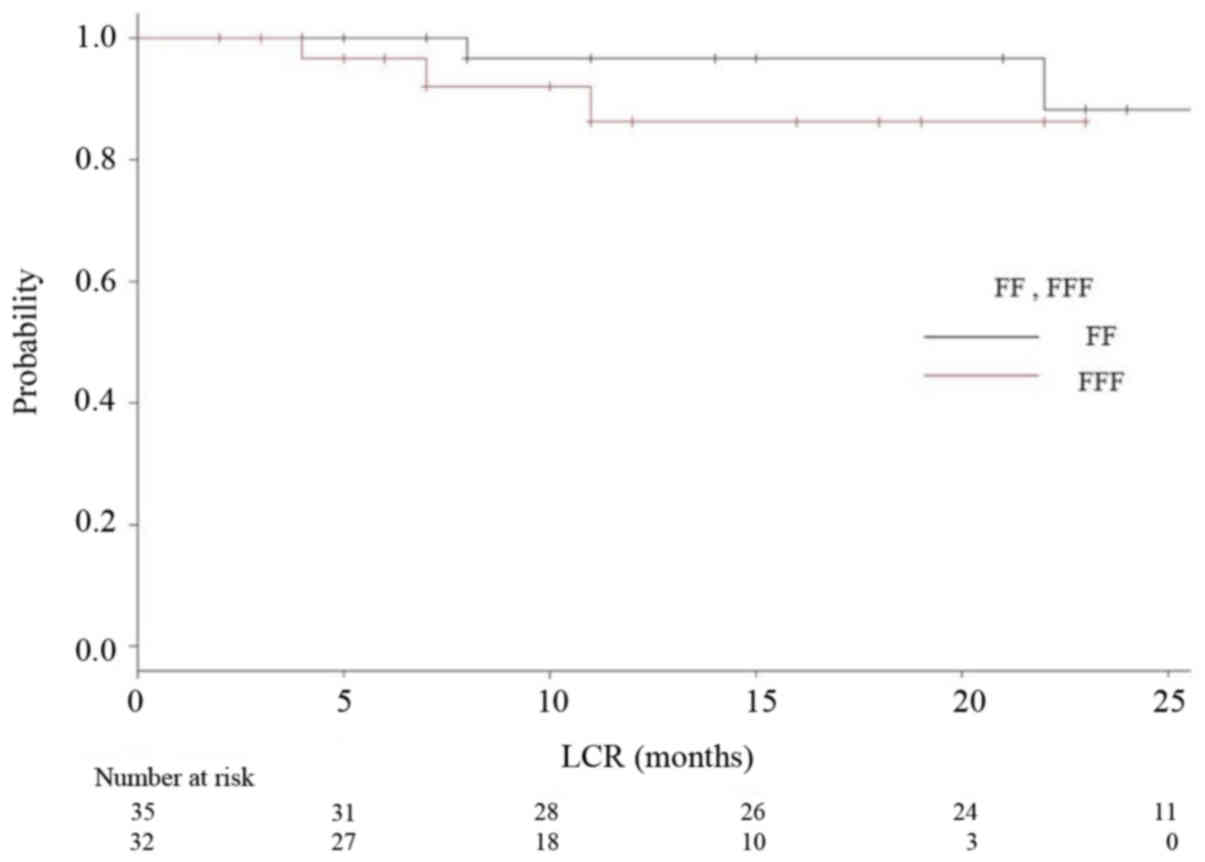
are shown in Figs. 3 and 4, respectively. Fig. 5 is a Kaplan-Meier curve for local
control. One-year LCRs were 97.1% (1 case relapsed) and 90.0% (3
cases relapsed) in the FF and FFF groups, respectively.
