Introduction
As the most prevalent gynecologic malignancy,
endometrial cancer (EC) is increasingly populated in the world,
especially in the United States (1).
According to associated report, EC can be classified into type I
and II tumors in general (2).
Moreover, with regard to different disease stages, the therapies of
EC are various. At the early stage of most EC patients, only
surgery is considered as the effective treatment method (3). As a result of tumor recurrence and
metastasis, even under surgical resection plus systemic
chemotherapy, the prognosis is still at a poor level for the newly
diagnosed EC patients. Therefore, it is significant to explore
novel therapies and elucidate the underlying molecular
mechanism.
Cannabis sativa plant has been exploited to provide
recreational and medicinal usage from the very beginning (4). It consists of over 60 kinds of
cannabinoids, who exert a wide spectrum of psycho-active and
immuno-active effects. ∆9-tetrahydrocannabinol (THC) is
known to be the most active constituent of cannabinoids (5). Cannabinoids exert most of their actions
by binding to specific Gαi protein-coupled receptors,
CB1 receptor (central-type receptor) (6) and CB2 receptor
(peripheral-type receptor) (7),
respectively. CB1 receptor is widely distributed in the
central nervous system where they mediate psychoactive effects
although it has also been detected in reproductive organs such as
uterus and testis (8). CB2
receptor mainly subjects to specific constituent of the immune
system (9).
It is well-known that cannabinoids can act as
anti-inflammatory agents and suppress the antitumor immune
response. Accordingly, THC has been employed in the field of cancer
research recently. Several preclinical studies suggest THC shows
anti-cancer performance in vitro against breast cancer, lung
carcinoma, skin carcinoma, pancreatic cancer and prostate carcinoma
(10), while researches seldom relate
to EC.
In the present study, we analyze the expression of
CB1 and CB2 receptors in human EC patient
samples over their normal counterparts. We further analyze the
biological consequences of THC on aggressive human EC cell lines
in vitro. Moreover, we also determine the mechanisms of THC
that regulate tumor growth and migration of EC. These results shed
light on the mechanisms and pathways by which EC occurs and
develops, providing evidence that THC prevented EC growth and
metastasis through inhibiting epithelial-mesenchymal transition
(EMT) and matrix metalloproteinase-9 (MMP-9) signaling pathway.
Materials and methods
Patients and samples collection
Tissue samples were obtained from 6 Chinese patients
who underwent surgical resection for primary EC and para-tumor
normal endometrial tissues at The Second Affiliated Hospital of
Zhejiang University (Hangzhou, China) between 2014 and 2016. None
of the patients had received preoperative treatments such as
irradiation or chemotherapy. Written informed consent was obtained
from all patients and the study was approved by The Second
Affiliated Hospital of Zhejiang University ethics committee. Small
pieces (~0.5 cm3) were cut and washed briefly in sterile
PBS to remove blood contamination. All the samples were frozen
within 20 min of delivery and stored in liquid nitrogen for western
blotting and quantitative PCR analysis. The clinical pathological
data of all patients are summarized in Table I.
 | Table I.Clinical characteristics of all
patients. |
Table I.
Clinical characteristics of all
patients.
| Demographics | N=6 |
|---|
| Age (mean ±
SD) |
59.2±8.23 |
| BMI (mean ±
SD) | 30.62±5.82 |
| FIGO |
|
| IA | 3 |
| IB | 3 |
Drugs
Δ9-tetrahydrocannabinol (THC, 10 mg/ml in
ethanol) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany) and store at −20°C.
RNA isolation and RT-qPCR
Briefly, total cellular RNA was extracted using
TRIzol® (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) following the supplier's instructions. cDNA was generated
using 1 mg total RNA and a QuantiTect Reverse Transcription kit
(Qiagen, Berlin, Germany). qPCR was performed using SYBR-Green
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) methods. The primer
sequences for qPCR analysis are shown in Table II.
 | Table II.Gene primers for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table II.
