Introduction
The foot is an uncommon location for osseous tumour
types, which comprise ~3% of all skeletal tumour types (1). In particular, primary malignancies
located in the calcaneus are notably rare, accounting for 31% of
benign and 35% of osseus tumor types in the foot. Current studies
on calcaneus tumour types are limited, consisting mostly of
individual case reports describing a single primary tumour of the
calcaneus (2–4). Due to the rarity and lack of familiarity
with calcaneal tumour types, delays in diagnosis and inadequate
treatment are frequently reported (2). Furthermore, improper diagnosis and
treatment even result in unnecessary amputation (1).
In the present review, currently reported types,
clinical manifestations, radiological and histopathologic
appearances, in addition to appropriate treatment options for a
variety of benign and malignant tumour types in the calcaneus are
presented. The purpose of the present review is to increase
clinical awareness of primary tumour types in the calcaneus, as the
stage of progression at which the tumour is at, at which it is
identified, diagnosed and treated, is associated with the outcome
and prognosis. To the best of our knowledge, the present review
represents a relatively exhaustive review describing the known
spectrum of tumour types of the calcaneus. Numerous other, more
comprehensive reviews of the calcaneus are expected to be reported
in the future.
Benign calcaneal tumour types
Simple bone cyst (SBC)
SBCs are benign, fluid-containing skeletal lesions
and are referred to as unicameral bone cysts or juvenile bone cysts
(3). In a review of 258 patients with
SBCs, the calcaneus was identified to be a relatively common site
for the formation of SBCs, with a morbidity rate of 14% (4).
A majority of SBCs of the calcaneus were
asymptomatic and diagnosed incidentally as a result of minor trauma
to the foot or ankle (3–5). Pain is the major complaint in ~44.4% of
cases, and those who were middle aged experienced more severe pain
(4). Pathological fracture is rare
(3). On physical examination,
patients demonstrated normal ankle and subtalar motion. There was
no tenderness or instability in the ankle (6).
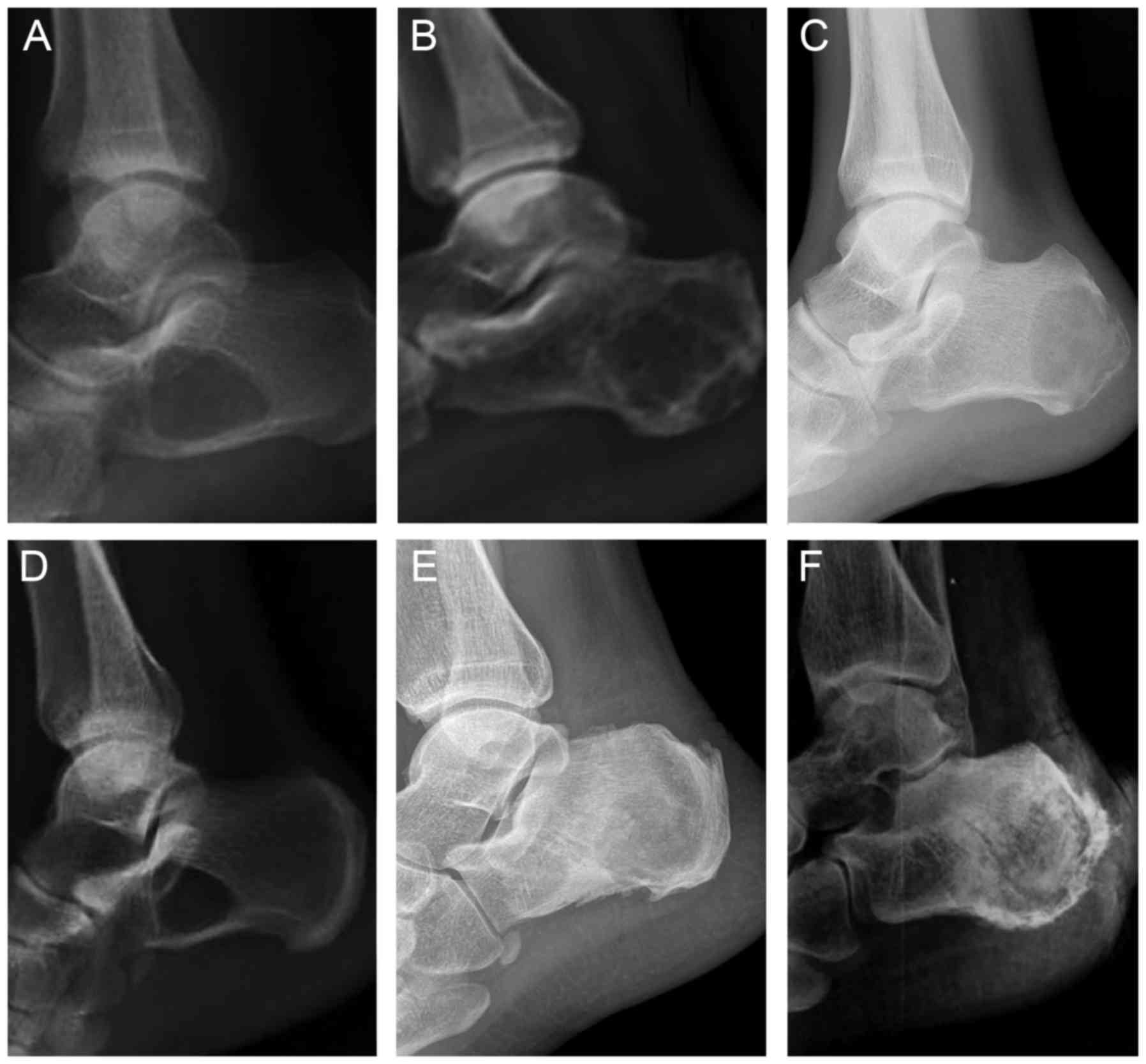
X-ray analysis (Fig.