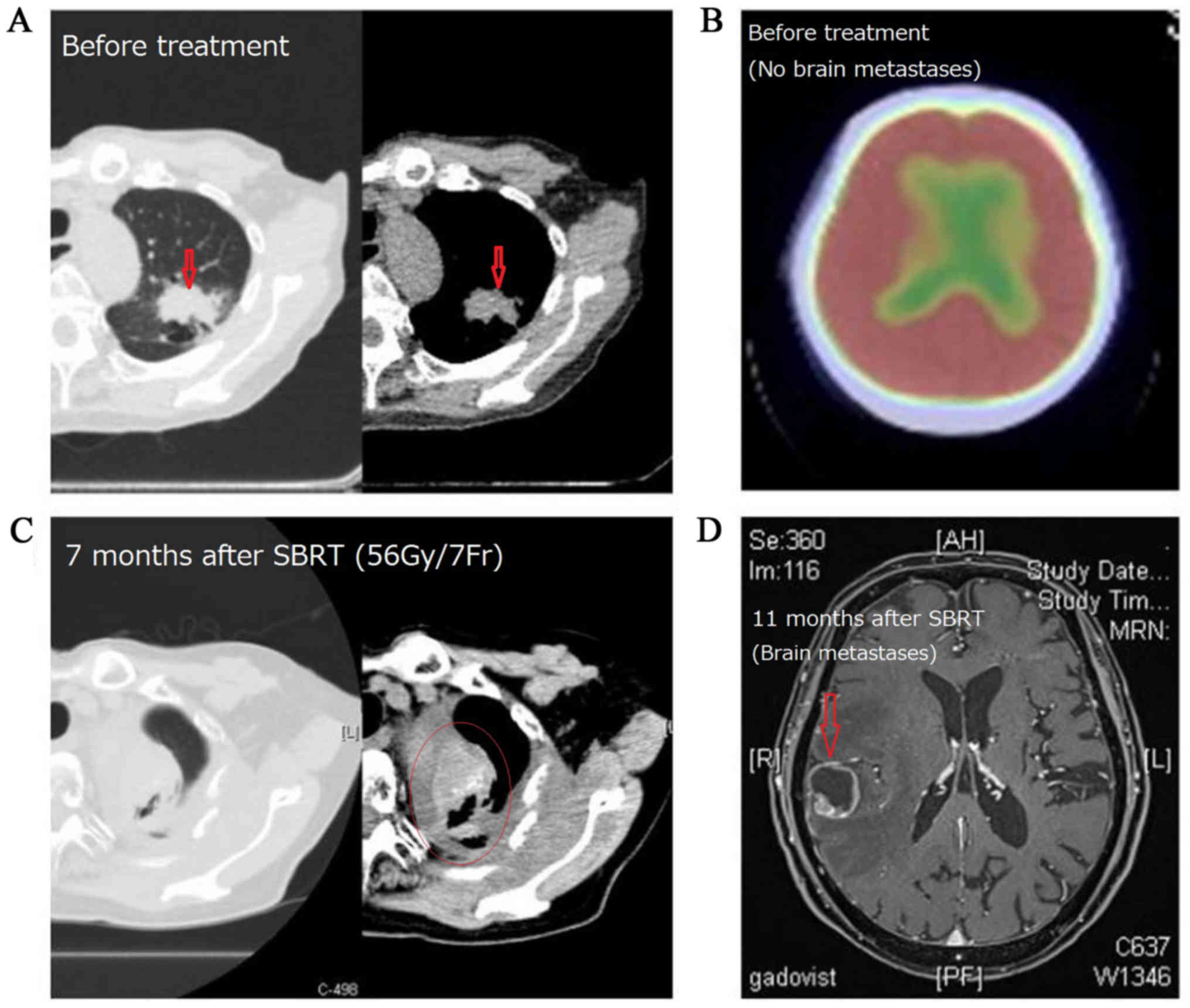
Out of 67 subjects, recurrence was observed in 10
cases (7 cases in the FF group and 3 cases in the FFF group). There
were 6 cases of local recurrence and 10 cases of distant
metastasis. All cases with local recurrence also had distant
metastases. One case of recurrence is shown in Fig. 6.
Adverse events
We investigated the frequency of adverse events
(AEs) based on the information in the medical records using CTCAE
v4.0. Toxicity observed within 90 days of SBRT was categorized as
acute, whereas toxicity observed >90 days after SBRT was
categorized as late.