Gene primers for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer |
|---|
|
CB1R | F:
5′-TTACAACAAGTCTCTCTCGTCCT-3′ |
|
| R:
5′-GGCTGCCGATGAAGTGGTA-3′ |
|
CB2R | F:
5′-GGGTGACAGAGATAGCCAATGG-3′ |
|
| R:
5′-TGAACAGGTATGAGGGCTTCC-3′ |
| MMP-9 | F:
5′-GGGACGCAGACATCGTCATC-3′ |
|
| R:
5′-TCGTCATCGTCGAAATGGGC-3 |
| E-cadherin | F:
5′-AAAGGCCCATTTCCTAAAAACCT-3′ |
|
| R:
5′-TGCGTTCTCTATCCAGAGGCT-3′ |
| N-cadherin | F:
5′-TCAGGCGTCTGTAGAGGCTT-3′ |
|
| R:
5′-ATGCACATCCTTCGATAAGACTG-3′ |
| Vimentin | F:
5′-TCCACACGCACCTACAGTCT-3′ |
|
| R:
5′-CCGAGGACCGGGTCACATA-3′ |
| β-actin | F:
5′-CCACACCCGCCACCAGTTCG-3′ |
|
| R:
5′-TACAGCCCGGGGAGCATCGT-3′ |
Western blot analysis
For analysis of CB1R, CB2R,
E-cadherin, N-cadherin, Vimentin (VIM), MMP-2, MMP-9 expressions,
tissues and cells were lysed in RIPA Lysis buffer, then homogenized
by vigorous mixing for 30 min on ice, and centrifuged at 12,000 × g
for 30 min. Total protein concentration was measured using the
bicinchoninic acid assay (Pierce; Thermo Fisher Scientific, Inc.,
Bonn, Germany). Proteins were separated on a 10% sodium dodecyl
sulfate polyacylamide gel. Following transfer to PVDF membrane and
blocking with 5% non-fat milk powder, blots were probed with
specific antibodies CB1R (1:1,000; Abcam, Cambridge,
UK), CB2R (1:1,000; Abcam), E-cadherin (1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), N-cadherin (1:1,000;
Cell Signaling Technology, Inc.), VIM (1:1,000; Cell Signaling
Technology, Inc.), MMP-2 (1:1,000; Abcam), MMP-9 (1:1,000; Abcam)
and GAPDH (1:5,000; Abcam) at 4°C overnight. Subsequently,
membranes were washed and incubated with anti-rabbit IgG (1:5,000;
Cell Signaling Technology, Inc.) or anti-mouse IgG (1:5,000; Cell
Signaling Technology, Inc.). Ultimately, proteins were visualized
using the enhanced chemiluminescence reagents (Thermo Fisher
Scientific, Inc.), and the relative expression of CB1R
and CB2R, protein levels were analyzed by densitometry
using the Image-J imaging analysis software (National Institutes of
Health, Bethesda, MD, USA).
ELISA
MMP-9 level was measured in supernatants from cells
treated with for 24 h. Protein levels in the supernatants were
assayed using a MMP-9 ELISA kit (R&D Systems, Inc.,
Minneapolis, MN, USA) following the manufacturer's instruction.
Optical density was measured at 450 nm. MMP-9 concentration was
calculated by comparing the data to the known standards for MMP-9
proteins.
Cell culture
The EC cell lines HEC-1B, and An3ca were obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). All cells were maintained in Dulbecco's modified Eagle's
medium (DMEM)-F12 (Gibco; Thermo Fisher Scientific, Inc., Auckland,
New Zealand) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere with 5%
CO2.
Cell proliferation assay
Cells were seeded in 96-well plates (2,000
cells/well) in 100 µl of DMEM-F12 medium. Then the medium was
changed to one that contained different dose of 0.1–20 µM THC for
24 h, and 20 µl MTS reagent (Promega Corporation, Madison, WI, USA)
was added to each well before incubation at 37°C for 2 h. The
absorbance at 450 nm was measured using a SpectraMax 190 microplate
reader (Bio-Rad Laboratories, Inc.).
Cell migration assay
Cells were suspended in serum-free DMEM-F12 medium
and plated at a density of 5×104 cells/well in transwell
chambers equipped with 8.0 µm pore polycarbonate membranes (Corning
Incorporated, Corning, NY, USA). Complete medium (800 µl) was added
to the lower chamber. After incubation for 16 h, fluorescent stain
(calcein-AM) was added to each chamber and incubated for 30 min.
Then, the cells that migrated to the basal side of the membrane
were counted at 100× magnification by fluorescence analysis (Nikon
Corporation, Tokyo, Japan).
Cell infection
Oligonucleotides for human MMP-9, CB1R,
CB2R siRNA kit were purchased from GenePharma (Shanghai,
China). The kit contains three predesigned duplexes targeting a
specific MMP-9 gene. Cells were transfected with MMP-9,
CB1R, CB2R siRNA or NC using the opti-MEM
plus X-treme GENE siRNA transfection reagent (Roche, Mannheim,
Germany) according to the instruction. Stably infected cells were
selected and processed for further analysis by western blotting.
After 48 h of post-transfection, western blot analyses were further
performed. For gene overexpression, the recombinant lentiviruses
carrying MMP-9 or control were obtained as gifts from colleague and
used according to the manufacturer's protocol. Briefly, HEC-1B
cells were seeded at 2×105 cells/well in a 6-well plate.
After adherent cells reached ~40% confluence, they were infected
with an LV-MMP-9 or a control supplemented with 8 µg/ml polybrene
(Sigma-Aldrich; Merck KGaA). Treated cells were selected with
puromycin to generate puromycin-resistant clones, which were
assayed by qPCR and western blotting.
Statistical analysis
All data were expressed as the mean ± the standard
error (SEM) and analyzed using the SPSS 19.0 statistical analysis
software (SPSS, Inc., Chicago, IL, USA). Statistical significance
was determined using an unpaired Student's t-test and one-way ANOVA
followed by Dunett's post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
The cannabinoid receptors are highly
expressed in EC tissues
Cannabinoid receptors are overexpressed in different
cancers, including skin, breast and lung cancers (11–13).
Nevertheless, so far as we are concerned that none work has been
indicated to present the expression of cannabinoid receptors in EC.
