Introduction
Malignant gliomas are the most common type of brain
tumor, with high rates of recurrence and mortality (1). Despite the aggressive use of surgery,
radiotherapy and chemotherapy, the average life expectancy of
patients with high-grade glioma is 14 months after diagnosis
(2). Novel treatments are therefore
required to improve this figure. In recent years, natural products
isolated from herbs and plants have received substantial attention
for their potential as novel anticancer drugs (3).
Ganoderma lucidum is a mushroom that has been
used for medicinal purposes for centuries in China and other
oriental countries. G. lucidum polysaccharides (GL-PS) are
primary bioactive components of the fungus that can exert various
pharmacological effects, including immunomodulation (4–10),
antitumor (11–16), anti-angiogenesis (17,18),
anti-oxidation (19–22), anti-inflammatory (23) and hepatoprotective effects (24,25).
The potential antitumor effects of GL-PS have
received substantial attention and have been the subject of
extensive investigation. It has been demonstrated that GL-PS is
effective at inhibiting tumor growth in vivo via multiple
mechanisms, particularly via the activation of immune effector
cells such as lymphocytes (9,26–29),
macrophages (9) and natural killer
cells (29,30). GL-PS has also been revealed to inhibit
tumor-induced neovascularization processes. However, GL-PS is
considered to possess weak or no antitumor activity in vitro
(17,28,31–34).
Previous studies have demonstrated that GL-PS may directly inhibit
the in vitro proliferation of certain types of cancer cell,
including breast cancer (14),
ovarian cancer (15), colorectal
cancer (23) and hepatoma cells
(11). However, whether GL-PS elicits
antitumor effects on gliomas remains unknown. In the present study,
we isolated and identified a 42,635 Da G. lucidum
polysaccharide peptide (GL-PP) from G. lucidum cultivated
with JUNCAO grasses and determine its antitumor effects on gliomas
for the first time. It was revealed that this GL-PP exhibited
anti-proliferative and apoptosis-inducing effects in human U251
glioma cells.
Materials and methods
Preparation of GL-PP
GL-PP was isolated from the boiled extract of G.
lucidum cultivated with JUNCAO grasses (National Engineering
Research Center of Juncao Technology, Fujian, China). An ethanol
precipitation was then performed, followed by dialysis and
de-proteination according to the Sevag method, as previously
described (35).
The homogeneity and molecular weight of GL-PP was
identified using high-performance gel permeation chromatography
(HPGPC) with a Waters 2695 HPLC apparatus (Waters, Milford, MA,
USA), equipped with a Waters 2515 HPLC pump (Waters), a gel
permeation column TSK4000PW (21.5×300 mm, 10 µm; Tosoh, Tokyo,
Japan) and a Waters 2414 refractive index detector (Waters). Water
(HPLC grade) was used as the mobile phase with a gradient elution
(flow rate of 0.8 ml/min) at 35°C. Dextran standards were obtained
from the National Institutes for Food and Drug Control and
molecular weights ranging from 2,500 to 84,400 Da were used to
generate a calibration curve (36).
The molecular weight of GL-PP was estimated using Waters Empower
software (version 5.0; Waters).
Monosaccharides were determined using hydrophilic
interaction liquid interface chromatography and an evaporative
light scattering detector (HILIC-ELSD) via a Waters Alliance 2695
HPLC system and 2424 ELSD (both from Waters). Chromatographic
separation was performed on a Sugar-D column (4.6×250 mm; 5 µm;
Nacalai Tesque, Inc., Kyoto, Japan). The column was run at 35°C and
the mobile phase consisted of 75% acetonitrile and 25% water, at a
flow rate of 0.8 ml/min. The drift tube temperature and air carrier
gas pressure of the ELSD was set to 55°C and 45 psi, respectively.
The injection volume was 10 µl. The identity of sample
monosaccharides were determined by comparing the retention time of
peaks with those of known standards including, rhamnose, fructose,
xylose, arabinose, glucose, galactose and mannose.
The amino acid composition of GL-PP was determined
using the Hitachi-L8800 amino acid analyzer (Hitachi
High-Technologies Co., Tokyo, Japan) according to the Chinese
National Standard (37).
GL-PP was dissolved in serum-free Dulbecco's
modified Eagle's medium (DMEM; HyClone; GE Healthcare Life
Sciences, Chicago, IL, USA), then filtered through a 0.22-µm filter
and stored at 4°C. This medium was further diluted to the indicated
concentration prior to each assay.
Endotoxin test
The presence of an endotoxin in GL-PP was detected
using Tachypleus Amebocyte Lysate (TAL; Fuzhou Xin Bei Biochemical
Industry Co., Ltd., Fuzhou, China) according to the manufacturer's
instructions. A total of 100 µl TAL reagent was added to 100 µl of
800 µg/ml GL-PP, 100 µl endotoxin standard (Fuzhou Xin Bei
Biochemical Industry Co., Ltd.) and 100 µl endotoxin-free water.
The mixture was gently agitated, covered with foil and incubated at
37°C for 1 h. Since endotoxin contamination results in the
formation of a hard gel substance, samples were observed for the
formation of this gel by performing a gentle 180° tube inversion
following the incubation period.
Cell culture
The human glioma U251 cell line was obtained from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) where it was routinely maintained in DMEM
containing 10% inactivated fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences) and 100 U/ml penicillin/streptomycin.
Cells were grown at 37°C in an atmosphere of 5% CO2.