The appearance of AEs in each group is shown in
Table V. Acute adverse events of
grade ≥3 were not observed. There were six cases (9.5%) of
dermatitis, seven cases (11.1%) of pleurisy and two cases (3.2%) of
esophagitis among all subjects. Late adverse events were
investigated in 63 cases (FF: 34 cases, FFF: 29 cases) that could
be observed for >90 days from the start of treatment. In these
63 cases, grade ≥1 radiation-induced pneumonitis were observed in
33 cases (97%) in the FF group and 27 cases (93%) in the FFF group.
Most of the ‘pneumonitis’ showed only changes on the image, in
other words, grade 1. Of the FF group, there were twenty-nine cases
(85%) of grade1 pneumonitis, three cases (9%) of grade 2, and two
cases (6%) of grade 3. Of the FFF group, there were twenty-two
cases (76%) of grade1 pneumonitis, four cases (14%) of grade 2 and
one case (4%) of grade 3. Pneumonitis of grade ≥4 was not
observed.
 | Table V.The appearance of side effects in
each group. |
Table V.
The appearance of side effects in
each group.
| Component and
disorders | Grade (CTCAE
vol4.0.) | FF-group number
(%) | FFF-group number
(%) |
|---|
| Acute adverse
events |
| 35 | 30 |
| Dermatitis | ≤2 | 5
(14.3) | 1 (3.3) |
|
| 3 | 0 (0) | 0 (0) |
| Esophagitis | ≤2 | 2 (5.7) | 0 (0) |
|
| 3 | 0 (0) | 0 (0) |
| Late adverse
events |
| 34 | 29 |
| Pneumonitis | 1 | 28 (82.4) | 3
(10.3) |
|
| 2 | 3 (8.8) | 1 (4.3) |
|
| 3 | 2 (5.9) | 1 (4.3) |
| Tracheal
stenosis | ≤2 | 0 (0) | 0 (0) |
|
| 3 | 0 (0) | 2 (8.7) |
| Pleuritis | ≤2 | 5
(14.7) | 0 (0) |
|
| 3 | 0 (0) | 1 (4.3) |
| Pericarditis | ≤2 | 0 (0) | 0 (0) |
|
| 3 | 0 (0) | 0 (0) |
| Rib fracture | all | 0 (0) | 0 (0) |
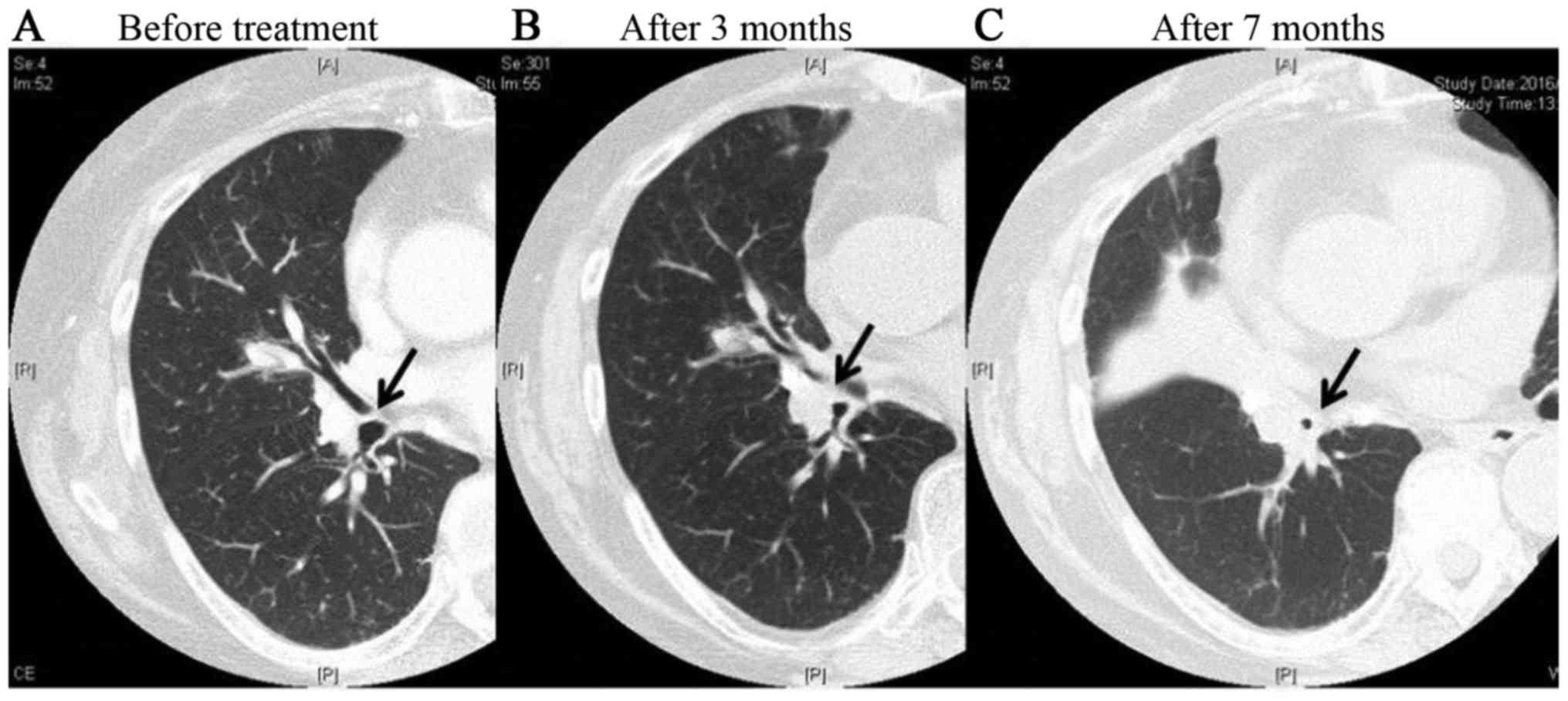
We experienced one case of grade 2 tracheal stenosis
in the FFF group. In this case, wheezing appeared 3 months after
treatment, and the patient received steroid medication. At 7 months
after treatment, thickening of the bronchial walls and tracheal
stenosis were observed, but symptoms were controlled by the use of
inhaled steroids (Fig. 7). There were
no late adverse events of grade ≥3 involving the heart or
esophagus.
Discussion
Clinical reports on FFF are sparse, and few of these
compare the clinical results of FF and FFF in the same facility.