Therefore, we first investigated CB1R and
CB2R expressions in normal endometrium (6 samples) and
paired adjacent normal tissues (6 samples) using PCR and western
blot. These data indicated both CB1R and CB2R
were overexpressed in EC tissues compared with the normal
endometrium (Fig. 1A and B).
Moreover, the expressions of CB1R and CB2R
were positively correlated with VIM, the marker of mesenchymal
cells (Fig. 1A and C).
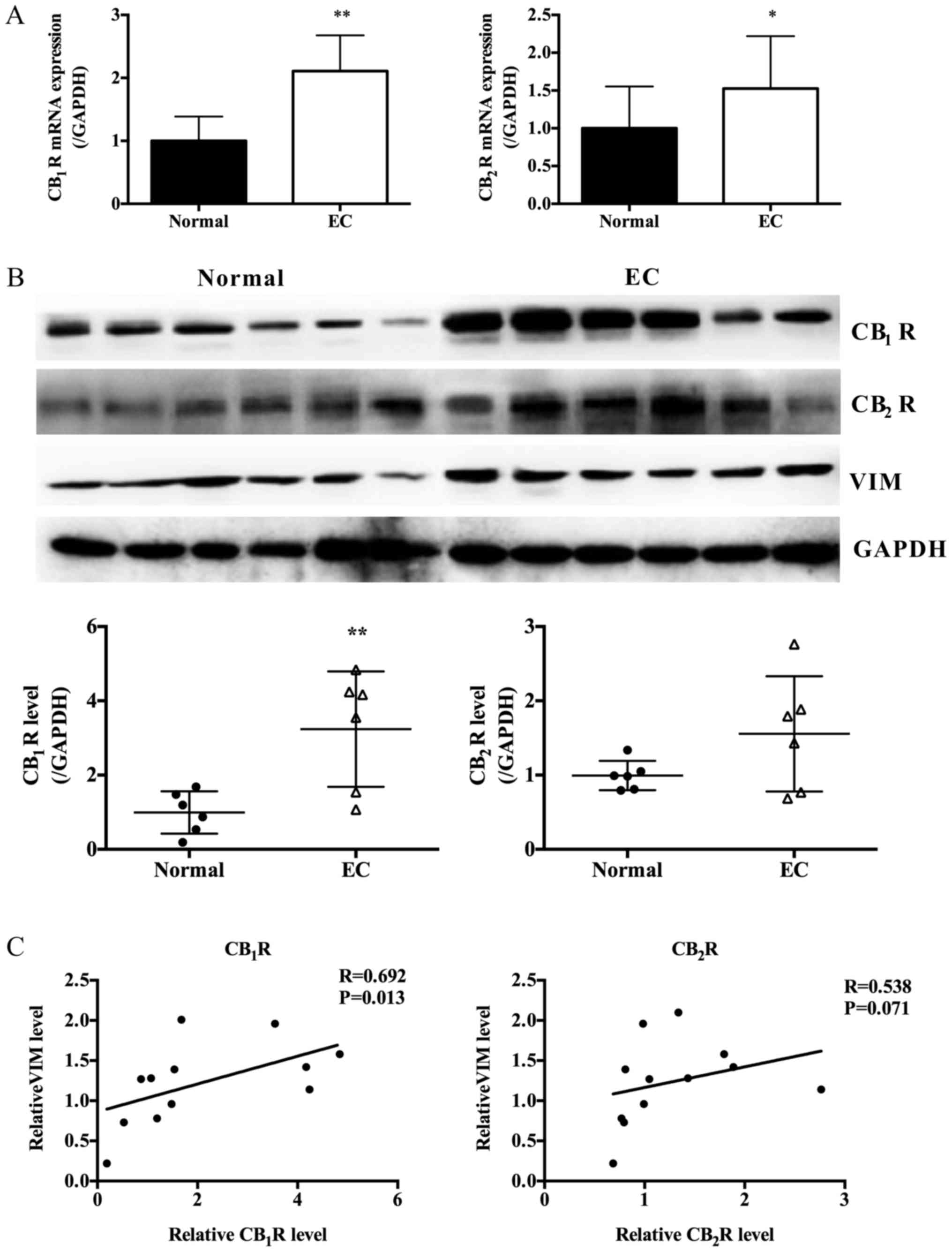 | Figure 1.Expression of CB1R,
CB2R and EMT marker in EC tissues and paired adjacent
normal tissues. (A) Relative CB1R and CB2R
mRNA expression in EC tissues and paired adjacent normal tissues
(n=6, Paired Student's t-test, *P<0.05, **P<0.01 vs. normal).
(B) Protein expression levels of CB1R, CB2R
and VIM in EC tissues and paired adjacent normal tissues as
determined by western blot analysis; GAPDH was included as an
internal control (n=6, Paired Student's t-test, **P<0.01). (C)
Expression correlations between CB1R or CB2R
and VIM, performed using the Spearman's correlation coefficient
test. VIM, vimentin; EMT, epithelial-mesenchymal transition; EC,
endometrial cancer. |
THC regulates EC cell proliferation
and migration
In the present study, the effect of THC on cell
proliferation was analyzed in EC cell lines HEC-1B and An3ca cells.