Cell proliferation assay
Cell proliferation and viability were analyzed using
the Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). U251 cells were seeded onto 96-well culture
plates at a density of 2,000 cells/well and cultured for 24 h prior
to treatment. GL-PP was added to the culture at a final
concentration of 50, 100, 200, 400 or 800 µg/ml and cells were
incubated for a further 24, 48 or 72 h. Wells that were not treated
with GL-PP were used as negative controls. The CCK-8 reagent was
then added to the cultures according to manufacturer's instructions
and absorbance was measured at 450 nm using an ELISA MK3 microplate
reader (Thermo Fisher Scientific Inc., Waltham, MA, USA). All
measurements were performed in triplicate.
Cell cycle assay
U251 cells (2.5×104 cells/ml) were plated
onto a 6-well plate and harvested at 48 h following treatment.
Cells were fixed in 70% ice-cold ethanol, washed with ice cold PBS
and stained with 50 mg/ml propidium iodide in the presence of 50
mg/ml RNase A for 30 min. Staining procedures were performed under
low light at room temperature. Analyses were performed using a flow
cytometer (Cytomics FC 500; Beckman Coulter Inc., Brea, CA, USA).
Cell proportions in sub-G1, G0/G1,
S and G2/M phases were analyzed using ModFit LT software
(version 2.0; Verity Software House, Topsham, ME, USA). Tests were
performed in triplicate for each sample.
Western blotting
A 6-well cell culture plate was prepared as
aforementioned. Total protein was purified from cells using a cell
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
containing 1 mM phenylmethylsulfonyl fluoride. Protein
concentrations were subsequently measured using a BCA kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. Samples (150 µg protein/lane) were then separated on
12% SDS-PAGE gel and transferred onto a nitrocellulose membrane.
β-actin (Cell Signaling Technology Inc., Danvers, MA, USA) was used
as a loading control. The membrane was blocked with 5% bovine serum
albumin (Sangon Biotech, Shanghai, China) at room temperature for 2