The present study is a retrospective observation study of 67 cases
where VMAT-SBRT was performed in our hospital from 2013 to 2015.
The cases during the period were divided into FF and FFF groups,
and beam data and clinical results were compared between the two
groups. Although the two groups underwent treatment at different
times in the same facility, no change was made except for the
presence or absence of the flattening filter.
Shortening of treatment time
We have performed SBRT using VMAT-IMRT since 2010,
and FFF since 2014. Yamashita, et al already reported
details of treatment from our hospital (20). VMAT has been reported to be a novel
rotational technique and an extension of IMRT, and is applicable
for SBRT for lung tumors (23,30–34).
By introducing VMAT, we could reduce the dose delivery time down to
210 sec for a D95 prescription dose of 50 Gy in four fractions
(35). Furthermore, we added a
FFF-system to our LINAC (Elekta AB, Stockholm, Sweden), and
succeeded further reduction of treatment time with the beam-on time
reduced 50% or more (22) (Table III, Fig.
2).
Clinical Merit of FFF system
Increasing the dose rate and shortening the
treatment time reduces the patient's pain. As Thomas et al
stated, shortening treatment time enables more accurate dose
delivery because body motion and irregular breathing during
treatment time are reduced (36).
Another advantage of the FFF method is reported to
be a reduction in the dose to surrounding normal tissue, in
particular lungs, by drawing sharp slope on the periphery of the
tumor (37,38). It is expected to lead to reduce side
effects eventually. In addition, adopting FFF methodology to volume
prescription, a high dose (so-called ‘hot spot’) will be aimed at
the central part of the target. This may improve the treatment of
radio-resistant lesions (e.g., colon cancer lung oligo-recurrence)
(39).
These advantages of using FFF-method were not
directly reflected in our clinical data, however. There was no
significant difference between the FF and FFF groups in MU value,
dose to OARs and targets. It seems to be partly because the number
of the subjects of this study was small, the stage and tumor
diameters had a wide range, and the observation period was short.