Fig. 2A showed that THC acted as a
concentration-dependent inhibitor of cell growth. Tumor growth and
metastasis are the leading causes of cancer-related mortality,
specifically, tumor cells can migrate from the site of primary
tumor and then invade neighboring tissues. To determine the role of
THC in EC progression, the transwell assay was designed to test
whether the treatment of THC altered the locomotive potential of
tumor cells. After 16 h of incubation, THC resulted in a
significant decrease in cell migration (Fig. 2B and C). In conclusion, THC obviously
suppressed the proliferation and migration capabilities of HEC-1B
and An3ca cells.
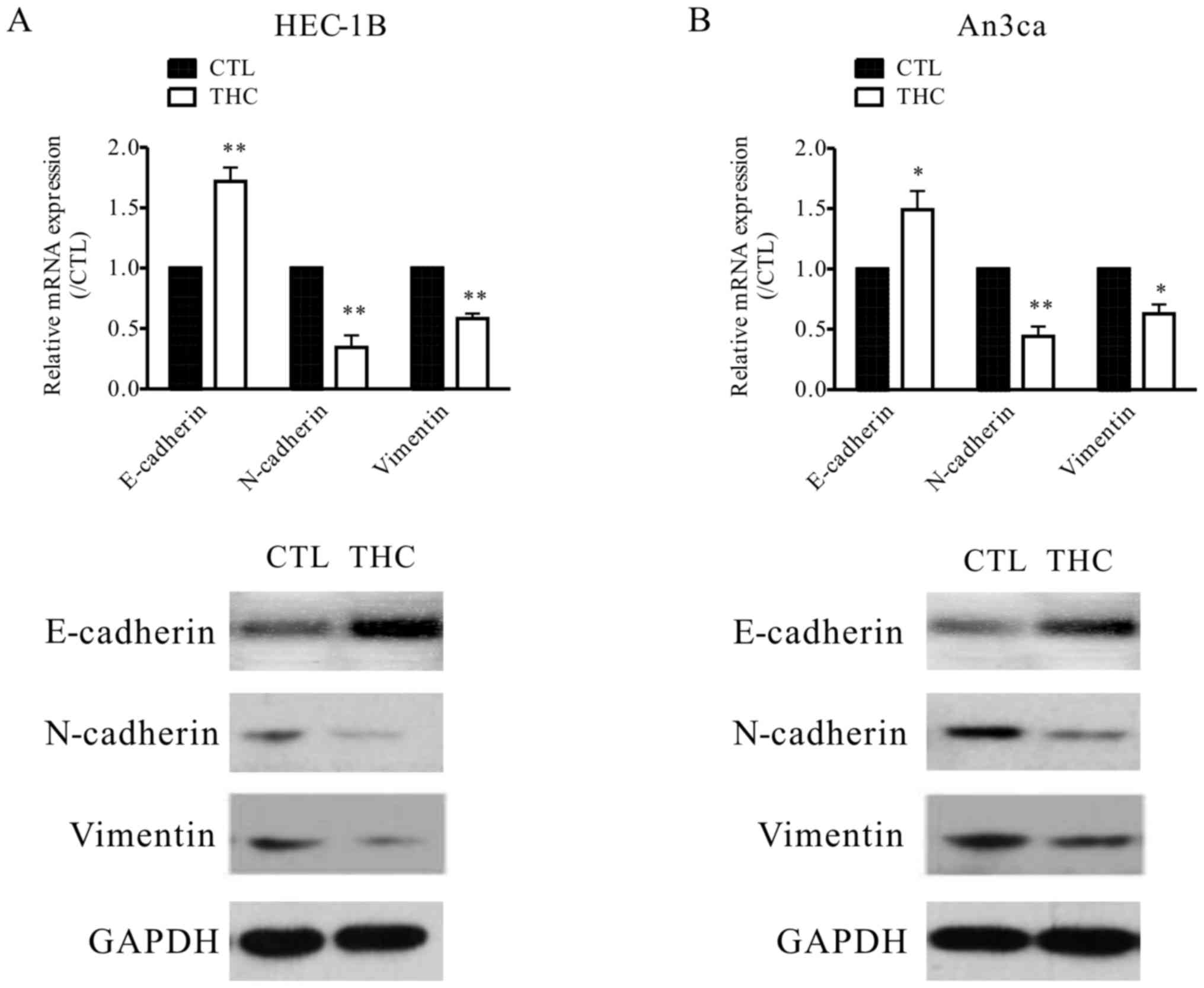
THC regulates the transition between
epithelial and mesenchymal phenotypes in EC cells
Tumor EMT, a key step for tumor progress, is always
defined as specific phenotypic and morphological alterations in
epithelial cancer cells, causing them to transform into mesenchymal
type cells during metastasis in many cancers (14). In tumor EMT, epithelial molecular
markers, including E-cadherin, β-catenin are downregulated, whereas
the levels of mesenchymal molecular markers, including N-cadherin,
VIM are upregulated (15), we also
get the similar result that VIM was elevated in EC tissues
(Fig. 1B). In our study, the
expressions of EMT protein markers were evaluated to explore the
potential relationship between THC and EMT. In the THC-treated
HEC-1B and An3ca cells, the level of epithelial cell marker
(E-cadherin) was increased, while the mesenchymal cell markers
(N-cadherin, and VIM) were both decreased, as determined by western
blot analysis and qPCR (Fig. 3A and
B) assay. This evidence suggested that THC plays crucial roles
in tumor EMT.