h and incubated overnight at 4°C with a rabbit polyclonal
anti-active caspase-3 antibody (1:1,000, cat. no. ab2302; Abcam,
Cambridge, UK). The membrane was then incubated with a horseradish
peroxidase conjugated goat anti-rabbit immunoglobulin G (1:2,000,
cat. no. sc-2004; Santa Cruz Biotechnology Inc., Dallas, TX, USA)
at room temperature for 2 h. Protein expression was detected using
a Chemiluminescent HRP Substrate (EMD Millipore, Billerica, MA,
USA) and exposed on X-ray film. The optical density of each band
was determined using Quantity One software (version 4.6.1; Bio-Rad
Laboratories Inc., Hercules, CA, USA). The expression of cleaved
caspase-3 was expressed as a ratio to β-actin used as an internal
control. Tests were performed in triplicate for each sample.
Caspase-3 activity assay
The activity of caspase-3 was determined using a
Caspase 3 Activity Assay kit (Beyotime Institute of Biotechnology).
To evaluate the activity of caspase-3, cell lysates were prepared
following cell treatment with GL-PP for 48 h. Each 10 µl cell
lysate was incubated with 80 µl reaction buffer [1% NP-40, 20
mmol/l Tris-HCl (pH 7.5), 137 mmol/l NaCl, 10% glycerol] containing
10 µl caspase-3 substrate [2 mmol/l acetyl-Asp-Glu-Val-Asp
p-nitroanilide (Ac-DEVD-pNA)] at 37°C for 2 h. Caspase-3 can
catalyze the substrate Ac-DEVD-pNA to produce pNA. Thereafter,
absorbance of pNA at 405 nm was measured using an ELISA plate
reader (MK3; Thermo Fisher Scientific Inc.). The analysis procedure
followed the manufacturer's instructions. All experiments were
performed in triplicate.
Statistical analysis
The data were expressed as mean ± standard
deviation. Multigroup comparisons of the means were carried out by
one-way analysis of variance test with post hoc
Student-Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of GL-PP
GL-PP was determined to be a polysaccharide peptide
with a mean molecular weight (Mw) of 42,635 Da (Fig. 1), a purity of 96.89% and a
polysaccharide to peptide ratio of 88.70:11.30%. HILIC-ELSD data
indicated that polysaccharides were primarily composed of