Prendergast et al reported that there was no significant
difference between the two treatments, and Rieber et al
pointed out the same cause as us (40,41).
As for the clinical result in our research, one-year
LCR was 95% for all, 93% for the FF group and 97% for the FFF
group. One-year OS was 97% for all, 94% for FF and 100% for FFF
group, respectively. Rieber, et al reported the results
similar to ours. They achieved 1-year LCR of 92.8% and 1-year OS of
94.4% with FFF-VMAT-SBRT using 8×7.5 Gy (D80%) for
central lesions and 3×15 Gy (D65%) for peripheral
lesions (14). Navarria et al
reported on 132 VMAT-SBRT cases with 48 Gy/4 fr prescriptions with
a 1 year LCR of 100% with FFF and 92.5% with FF (27). In their report, they stated that the
FFF achieved a significantly better one-year LCR. However, most of
the presentations so far are only saying that there is no obvious
difference for the same reasons as ours (14,28,29). These
results can lead to no definite conclusion at the moment, but at
least, we can say that the performance of FFF was not inferior to
that of FF.
Speaking of AEs of this study using FFF, grade 3 AEs
appeared as two cases of pneumonitis (3%) and one case of
esophagitis (1.5%). No AEs >grade 3 were observed. Although the
observation period was short and no conclusions can be drawn, no
serious AEs have been observed in either group to date. Although
most reports targeted peripheral early stage lung cancer, the
target of our study was not limited to early stage, and also
included central lesions that are said to have higher risk of AEs
(5,42–43). In
addition, most studies of lung SBRT in Japan adopt a 48 Gy/4 fr
prescription (5,44), whereas we prescribed 55 Gy/4 fr for
peripheral lung cancers and 56 Gy/7 fr for central ones (that is,
BED10 >100 in all cases). Taking these conditions
into consideration, safety results were not inferior to those of
other reports about SBRT using FF technique.
Limitations of this study are its small number of
cases and short observation period. Further, the study was
conducted at a single facility and the observation period between
the two groups varied. It is inevitable for retrospective
observational studies to have missing data, such as loss of
pathological diagnosis. Although the results of the SBRT of each
facility cannot be compared simply because the techniques,
prescription dose, and objects are greatly different from each
other, it will be necessary to conduct investigations with larger
numbers of cases and longer observation periods.
VMAT-SBRT using FFF enabled a shortened treatment
time without lowering local control or increasing AEs.
Acknowledgements
This study was partially supported by a Grant-in-Aid
from JSPS (Japan Society for the Promotion of Science) KAKENHI JP
Scientific Research (C), grant no. 15K08692.
Glossary
Abbreviations
Abbreviations:
|
AE
|
adverse event
|
|
BED
|
biologically equivalent dose
|
|
CT
|
computed tomography
|
|
DVH
|
dose volume histogram
|
|
FF
|
flattening filter
|
|
FFF
|
flattening filter-free
|
|
GGO
|
ground glass opacity
|
|
GTV
|
gross tumor volume
|
|
HI
|
homogeneity index
|
|
ITV
|
internal target volume
|
|
LCR
|
local control rate
|
|
MLC
|
multileaf collimator
|
|
MU
|
monitor unit
|
|
NSCLC
|
non-small cell lung cancer
|
|
OARs
|
organs at risk
|
|
PFS
|
progression-free survival
|
|
PTV
|
planning target volume
|
|
RFS
|
relapse-free survival
|
|
SBRT
|
stereotactic body radiotherapy
|
|
TPS
|
treatment planning system
|
|
TT
|
treatment time
|
|
VMAT
|
volumetric modulated arc therapy
|
References
|
1
|
Maquilan G and Timmerman R: Stereotactic
body radiation therapy for early-stage lung cancer. Cancer J.
22:274–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ginsberg RJ and Rubinstein LV: Randomized
trial of lobectomy versus limited resection for T1 N0 non-small
cell lung cancer. Lung cancer study group. Ann Thorac Surg.