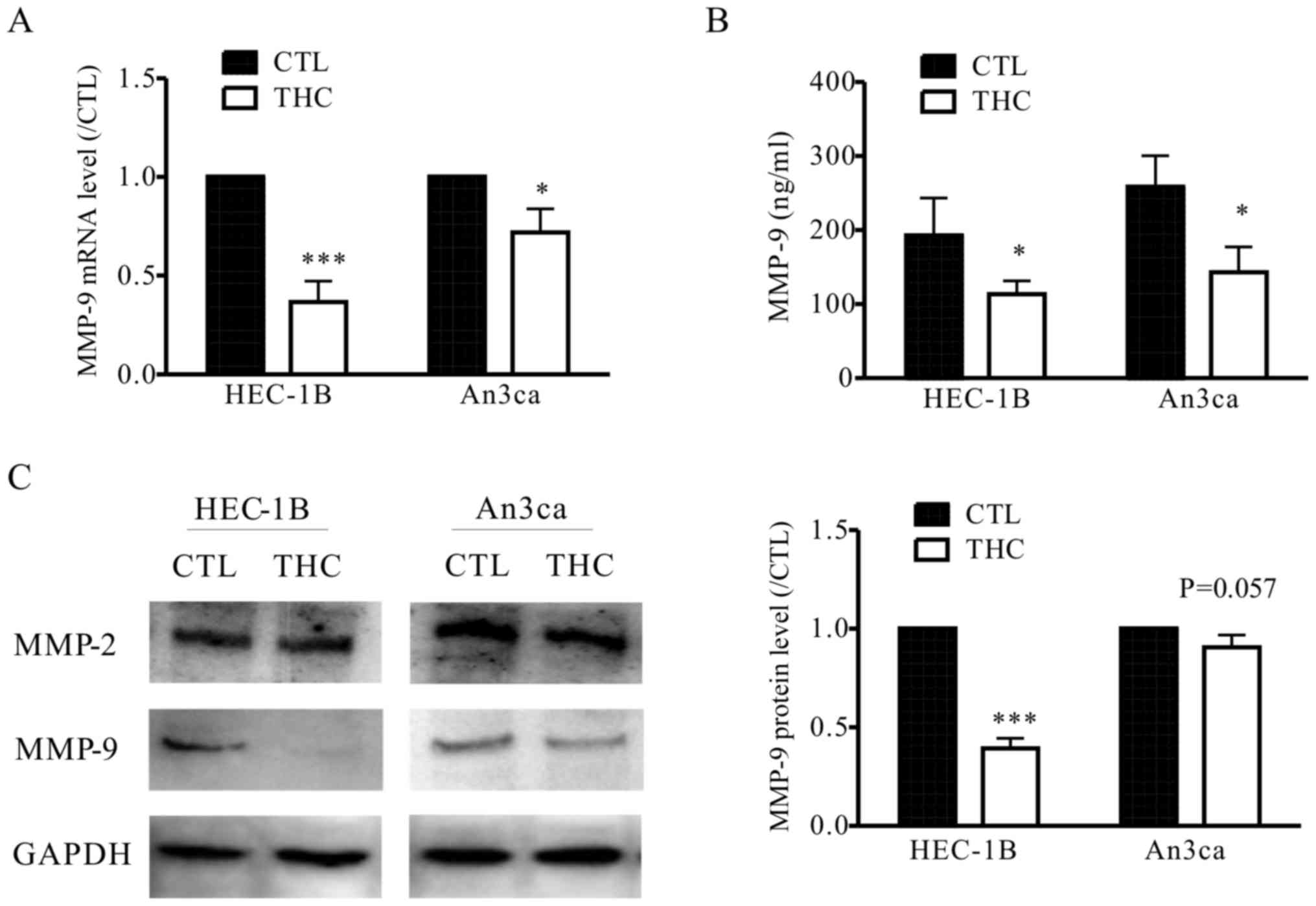
THC inhibits MMP-9 expression in EC
cells
To elucidate the mechanisms by which THC is engaged
in the metastasis of EC cells, we detected the level of MMPs after
treated with THC. MMPs are functionally related to tissue
remodeling processes, where MMP-2 and MMP-9 can be utilized as
catalytic in these processes in vivo. Recent researches have
been tried to demonstrate the molecular basis and pathophysiology
of EC, MMP-9 plays important roles in invasion and metastasis by
regulating the signaling pathways that control cell growth and
invasion (16). In our study, we also
observed THC significantly inhibited the secretion of MMP-9 in
HEC-1B and An3ca cells by qPCR, western blot and ELISA assay, while
the expression of MMP-2 was not significantly altered (Fig. 4A-C). This result supports the
hypothesis that THC regulates EMT and the EC cell metastasis is
mediated by MMP-9.