l-arabinose, d-mannose and d-glucose at a molar ratio of
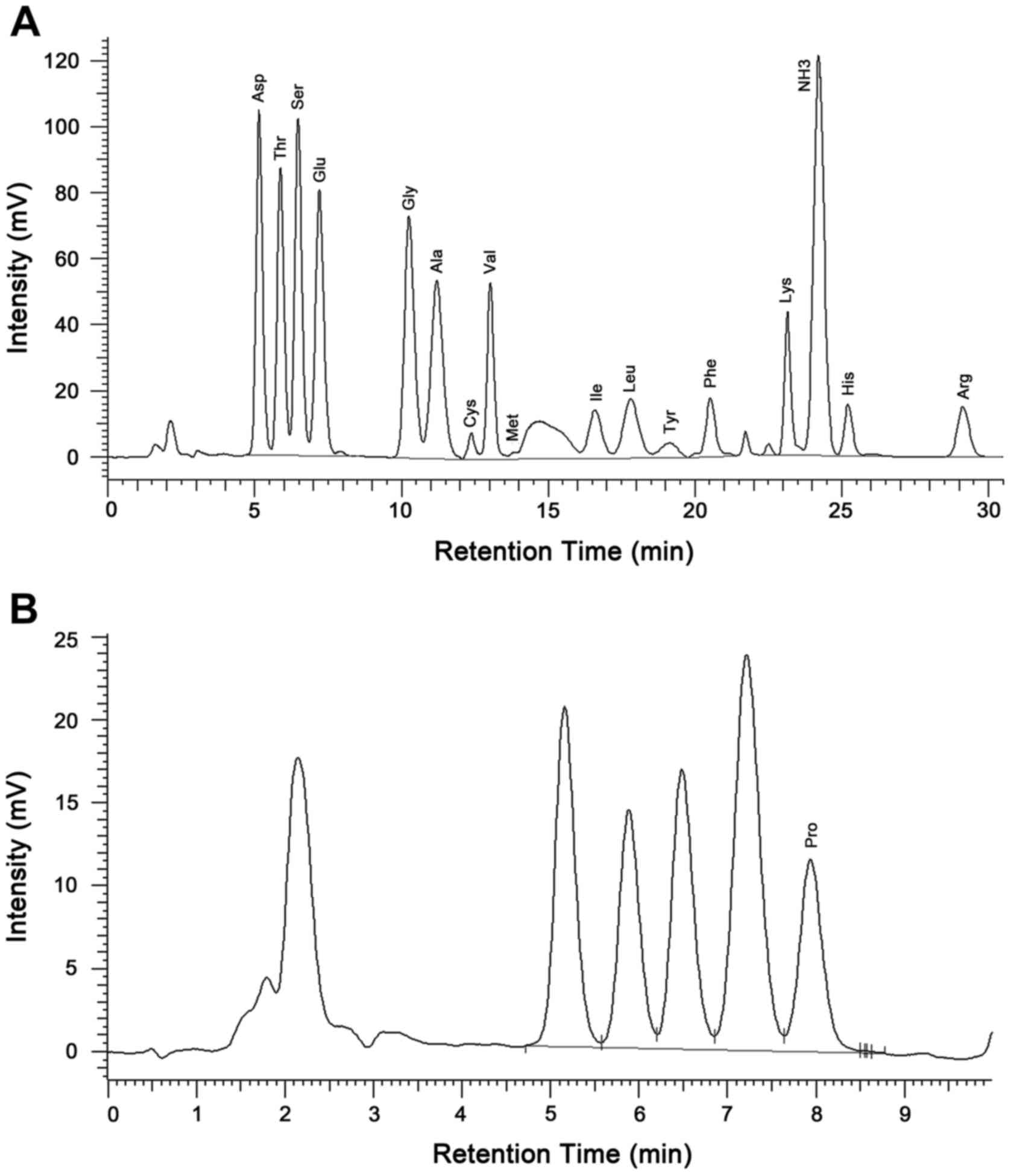
1.329:0.372:2.953 (Fig. 2). The amino
acid automatic analyzer indicated that the peptide contained 17
amino acids, including Asp, Thr, Ser, Glu, Gly, Ala, Cys, Val, Met,
Ile, Leu, Tyr, Phe, Lys, His, Arg and Pro, with a respective mass
ratio of:
1.45:1.08:1.12:1.45:0.84:0.87:0.19:0.64:0.01:0.38:0.56:0.23:0.47:0.58:0.30:0.53:0.60
(Table I and Fig. 3). No detectable level of endotoxin
(≤0.10 EU/ml) was identified in the GL-PP samples.
 | Table I.Amino acid comparisons of
Ganoderma lucidum polysaccharide peptide. |
Table I.
Amino acid comparisons of
Ganoderma lucidum polysaccharide peptide.
| Amino acid | Category | Contents (%) |
|---|
| Asparagine | N | 1.45 |
| Threonine | N | 1.08 |
| Serine | N | 1.12 |
| Glutamic acid | N | 1.45 |
| Glycine | N | 0.84 |
| Alanine | N | 0.87 |
| Cystine | N | 0.19 |
| Valine | E | 0.64 |
| Methionine | E | 0.01 |
| Isoleucine | E | 0.38 |
| Leucine | E | 0.56 |
| Tyrosine | N | 0.23 |
| Phenylalanine | E | 0.47 |
| Lysine | E | 0.58 |
| Histidine | N | 0.30 |
| Arginine | N | 0.53 |
| Proline | N | 0.60 |
| Total | – | 11.3 |
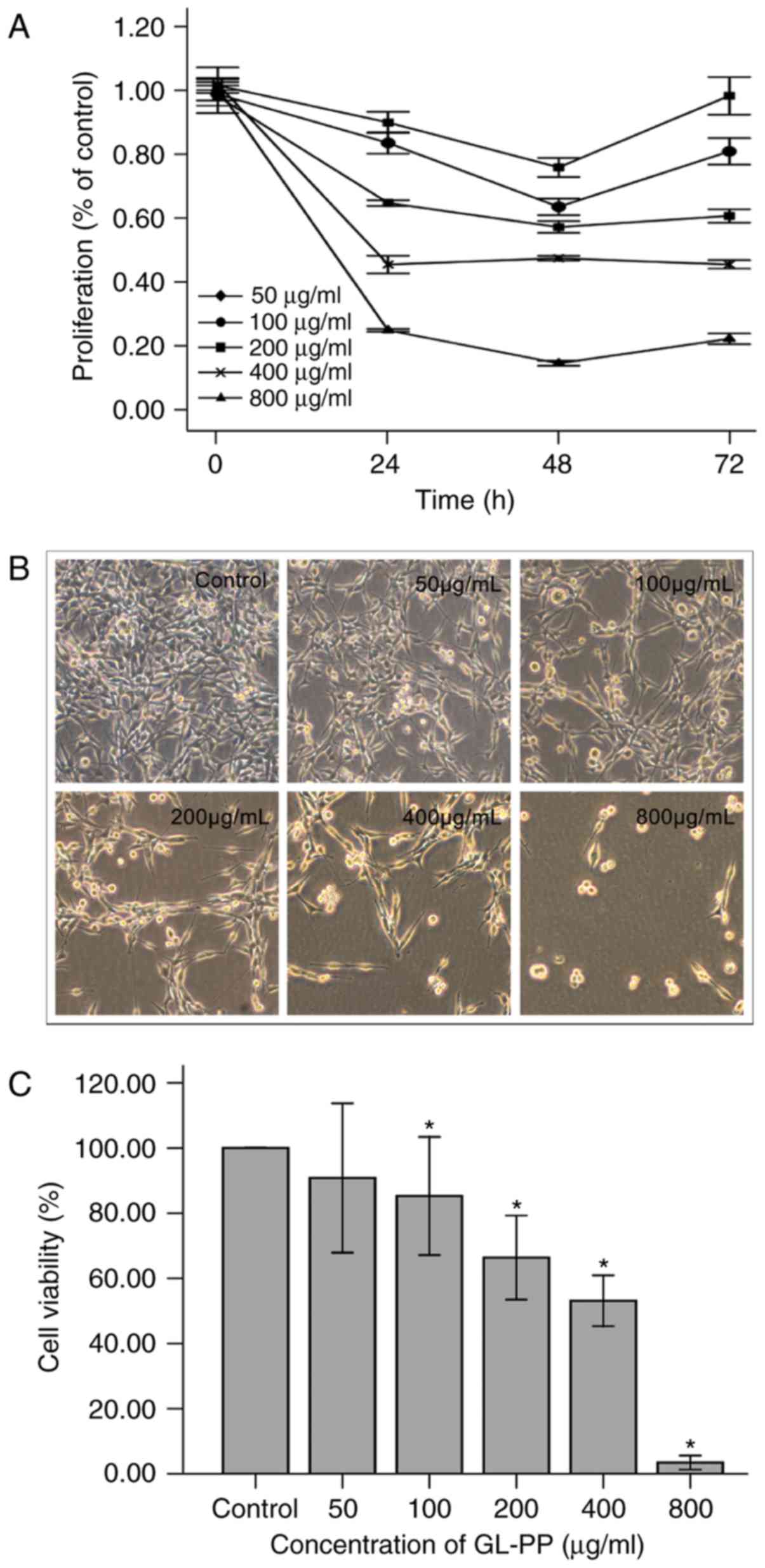
GL-PP inhibits the proliferation of
U251 cells
Cells were incubated with varying concentrations of
GL-PP (50, 100, 200, 400 and 800 µg/ml) for 24, 48 or 72 h. GL-PP
markedly inhibited the proliferation of U251 cells in a
dose-dependent manner compared with untreated controls. The maximum
inhibition observed at 48 h among all treatment groups except 400
µg/ml group (Fig. 4). The
half-maximal inhibitory concentration of GL-PP at 48 h was 274.1
µg/ml.
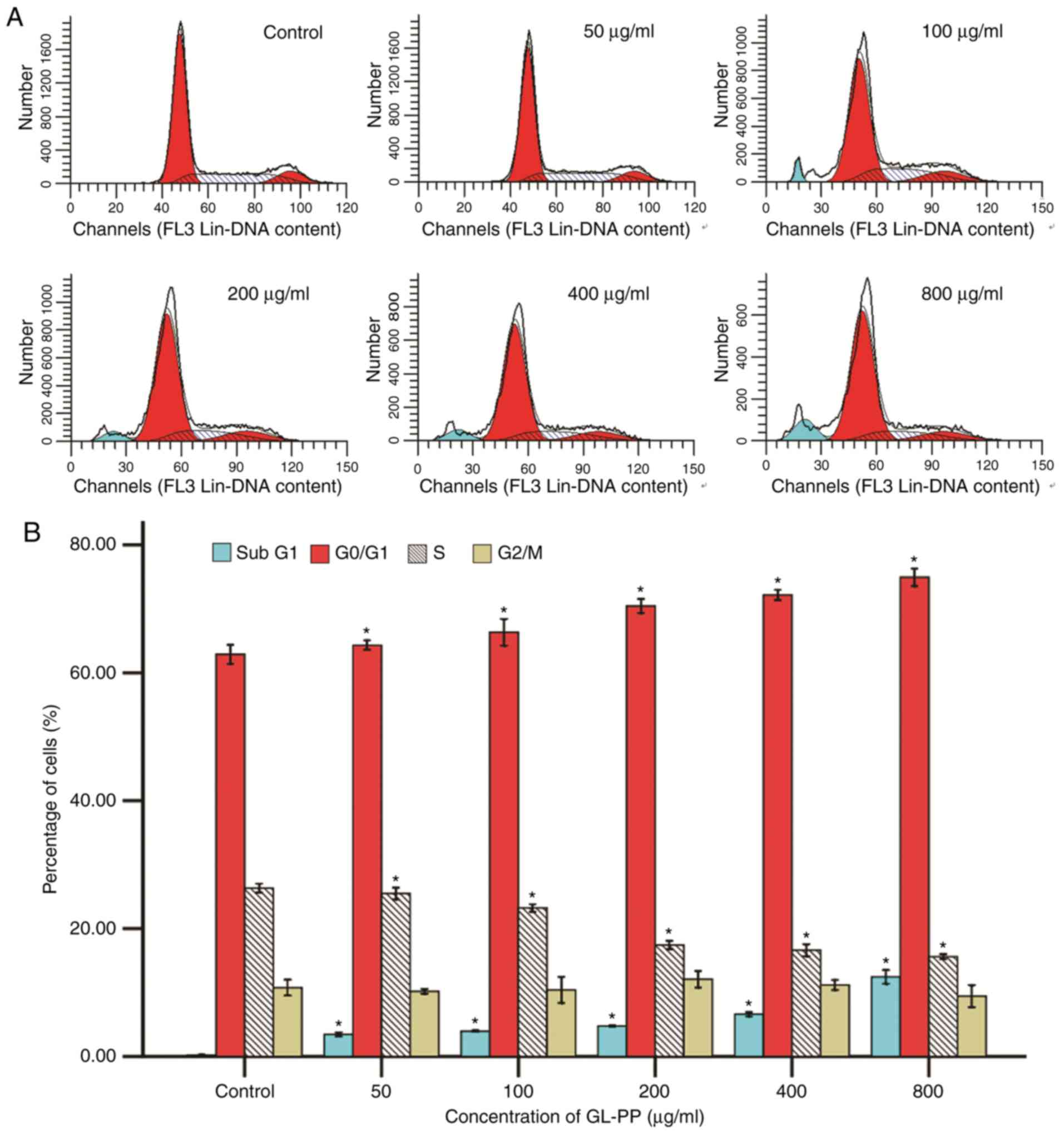
GL-PP blocks cell cycle progression at
the G0/G1 phase and induces apoptosis
To examine how GL-PP inhibited cell proliferation
and viability, flow cytometry was utilized to determine the effect
of GL-PP on cell cycle progression. Results revealed that the
presence of G0/G1 phase cells was increased from 62.9 to 74.94% at
48 h with increasing concentrations of GL-PP among all groups. This