60:615–623. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Falkson CB, Vella ET, Yu E, El-Mallah M,
Mackenzie R, Ellis PM and Ung YC: Radiotherapy with curative intent
in patients with early-stage, medically inoperable, non-small-cell
lung cancer: A systematic review. Clin Lung Cancer. 18:105–121.e5.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nanda RH, Liu Y, Gillespie TW, Mikell JL,
Ramalingam SS, Fernandez FG, Curran WJ, Lipscomb J and Higgins KA:
Stereotactic body radiation therapy versus no treatment for early
stage non-small cell lung cancer in medically inoperable elderly
patients: A National Cancer Data Base analysis. Cancer.
121:4222–4230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagata Y, Hiraoka M, Shibata T, Onishi H,
Kokubo M, Karasawa K, Shioyama Y, Onimaru R, Kozuka T, Kunieda E,
et al: Prospective trial of stereotactic body radiation therapy for
both operable and inoperable T1N0M0 non-small cell lung cancer:
Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol
Biol Phys. 93:989–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang JY, Senan S, Paul MA, Mehran RJ,
Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, et al:
Stereotactic ablative radiotherapy versus lobectomy for operable
stage I non-small-cell lung cancer: A pooled analysis of two
randomised trials. Lancet Oncol. 16:630–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baumann P, Nyman J, Hoyer M, Wennberg B,
Gagliardi G, Lax I, Drugge N, Ekberg L, Friesland S, Johansson KA,
et al: Outcome in a prospective phase II trial of medically
inoperable stage I non-small-cell lung cancer patients treated with
stereotactic body radiotherapy. J Clin Oncol. 27:3290–3296. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niibe Y and Hayakawa K: Oligometastases
and oligo-recurrence: The new era of cancer therapy. Jpn J Clin
Oncol. 40:107–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niibe Y and Chang JY: Novel insights of
oligometastases and oligo-recurrence and review of the literature.
Pulm Med. 2012:2610962012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamashita H, Niibe Y, Yamamoto T, Katsui
K, Jingu K, Kanazawa S, Terahara A and Nakagawa K: Lung
stereotactic radiotherapy for oligometastases: Comparison of
oligo-recurrence and sync-oligometastases. Jpn J Clin Oncol.
46:687–691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niibe Y, Yamashita H, Sekiguchi K,
Takahashi W, Shiraishi K, Okuma K, Terahara A, Kawamori J and
Nakagawa K: Stereotactic body radiotherapy results for pulmonary
oligometastases: A two-institution collaborative investigation.
Anticancer Res. 35:4903–4908. 2015.PubMed/NCBI
|
|
12
|
Pasqualetti F, Montrone S, Vivaldi C, Zani
M, Fedele D, Fornaro L, Pasqualetti G, Salvatore L, Manfredi B,
Laliscia C, et al: Stereotactic body radiotherapy in patients with
lung oligometastases from colorectal cancer. Anticancer Res.
37:315–319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang JY, Lu JY, Wu LL, Hong DL, Ma CC,
Peng X and Lin ZX: A dosimetric and treatment efficiency evaluation
of stereotactic body radiation therapy for peripheral lung cancer
using flattening filter free beams. Oncotarget. 7:73792–73799.
2016.PubMed/NCBI
|
|
14
|
Rieber J, Tonndorf-Martini E, Schramm O,
Rhein B, König L, Adeberg S, Meyerhof E, Mohr A, Kappes J, Hoffmann
H, et al: Establishing stereotactic body radiotherapy with
flattening filter free techniques in the treatment of pulmonary
lesions-initial experiences from a single institution. Radiat
Oncol. 11:802016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoogeman MS, Nuyttens JJ, Levendag PC and
Heijmen BJ: Time dependence of intrafraction patient motion
assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol
Phys. 70:609–618. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi W, Yamashita H, Kida S, Masutani
Y, Sakumi A, Ohtomo K, Nakagawa K and Haga A: Verification of
planning target volume settings in volumetric modulated arc therapy
for stereotactic body radiation therapy by using in-treatment
4-dimensional cone beam computed tomography. Int J Radiat Oncol
Biol Phys. 86:426–431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stieler F, Fleckenstein J, Simeonova A,
Wenz F and Lohr F: Intensity modulated radiosurgery of brain
metastases with flattening filter-free beams. Radiother Oncol.