MMP-9 signaling pathway is involved in
THC-decreased cell migration
To further confirm that THC decreased EC cell
mobility through MMP-9 signaling pathway, siRNA was used to silence
the MMP-9 expression in HEC-1B and An3ca cells. The presences of
HEC-1B and An3ca cells with MMP-9 silencing was verified by qPCR
(Fig. 5A) and western blot analyses
(Fig. 5B). Furthermore, the
transfection with MMP-9 siRNA decreased the migration capacity of
HEC-1B and An3ca cells, as shown by the transwell assay (Fig. 5C and D). In addition, the lentivirus
of MMP-9 gene overexpression vector and control vector were
infected into HEC-1B cells. Stably infected cells were used to
perform cell migration assay (Fig. 5E and
F). HEC-1B cells overexpress MMP-9 exhibited significantly
higher migratory ability than the control group and also slightly
reverse the THC-reduced cell migration (Fig. 5G). Furthermore, MMP-9 overexpression
in HEC-1B cells can reverse the EMT protein markers which were
changed by THC treatment (Fig. 5H).
Overall, these data further indicated that MMP-9 mediates
THC-reduced EMT and cell mobility in EC cells.
 | Figure 5.MMP-9 is involved in THC-impaired EC
cell metastasis. (A) qPCR and (B) western blotting results show
that the expression of MMP-9 was reduced in HEC-1B and An3ca cells
transfected with MMP-9 siRNA. The graph shows the relative
expression of MMP-9 fold of the negative control siRNA. (Student's
t-test, ***P<0.001 vs. NC). In the presence of siRNA targeting
MMP-9, transwell assay was conducted to evaluate (C) HEC-1B and (D)
An3ca cell migration after transfection. The graph shows the
relative migrated cell number fold of the negative control siRNA,
scale bar: 10 µm (Student's t-test, **P<0.01, ***P<0.001 vs.
NC). (E) qPCR and (F) western blotting results show that the
expression of MMP-9 was increased in HEC-1B cells transfected with
MMP-9 lentiviruses. The graph shows the relative expression of
MMP-9 fold of the negative control. (Student's t-test,
***P<0.001 vs. NC). (G) Transwell assay was conducted to
evaluate effect of THC on OV-MMP-9 HEC-1B cell migration. The graph
shows the relative migrated cell number fold of the negative
control, scale bar: 10 µm (one-way ANOVA and Dunett's post-hoc
test, *P<0.05, **P<0.01, ***P<0.001 vs. NC;
#P<0.05 vs. THC). (H) Western blot analysis of
E-cadherin and Vimentin levels in HEC-1B cells after THC (20 µM)
treatment. The graph shows the relative concentrations of
E-cadherin and Vimentin fold of the negative control (one-way ANOVA
and Dunett's post-hoc test, *P<0.05, **P<0.01, ***P<0.001
vs. NC; #P<0.05 vs. THC). THC,
∆9-tetrahydrocannabinol; MMP, matrix metalloproteinase; EC,
endometrial cancer. |
CB1R and CB2R
knockdown by siRNA inhibit HEC-1B cell migration and MMP-9
expression
Previous studies (17,18) have
shown that chronic THC administration causes downregulation of
CBRs. Next, siRNA was used to decrease the active level of
CB1R and CB2R, which may be the result of THC
exposure (Fig. 6A). In addition,
downregulation of CB1R and CB2R attenuated
the MMP-9 expression in HEC-1B cell (Fig.
6B) and also inhibited the migration of HEC-1B cells (Fig. 6C). The data potentially revealed that
THC signal transfer into cells through CB1R and
CB2R to develop anti-tumor ability.
Discussion
EC is still a major clinical challenge because of
its aggressive metastasis and the limited effective strategies
available against metastasis (19).
Cannabinoids have been used both in vivo preclinical models
and in various human cancer cell lines in the past two decades,
which significantly contributes to the development of antitumor
drugs. Our study first reported that THC plays a functional role in
EC metastasis and EMT. Moreover, THC regulates MMP-9 expression in
EC cells. Effects of silencing MMP-9 in EC cells are similar to
those caused by THC exposure. In conclusion, our data reveal a new
target for intervention in EC metastasis and may increase the
future treatment options of EC.
In the present study, we first demonstrated that two
main cannabinoid receptors, both CB1 and CB2
receptors were highly expressed in EC tissues. Previous studies
show that cannabinoid receptors are overexpressed in different
cancers including lung and breast cancers. It is demonstrated in
(20) hat the expression of
CB1R is high in the primary tumor of esophageal squamous
cell carcinoma and is remarkably related to metastasis to lymph
nodes and distant organs. Next, we need to expand the sample size
to further analyze the relationship between cannabinoid receptors
expression and EC progression.
The important aspects of effective anti-tumor drugs
are their ability to inhibit cancer cell proliferation and
migration. Through obstructing the cell cycle and inducing
apoptotic cell death, THC prevents breast tumor cell growth
(21). Thus, we detected and observed
that THC significantly inhibited EC cell proliferation and
migration. Similar to THC, CBD can impair cell survival in
vitro in A549 lung cancer cell line and in primary cells from
patients with lung cancer. Meanwhile, CBD can result in tumor
regression in A549-xenografted nude mice (22). In addition, Chang et al show
that THC inhibited BeWo (choriocarcinoma cell line) cell migration
via STAT3 signaling pathway (18).