increase was accompanied by a significant decrease in the
percentage of S phase cells, whereas the fraction of
G2/M phase cells was primarily unchanged. The
sub-G1 phase cell population, which indicate the number
of late-stage apoptotic cells, also increased significantly in
GL-PP treated cells in a dose-dependent manner (Fig. 5). This result demonstrated that GL-PP
induced G0/G1 phase arrest and apoptosis
induction.
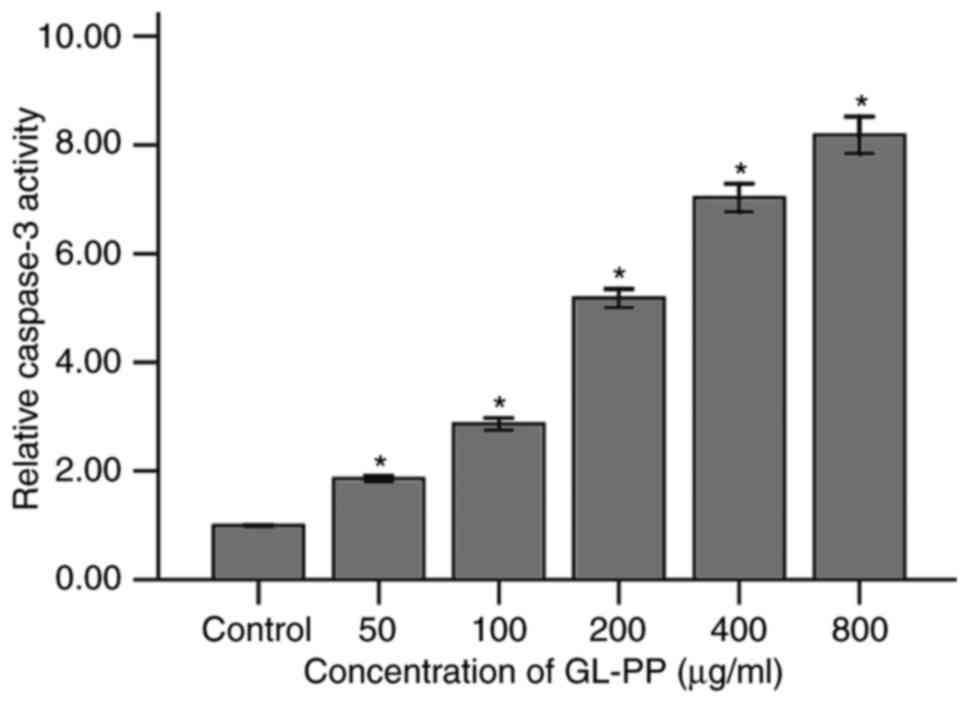
GL-PP induces apoptosis specific
caspase-3 activity in U251 cells
To examine the induction of apoptosis by GL-PP, the
activation of caspase-3, a primary enzyme in cell apoptosis, was
assessed. The expression of active caspase-3 in GL-PP-treated U251
cells was determined using western blotting. The active caspase-3
level was increased in a dose-dependent manner following treatment
with 50, 100, 200, 400 or 800 µg/ml GL-PP for 48 h (Fig. 6). In addition, the activation of
caspase-3 was analyzed by measuring the catalytic capability to the
substrate Ac-DEVD-pNA. The result showed the activation of
caspase-3 significantly increased in GL-PP treated groups in a
dose-dependent manner (Fig. 7). This
agrees with the result obtained by western blot analysis (Fig. 6).
Discussion
GL-PS and G. lucidum glycopeptides have been
identified as potential natural sources of anticancer compounds
(38). The present study identified,
purified and tested the anti-glioma activity of a novel
glycopeptide from grass-cultured G. lucidum, GL-PP, in
vitro. GL-PP strongly inhibited human U251 glioma cell
proliferation in a dose-dependent manner through cell cycle arrest
and induction of apoptosis.
The sustainment of proliferative signaling and the
resistance to apoptosis are two hallmarks of cancer (39). In the present study, human U251 glioma
cells treated with GL-PP, exhibited G0/G1
cell cycle arrest and inhibited proliferation. Furthermore, the
proportion of hypoploid cells (sub-G1) and the activity
of caspase-3 markedly increased, indicating that GL-PP induced U251
glioma cell apoptosis.