109:448–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Timmerman RD, Paulus R, Galvin J,
Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G,
Johnstone D, et al: Stereotactic body radiation therapy for
medically inoperable early stage lung cancer. JAMA. 303:1070–1076.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roesch J, Panje C, Sterzing F, Mantel F,
Nestle U, Andratschke N and Guckenberger M: SBRT for centrally
localized NSCLC-What is too central? Radiat Oncol. 11:1572016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamashita H, Takahashi W, Haga A, Kida S,
Saotome N and Nakagawa K: Stereotactic body radiotherapy for small
lung tumors in the University of Tokyo hospital. Biomed Res Int.
2014:1365132014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakagawa K, Haga A, Kida S, Masutani Y,
Yamashita H, Takahashi W, Sakumi A, Saotome N, Shiraki T, Ohtomo K,
et al: 4D registration and 4D verification of lung tumor position
for stereotactic volumetric modulated arc therapy using
respiratory-correlated cone-beam CT. J Radiat Res. 54:152–156.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakagawa K, Haga A, Sakumi A, Yamashita H,
Igaki H, Shiraki T, Ohtomo K, Iwai Y and Yoda K: Impact of
flattening-filter-free techniques on delivery time for lung
stereotactic volumetric modulated arc therapy and image quality of
concurrent kilovoltage cone-beam computed tomography: A preliminary
phantom study. J Radiat Res. 55:200–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamashita H, Haga A, Takahashi W, Takenaka
R, Imae T, Takenaka S and Nakagawa K: Volumetric modulated arc
therapy for lung stereotactic radiation therapy can achieve high
local control rates. Radiat Oncol. 9:2432014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dvorak P, Georg D, Bogner J, Kroupa B,
Dieckmann K and Pötter R: Impact of IMRT and leaf width on
stereotactic body radiotherapy of liver and lung lesions. Int J
Radiat Oncol Biol Phys. 61:1572–1581. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haga A, Kida S, Saotome N, Takahashi W,
Yamashita H, Masutani Y, et al Others: Four-dimensional Cone-Beam,
: CT During SBRTStereotactic Body Radiation Therapy. Springer;
Tokyo: pp. 225–236. 2015, View Article : Google Scholar
|
|
26
|
Miura H, Ozawa S, Hosono F, Sumida N,
Okazue T, Yamada K and Nagata Y: Gafchromic EBT-XD film: Dosimetry
characterization in high-dose, volumetric-modulated arc therapy. J
Appl Clin Med Phys. 17:312–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Navarria P, Ascolese AM, Mancosu P, Alongi
F, Clerici E, Tozzi A, Iftode C, Reggiori G, Tomatis S, Infante M,
et al: Volumetric modulated arc therapy with flattening filter free
(FFF) beams for stereotactic body radiation therapy (SBRT) in
patients with medically inoperable early stage non small cell lung
cancer (NSCLC). Radiother Oncol. 107:414–418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prendergast BM, Fiveash JB, Popple RA,
Clark GM, Thomas EM, Minnich DJ, Jacob R, Spencer SA, Bonner JA and
Dobelbower MC: Flattening filter-free linac improves treatment
delivery efficiency in stereotactic body radiation therapy. J Appl
Clin Med Phys. 14:41262013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lang S, Shrestha B, Graydon S, Cavelaars
F, Linsenmeier C, Hrbacek J, Klöck S, Studer G and Riesterer O:
Clinical application of flattening filter free beams for
extracranial stereotactic radiotherapy. Radiother Oncol.