Notably, the THC anticancer action in vitro also implicates
the effects observed in vivo (23). It is reported by preliminary clinical
investigation in 2006 that THC intratumoural injection was
effective and safe for the patients with glioblastoma who had
previously failed standard therapy (24). The medical and recreational usages of
cannabis currently become a controversial topic (25). The usage of cannabis is not allowed in
most countries, although different countries have different
attitudes for cannabis (26).
We further explored the potential mechanism by which
THC regulated EC cell motility. EMT, a phenotypic cellular process,
leads to loss of cell-cell adhesion. In tumor EMT, tumor cells
surrounding the epithelial cells and matrix lose their polarity and
adhesive properties, thereby enhancing the cells' migratory and
invasive abilities (27). MMPs are
regarded as key proteins in tumor EMT because they are paramount
for cancer proliferation and metastasis. In our present study,
MMP-9 protein level in EC cells and cell culture was decreased
seriously after treatment of THC, while MMP-2 expression was not
decreased. Researchers observed a tendency towards the high
expression of MMP-9 in the advanced stages of EC (FIGO IIIA-IV)
(28). Moreover, the impact of
cannabinoids on MMPs was recorded in (29), in which CB2R agonists
decrease MMP-9 expression in activated T cells without affecting
MMP-2. More specifically, cannabidiol has inhibitory effects on
tumor cell invasion, which is consequence of the induction of
tissue inhibitor of metalloproteinase 1 (TIMP1) and subsequent
inhibition on MMP-9 (30).
Additionally, we demonstrated that downregulation of MMP-9 can
inhibit EC cell motility and MMP-9 overexpression reversed the
THC-attenuated cell migration and EMT protein markers. Furthermore,
researchers found CB2R-selective agonists inhibited cell
motility in dendritic cells (DCs) microglia (31) by decreasing MMP-9 level. This was also
supported by the evident that knockdown of CB1R and
CB2R can inhibit cell migration via reducing MMP-9
expression. Therefore, we suggested that THC reduced EC cell
migration probably through targeting CBRs and suppression of MMP-9
activity.
Surgery is the standard treatment for early-stage EC
patients, but patients with lymph node or distant-organ metastases
often have poor clinical outcomes. Therefore, identifying novel
therapy to prevent EC cell metastatic potential will help to
optimize treating strategies. This current study found that
cannabinoid receptors were over expressed in EC tissues and the
protein levels were positively correlated with EMT markers.
Furthermore, THC has been proven to inhibit EC cell motility and
EMT through reducing MMP-9, which makes THC become a novel tumor
suppressor in EC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical and
Health Science and Technology project of Zhejiang Province (grant
no. 2014KYB239).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ designed the experiments and wrote the
manuscript. YZ collected the EC tissues and analyzed the data. KS
performed the cell function experiments and WS conducted the
molecular biological experiments.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Second Affiliated Hospital of Zhejiang University. Written
informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Creasman WT, Ali S, Mutch DG, Zaino RJ,
Powell MA, Mannel RS, Backes FJ, DiSilvestro PA, Argenta PA, Pearl
ML, et al: Surgical-pathological findings in type 1 and 2
endometrial cancer: An NRG Oncology/Gynecologic Oncology Group
study on GOG-210 protocol. Gynecol Oncol. 145:519–525. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Bakel H, Stout JM, Cote AG, Tallon CM,
Sharpe AG, Hughes TR and Page JE: The draft genome and
transcriptome of Cannabis sativa. Genome Biol. 12:R1022011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deiana S: Medical use of cannabis.
Cannabidiol: A new light for schizophrenia? Drug Test Anal.
5:46–51. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuda LA, Lolait SJ, Brownstein MJ,
Young AC and Bonner TI: Structure of a cannabinoid receptor and
functional expression of the cloned cDNA. Nature. 346:561–564.
1990. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Devane WA, Hanus L, Breuer A, Pertwee RG,
Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A and
Mechoulam R: Isolation and structure of a brain constituent that
binds to the cannabinoid receptor. Science. 258:1946–1949. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pertwee RG, Howlett AC, Abood ME,
Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos
G, Mackie K, et al: International Union of Basic and Clinical
Pharmacology. LXXIX. Cannabinoid receptors and their ligands:
Beyond CB1 and CB2. Pharmacol Rev.
62:588–631. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mackie K: Distribution of cannabinoid
receptors in the central and peripheral nervous system. Handb Exp
Pharmaco. 299–325. 2005. View Article : Google Scholar
|
|
10
|
Velasco G, Sánchez C and Guzmán M: Towards
the use of cannabinoids as antitumour agents. Nat Rev Cancer.
12:436–444. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caffarel MM, Sarrió D, Palacios J, Guzmán
M and Sánchez C: Delta9-tetrahydrocannabinol inhibits cell cycle
progression in human breast cancer cells through Cdc2 regulation.