Although the antitumor activity of GL-PS/GL-PP has
been demonstrated in previous studies (8,10,14,38,40,41),
whether GL-PS/GL-PP directly inhibits cancer cell proliferation or
induces cellular apoptosis remains unknown. A study by Cao et
al (31) indicated that GL-PP
inhibited human lung carcinoma cell migration in a dose-dependent
manner by inhibiting the expression and activity of matrix
metalloproteinase-9. However, no inhibitory effect on cellular
proliferation was identified. Li et al (42) also demonstrated that GL-PS had no
cytotoxic effect on human prostate carcinoma PC-3M cells. In
addition, a study by Oliveira et al (32) revealed that polysaccharide extracts
from G. lucidum exhibited no cytotoxic activity, as all
tumor cell lines (AGS, MCF-7, NCI-H460 and HCT-15) exhibited a
concentration causing a 50% reduction in proliferation >400
µg/ml. However, other studies have demonstrated that GL-PS/GL-PP
significantly inhibited the proliferation of certain types of
cancer cell in vitro, including breast cancer (14), ovarian cancer (15), hepatoma (11) and colorectal adenocarcinoma cells
(11). The findings of the present
study are similar to these results, but concern glioma cells.
The apparent discrepancies of previous studies on
the anti-proliferative effects of GL-PS/GL-PP may be due to the
variability in the chemical constituents of GL-PS/GL-PP and the
different origins of the cancer cell lines utilized. It has been
determined that the biological activities of polysaccharides are
associated with their chemical conformation, molecular weight,
chemical modification and content of bound protein (43–46). For
example, the native triple-helix conformation with β-(1–3) linkages
in the primary glucan chain and additional β-(1–6) branch
points of polysaccharides are considered to be necessary for
antitumor activity (47).
Furthermore, analysis performed by Yeh et al (9) compared de-proteinized polysaccharides
with ling zhi-8 (LZ-8) and determined that LZ-8 activates murine
macrophages and T lymphocytes, but a de-proteinized polysaccharide
only acts as a macrophage activator, indicating that retaining
proteins and the natural complex of polysaccharides may serve a
vital role in the biological activities that they exhibit.
The GL-PP used in the present study was isolated
from G. lucidum and cultivated with JUNCAO grasses.
According to a previous study (48),
the glycopeptides obtained from grass-cultured G. lucidum
(GLPG) have a similar molecular weight, monosaccharide constituent
and amino acid composition to those obtained from wood-log-cultured
G. lucidum (GLPW), which are each primarily attached by
β-glycosidic linkages. GLPG has a higher purification rate (2.8
times that of GLPW) and is more environmentally friendly than GLPW,
which relies on a large consumption of forest resources. Compared
with previous studies (15,17,26), the
GL-PP extract utilized in the present study has a smaller molecular
weight (42,635 kDa), contained fewer monosaccharides and exhibited
a higher proportion of peptide (11.3%). Only glucose, mannose and
arabinose were detected in the GL-PP isolated in the current study.
The other monosaccharides often present in GL-PS/GL-PP extracts,
including galactose, rhamnose, fucose, xylose (15,17,26), were
not detected. In addition, 17 different amino acids were detected
in the GL-PP extract utilized in the present study, including
tyrosine, which has not been detected in GL-PS/GL-PP in previous
studies. These differences may be partly due to the different
isolation procedures and detection methods used, and may ultimately
serve a role in the unique biological activities identified in the
present study.
Although the present study determined the molecular
weight, and monosaccharide and amino acid composition of GL-PP, to
clearly ascribe the anti-proliferative effects to its chemical
structure, a more thorough characterization is required.
Elucidation of the molecular mechanisms and signaling pathways
involved in GL-PP anti-glioma activity should also be performed in
future studies. Additionally, although the direct anti-glioma
activity of GL-PP has been observed in vitro, it may be
limited by a number of elements in vivo, including
biotransformation and the blood-brain barrier. Addressing these
limitations may clarify the immunomodulatory activities of GL-PP
and its inhibitory role in glioma growth in vivo.
In conclusion, the present study demonstrated that
the GL-PP isolated from the G. lucidum cultivated with
Juncao grasses demonstrates a potent inhibitive capacity on human
U251 glioma cell lines. The demonstrated in vitro
anti-glioma activity of GL-PP was determined to be dose-dependent,
and mediated by cell cycle arrest and induction of apoptosis.
Therefore, GL-PP may represent a natural addition to the treatment
regimen of patients with glioma.
Acknowledgements
The present study was supported by the China
National Engineering Research Center of JUNCAO Technology (grant
no. JCJJ14008). The authors would like to thank Saizhen Wang, the
Senior Engineer from the Fuzhou Institute of Green Valley Bio-Pharm
Technology, for her technical assistance in the preparation of
GL-PP. The authors would also like to thank the Central Laboratory
of Fujian Academy of Agricultural Science for performing amino acid
analyses.
References
|
1
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song YH, Sun H, Zhang AH, Yan GL, Han Y
and Wang XJ: Plant-derived natural products as leads to anti-cancer
drugs. J Med plant Herbal Ther Res. 2:6–15. 2014.
|
|
4
|
Habijanic J, Berovic M, Boh B, Plankl M
and Wraber B: Submerged cultivation of Ganoderma lucidum and the
effects of its polysaccharides on the production of human cytokines
TNF-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. N Biotechnol.