106:255–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang GG, Ku L, Dilling TJ, Stevens CW,
Zhang RR, Li W and Feygelman V: Volumetric modulated arc planning
for lung stereotactic body radiotherapy using conventional and
unflattened photon beams: A dosimetric comparison with 3D
technique. Radiat Oncol. 6:1522011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Macchia G, Deodato F, Cilla S, Cammelli S,
Guido A, Ferioli M, Siepe G, Valentini V, Morganti AG and
Ferrandina G: Volumetric modulated arc therapy for treatment of
solid tumors: Current insights. Onco Targets Ther. 10:3755–3772.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakagawa K, Haga A, Shiraishi K, Yamashita
H, Igaki H, Terahara A, Ohtomo K, Saegusa S, Shiraki T, Oritate T
and Yoda K: First clinical cone-beam CT imaging during volumetric
modulated arc therapy. Radiother Oncol. 90:422–423. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holt A, van Vliet-Vroegindeweij C, Mans A,
Belderbos JS and Damen EM: Volumetric-modulated arc therapy for
stereotactic body radiotherapy of lung tumors: A comparison with
intensity-modulated radiotherapy techniques. Int J Radiat Oncol
Biol Phys. 81:1560–1567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deodato F, Cilla S, Macchia G, Caravatta
L, Mignogna S, Massaccesi M, Picardi V, Digesu C, Sallustio G,
Bonomo P, et al: Extracranial radiosurgery with volumetric
modulated arc therapy: Feasibility evaluation of a phase I trial.
Oncol Lett. 5:1889–1896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakagawa K, Kida S, Haga A, Masutani Y,
Yamashita H, Onoe T, Imae T, Tanaka K, Ohtomo K and Yoda K: 4D
digitally reconstructed radiography for verifying a lung tumor
position during volumetric modulated arc therapy. J Radiat Res.
53:628–632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thomas EM, Popple RA, Prendergast BM,
Clark GM, Dobelbower MC and Fiveash JB: Effects of flattening
filter-free and volumetric-modulated arc therapy delivery on
treatment. Efficiency. 14:43282013.
|
|
37
|
Cashmore J: The characterization of
unflattened photon beams from a 6 MV linear accelerator. Phys Med
Biol. 53:1933–1946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vassiliev ON, Titt U, Pönisch F, Kry SF,
Mohan R and Gillin MT: Dosimetric properties of photon beams from a
flattening filter free clinical accelerator. Phys Med Biol.
51:1907–1917. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kinj R, Bondiau PY, François E, Gérard JP,
Naghavi AO, Leysalle A, Chamorey E, Evesque L, Padovani B, Ianessi
A, et al: Radiosensitivity of colon and rectal lung oligometastasis
treated with stereotactic ablative radiotherapy. Clin Colorectal
Cancer. 16:e211–e220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rieber J, Tonndorf-Martini E, Schramm O,
Rhein B, König L, Adeberg S, Meyerhof E, Mohr A, Kappes J and
Hoffmann H: Establishing stereotactic body radiotherapy with
flattening filter free techniques in the treatment of pulmonary
lesions-initial experiences from a single institution. Radiat
Oncol. 11:802016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prendergast BM, Dobelbower MC, Bonner JA,
Popple RA, Baden CJ, Minnich DJ, Cerfolio RJ, Spencer SA and
Fiveash JB: Stereotactic body radiation therapy (SBRT) for lung
malignancies: Preliminary toxicity results using a flattening
filter-free linear accelerator operating at 2,400 monitor units per
minute. Radiat Oncol. 8:2732013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Timmerman R, McGarry R, Yiannoutsos C,
Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C,
Williams M and Fletcher J: Excessive toxicity when treating central
tumors in a phase II study of stereotactic body radiation therapy
for medically inoperable early-stage lung cancer. J Clin Oncol.
24:4833–4839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Roesch J, Panje C, Sterzing F, Mantel F,
Nestle U, Andratschke N and Guckenberger M: SBRT for centrally
localized NSCLC-What is too central? Radiat Oncol. 11:1572016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kimura T, Nagata Y, Eba J, Ozawa S,
Ishikura S, Shibata T, Ito Y, Hiraoka M and Nishimura Y; Radiation
Oncology Study Group of the Japan Clinical Oncology Group, : A
randomized Phase III trial of comparing two dose-fractionations
stereotactic body radiotherapy (SBRT) for medically inoperable
Stage IA non-small cell lung cancer or small lung lesions
clinically diagnosed as primary lung cancer: Japan Clinical
Oncology Group Study JCOG1408 (J-SBRT trial). Jpn J Clin Oncol.
2017.(Epub ahead of print). View Article : Google Scholar
|