Cancer Res. 66:6615–6621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Casanova ML, Blázquez C, Martínez-Palacio
J, Villanueva C, Fernández-Aceñero MJ, Huffman JW, Jorcano JL and
Guzmán M: Inhibition of skin tumor growth and angiogenesis in vivo
by activation of cannabinoid receptors. J Clin Invest. 111:43–50.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Preet A, Qamri Z, Nasser MW, Prasad A,
Shilo K, Zou X, Groopman JE and Ganju RK: Cannabinoid receptors,
CB1 and CB2, as novel targets for inhibition of non-small cell lung
cancer growth and metastasis. Cancer Prev Res (Phila). 4:65–75.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang R, Zhang C, Liu G, Gu R and Wu H:
MicroRNA-126 inhibits proliferation, migration, invasion, and EMT
in osteosarcoma by targeting ZEB1. J Cell Biochem. 118:3765–3774.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie G, Ji A, Yuan Q, Jin Z, Yuan Y, Ren C,
Guo Z, Yao Q, Yang K, Lin X and Chen L: Tumour-initiating capacity
is independent of epithelial-mesenchymal transition status in
breast cancer cell lines. Br J Cancer. 110:2514–2523. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.PubMed/NCBI
|
|
17
|
Burston JJ, Wiley JL, Craig AA, Selley DE
and Sim-Selley LJ: Regional enhancement of cannabinoid
CB₁ receptor desensitization in female adolescent rats
following repeated Delta-tetrahydrocannabinol exposure. Br J
Pharmacol. 161:103–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang X, Bian Y, He Q, Yao J, Zhu J, Wu J,
Wang K and Duan T: Suppression of STAT3 signaling by
Δ9-Tetrahydrocannabinol (THC) induces trophoblast dysfunction. Cell
Physiol Biochem. 42:537–550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao R: Progesterone receptor isoforms A
and B: New insights into the mechanism of progesterone resistance
for the treatment of endometrial carcinoma. Ecancermedicalscience.
7:3812013.PubMed/NCBI
|
|
20
|
Hijiya N, Shibata T, Daa T, Hamanaka R,
Uchida T, Matsuura K, Tsukamoto Y, Nakada C, Iha H, Inomata M and
Moriyama M: Overexpression of cannabinoid receptor 1 in esophageal
squamous cell carcinoma is correlated with metastasis to lymph
nodes and distant organs, and poor prognosis. Pathol Int. 67:83–90.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caffarel MM, Andradas C, Pérez-Gómez E,
Guzmán M and Sánchez C: Cannabinoids: A new hope for breast cancer
therapy? Cancer Treat Rev. 38:911–918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramer R, Heinemann K, Merkord J, Rohde H,
Salamon A, Linnebacher M and Hinz B: COX-2 and PPAR-γ confer
cannabidiol-induced apoptosis of human lung cancer cells. Mol
Cancer Ther. 12:69–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salazar M, Carracedo A, Salanueva IJ,
Hernández-Tiedra S, Lorente M, Egia A, Vázquez P, Blázquez C,
Torres S, García S, et al: Cannabinoid action induces
autophagy-mediated cell death through stimulation of ER stress in
human glioma cells. J Clin Invest. 119:1359–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guzmán M, Duarte MJ, Blázquez C, Ravina J,
Rosa MC, Galve-Roperh I, Sánchez C, Velasco G and González-Feria L:
A pilot clinical study of Delta9-tetrahydrocannabinol in patients
with recurrent glioblastoma multiforme. Br J Cancer. 95:197–203.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kramer JL: Medical marijuana for cancer.
CA Cancer J Clin. 65:109–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilkie G, Sakr B and Rizack T: Medical
marijuana use in oncology: A review. JAMA Oncol. Mar 17–2016.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Araki K, Shimura T, Suzuki H, Tsutsumi S,
Wada W, Yajima T, Kobayahi T, Kubo N and Kuwano H: E/N-cadherin
switch mediates cancer progression via TGF-β-induced
epithelial-to-mesenchymal transition in extrahepatic
cholangiocarcinoma. Br J Cancer. 105:1885–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grybos A and Bar J: The relationships
between the immunoexpression of KAI1, MMP-2, MMP-9 and steroid
receptors expression in endometrial cancer. Folia Histochem
Cytobiol. 52:187–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghosh S, Preet A, Groopman JE and Ganju
RK: Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated
chemotaxis of T lymphocytes. Mol Immunol. 43:2169–2179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McAllister SD, Murase R, Christian RT, Lau
D, Zielinski AJ, Allison J, Almanza C, Pakdel A, Lee J, Limbad C,
et al: Pathways mediating the effects of cannabidiol on the
reduction of breast cancer cell proliferation, invasion, and
metastasis. Breast Cancer Res Treat. 129:37–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adhikary S, Kocieda VP, Yen JH, Tuma RF
and Ganea D: Signaling through cannabinoid receptor 2 suppresses
murine dendritic cell migration by inhibiting matrix
metalloproteinase 9 expression. Blood. 120:3741–3749. 2012.
View Article : Google Scholar : PubMed/NCBI
|