32:85–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lai CY, Hung JT, Lin HH, Yu AL, Chen SH,
Tsai YC, Shao LE, Yang WB and Yu J: Immunomodulatory and adjuvant
activities of a polysaccharide extract of Ganoderma lucidum in vivo
and in vitro. Vaccine. 28:4945–4954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng J, Hu X, Shan F, Hua H, Lu C, Wang E
and Liang Z: Analysis of maturation of murine dendritic cells (DCs)
induced by purified Ganoderma lucidum polysaccharides (GLPs). Int J
Biol Macromol. 49:693–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Y, Cai D, Wang X and Liu X:
Immunomodulatory effect of Ganoderma lucidum polysaccharides (GLP)
on long-term heavy-load exercising mice. Int J Vitam Nutr Res.
82:383–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Z, Chen X, Zhong Z, Chen L and Wang Y:
Ganoderma lucidum polysaccharides: Immunomodulation and potential
anti-tumor activities. Am J Chin Med. 39:15–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeh CH, Chen HC, Yang JJ, Chuang WI and
Sheu F: Polysaccharides PS-G and protein LZ-8 from Reishi
(Ganoderma lucidum) exhibit diverse functions in regulating murine
macrophages and T lymphocytes. J Agric Food Chem. 58:8535–8544.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Nie S, Huang D, Feng Y and Xie M:
A novel polysaccharide from Ganoderma atrum exerts antitumor
activity by activating mitochondria-mediated apoptotic pathway and
boosting the immune system. J Agric Food Chem. 62:1581–1589. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, Gao H, Chan E, Tang W, Xu A, Yang
H, Huang M, Lan J, Li X, Duan W, et al: Antitumor activity and
underlying mechanisms of ganopoly, the refined polysaccharides
extracted from Ganoderma lucidum, in mice. Immunol Invest.
34:171–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joseph S, Sabulal B, George V, Antony KR
and Janardhanan KK: Antitumor and anti-inflammatory activities of
polysaccharides isolated from Ganoderma lucidum. Acta Pharm.
61:335–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang CJ, Lee CW, Sung HC, Chen YH, Chiang
YC, Hsu HY, Tseng YC, Li CY, Wang SH and Chen YL: Ganoderma lucidum
polysaccharides reduce lipopolysaccharide-induced interleukin-1 β
expression in cultured smooth muscle cells and in thoracic aortas
in mice. Evid Based Complement Alternat Med. 2014:3051492014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shang D, Li Y, Wang C, Wang X, Yu Z and Fu
X: A novel polysaccharide from Se-enriched Ganoderma lucidum
induces apoptosis of human breast cancer cells. Oncol Rep.
25:267–272. 2011.PubMed/NCBI
|
|
15
|
Shang D, Zhang J, Wen L, Li Y and Cui Q:
Preparation, characterization, and antiproliferative activities of
the Se-containing polysaccharide SeGLP-2B-1 from Se-enriched
Ganoderma lucidum. J Agric Food Chem. 57:7737–7742. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Zhang L, Yu Y and Cheung PC:
Enhancement of antitumor activities in sulfated and
carboxymethylated polysaccharides of Ganoderma lucidum. J Agric
Food Chem. 57:10565–10572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao QZ and Lin ZB: Antitumor and
anti-angiogenic activity of Ganoderma lucidum polysaccharides
peptide. Acta Pharmacol Sin. 25:833–838. 2004.PubMed/NCBI
|
|
18
|
Cao QZ and Lin ZB: Ganoderma lucidum
polysaccharides peptide inhibits the growth of vascular endothelial
cell and the induction of VEGF in human lung cancer cell. Life Sci.
78:1457–1463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kan Y, Chen T, Wu Y and Wu J and Wu J:
Antioxidant activity of polysaccharide extracted from Ganoderma
lucidum using response surface methodology. Int J Biol Macromol.
72:151–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu W, Wang H, Pang X, Yao W and Gao X:
Characterization and antioxidant activity of two
low-molecular-weight polysaccharides purified from the fruiting
bodies of Ganoderma lucidum. Int J Biol Macromol. 46:451–457. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Q, Wang S, Xie Y, Sun J and Wang J:
HPLC analysis of Ganoderma lucidum polysaccharides and its effect
on antioxidant enzymes activity and Bax, Bcl-2 expression. Int J
Biol Macromol. 46:167–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao W, Jiang X, Deng W, Lai Y, Wu M and
Zhang Z: Antioxidant activities of Ganoderma lucidum
polysaccharides and their role on DNA damage in mice induced by
cobalt-60 gamma-irradiation. Food Chem Toxicol. 50:303–309. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang Z, Yi Y, Guo Y, Wang R, Hu Q and
Xiong X: Chemical characterization and antitumor activities of
polysaccharide extracted from Ganoderma lucidum. Int J Mol Sci.
15:9103–9116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang SS, Zhou D, Meng GL, Wu F, Wang S,
Chen X and Xu JL: Effect of Ganoderma lucidum polysaccharides on
oxidative stress of hyperlipidemic fatty liver in rats. Zhongguo
Zhong Yao Za Zhi. 37:3102–3106. 2012.(In Chinese). PubMed/NCBI
|
|
25
|
Zhang GL, Wang YH, Ni W, Teng HL and Lin
ZB: Hepatoprotective role of Ganoderma lucidum polysaccharide
against BCG-induced immune liver injury in mice. World J
Gastroenterol. 8:728–733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun LX, Lin ZB, Duan XS, Lu J, Ge ZH, Li
XJ, Li M, Xing EH, Jia J, Lan TF and Li WD: Ganoderma lucidum
polysaccharides antagonize the suppression on lymphocytes induced
by culture supernatants of B16F10 melanoma cells. J Pharm
Pharmacol. 63:725–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun LX, Lin ZB, Li XJ, Li M, Lu J, Duan
XS, Ge ZH, Song YX, Xing EH and Li WD: Promoting effects of
Ganoderma lucidum polysaccharides on B16F10 cells to activate
lymphocytes. Basic Clin Pharmacol Toxicol. 108:149–154. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang P, Ding R, Jiang S, Ji L, Pan M, Liu
L, Zhang W, Gao X, Huang W, Zhang G, et al: The adjuvanticity of
Ganoderma lucidum polysaccharide for Newcastle disease vaccine. Int
J Biol Macromol. 65:431–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu XL, Chen AF and Lin ZB: Ganoderma
lucidum polysaccharides enhance the function of immunological
effector cells in immunosuppressed mice. J Ethnopharmacol.
111:219–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng S, Jia Y, Zhao J, Wei Q and Liu Y:
Ganoderma lucidum polysaccharides eradicates the blocking effect of
fibrinogen on NK cytotoxicity against melanoma cells. Oncol Lett.
3:613–616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao QZ, Lin SQ and Wang SZ: Effect of
Ganoderma lucidum polysaccharides peptide on invasion of human lung
carcinoma cells in vitro. Beijing Da Xue Xue Bao Yi Xue Ban.
39:653–656. 2007.(In Chinese). PubMed/NCBI
|
|
32
|
Oliveira M, Reis FS, Sousa D, Tavares C,
Lima RT, Ferreira IC, dos Santos T and Vasconcelos MH: A methanolic
extract of Ganoderma lucidum fruiting body inhibits the growth of a
gastric cancer cell line and affects cellular autophagy and cell
cycle. Food Funct. 5:1389–1394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun LX, Lin ZB, Duan XS, Lu J, Ge ZH, Li
M, Xing EH, Lan TF, Jiang MM, Yang N and Li WD: Ganoderma lucidum
polysaccharides counteract inhibition on CD71 and FasL expression
by culture supernatant of B16F10 cells upon lymphocyte activation.
Exp Ther Med. 5:1117–1122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang PY, Zhu XL and Lin ZB: Antitumor and
immunomodulatory effects of polysaccharides from broken-spore of
Ganoderma lucidum. Front Pharmacol. 3:1352012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun LX, Chen LH, Lin ZB, Qin Y, Zhang JQ,
Yang J, Ma J, Ye T and Li WD: Effects of Ganoderma lucidum
polysaccharides on IEC-6 cell proliferation, migration and
morphology of differentiation benefiting intestinal epithelium
healing in vitro. J Pharm Pharmacol. 63:1595–1603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang SZ, Lin ZX, Lin DM, Lin SQ, Lin ZB
and Li J: Structure of polysaccharide peptide GL-PPS from Ganoderma
lucidum. Southwest China J Agricultural Sci. 28:793–796. 2015.
|
|
37
|
Gong Y, Huang Y, Gao L, Lu J, Hu Y, Xia L
and Huang H: Chinese standard GB/T 5009. 124-2003. Inspection of
grain and oilseeds: Method for determination of amino acids in
foods. Standards press of China, Beijing, China, 2003. J Food Nut
Res. 1:108–112. 2013.
|
|
38
|
Boh B: Ganoderma lucidum: A potential for
biotechnological production of anti-cancer and immunomodulatory
drugs. Recent Pat Anticancer Drug Discov. 8:255–287. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu YJ, Shen J, Xia YM, Zhang J and Park
HS: The polysaccharides from Ganoderma lucidum: Are they always
inhibitors on human hepatocarcinoma cells? Carbohydr Polym.
90:1210–1215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen J, Park HS, Xia YM, Kim GS and Cui
SW: The polysaccharides from fermented Ganoderma lucidum mycelia
induced miRNAs regulation in suppressed HepG2 cells. Carbohydr
Polym. 103:319–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li YB, Li YH, Wang R, Lin ZB and Li ZJ:
Efect of Ganoderma lucidumpolysaccharides (GlPS) on
tumor-endothelium interactions. Chin Pharmacol Bull. 39:250–253.
2008.
|
|
43
|
Chen X, Xu X, Zhang L and Zeng F: Chain
conformation and anti-tumor activities of phosphorylated
(1→3)-β-d-glucan from Poria cocos. Carbohydr Polymers. 78:581–587.
2009. View Article : Google Scholar
|
|
44
|
Surenjav U, Zhang L, Xu X, Zhang X and
Zeng F: Effects of molecular structure on antitumor activities of
(1→3)-β-d-glucans from different Lentinus Edodes. Carbohydr
Polymers. 63:97–104. 2006. View Article : Google Scholar
|
|
45
|
Wang Y, Zhang L, Li Y, Hou X and Zeng F:
Correlation of structure to antitumor activities of five
derivatives of a beta-glucan from Poria cocos sclerotium. Carbohydr
Res. 339:2567–2574. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang L, Li X, Xu X and Zeng F:
Correlation between antitumor activity, molecular weight, and
conformation of lentinan. Carbohydr Res. 340:1515–1521. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wasser SP: Medicinal mushrooms as a source
of antitumor and immunomodulating polysaccharides. Appl Microbiol
Biotechnol. 60:258–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin SQ, Wang SZ, Lin ZB and Lin YX:
Isolation and identification of active components of Ganoderma
lucidum cultivated with grassand wood log I. Extraction,
purification and characterization of glycopeptide. Chin Tradit
Herbal Drugs. 34:872–874. 2003.
|