1A) revealed a solitary bone cyst of the calcaneus that
presented as a sharply demarcated radiolucent osteolysis surrounded
by sclerotic borders (4,6,7). The
location of the cysts is usually the Ward's triangle of the
calcaneus, anterolaterally to the posterior facet of the subtalar
joint, where stresses are physiologically low (4,8). A
cortical destruction may arise from a fracture (9). A computed tomography (CT) scan revealed
a fluid-fluid level that represents the layering of fluids with
different densities (7,10). According to magnetic resonance imaging
(MRI), there is a low signal intensity on the T1-weighted image, a
high signal intensity (brighter compared with fat) on the
T2-weighted image, and a thin rim, occasionally with septal
enhancement, following the administration of intravenous contrast
(4–5,7,10,11). A
bone scan (BS) is a normal part of diagnosis and may be a useful
modality for the objective evaluation of symptomatic cysts and
differential diagnoses, particularly for patients without fractures
(5).
Macroscopically, a serous, fluid-filled cavity with
strip-like bony prominences and a thin fibrous membrane may be
identified during the surgery (7).
Microscopically, haemosiderin deposits and fibrous tissues are a
direct indicator of an SBC. Additionally, there should be no
cytological atypia (4,5,7). A
cholesterol cleft is a common microscopic observation in calcaneal
bone cysts, however, it is not detected in long bone cysts
(5).
In the majority of cases, conservative management of
calcaneal bone cysts is advocated due to the negligible symptoms
that they produce and the rarity of complications including
pathological fractures (3,4). Surgical treatments (Enneking stage 1 to
2) have included endoscopic curettage and grafting (8,12),
curettage and bone grafting (4,7,13), curettage, calcium sulphate pellet
cement and autogenous grafting (4),
multiple drilling (5), percutaneous
cyst curettage and injection of demineralized bone powder and
marrow (9,11,14),
minimal curettage combined with multiple drilling and continuous
decompression through a cannulated screw (15), steroid injection (16) and tricalcium phosphate ceramic
(17). Postoperative cases are almost
asymptomatic with no recurrence excluding mild heel pain in two
patients (2,4,5,7,8).
Aneurysmal bone cysts (ABCs)
ABCs in the calcaneus are benign, expansive, locally
destructive lesions that account for 1% of all primary bone tumour
types (18–20). Of all ABCs, ~30–35% are present in
association with another bone lesion including giant cell tumours,
osteoblastomas and/or chondroblastomas (18,19).
A majority of patients with ABC present complaints
of heel pain subsequent to minor trauma, discomfort while walking
and occasional swelling (18,19,21). The
heel pain may be chronic, worsening gradually following the trauma
(19). Pathological fractures are
rare but have been reported (18).
During clinical examination, tender swelling may be observed
without a local increase in temperature or lymph node swelling
(21).
Upon radiographic examination, the lesion (Fig. 1B) is generally identified as a lytic,
expansile lesion with an irregular or eccentric shape, thin
sclerotic borders or septations (18). A CT may help establish an anatomic
extent of the lesion, which is essential in order to delineate the
fracture line and the scheme of surgical management (18). MRI clearly demonstrates an expansile
and septate lesion with relatively well-defined margins that
contain multiple fluid-fluid levels. The fluid-fluid levels may be
best observed on the T2-weighted image with a varying signal
intensity due to the chronicity of the associated haemorrhage
(18,19,22). A BS
demonstrates increased uptake at the periphery in a number of cases
(23).
Upon gross examination, fragments of multiple
grey-brown or reddish-brown granular sections contain blood-filled
cystic spaces (22). Histopathologic
examination of the lesion reveals cavernous cavities of different
dimensions containing fibroblasts, histiocytes and osteoclastic
giant cells without any mitosis or atypia (19,21,22).
The choice of ABC treatments ranges from wide
resection of the cyst to curettage with bone grafting (Enneking
stage 2) (12,19). The use of adjuvants including
cryotherapy, phenol, polymethyl methacrylate and irradiation is
employed to remove an aggressive lesion (Enneking stage 3)
(24). All ABCs of the calcaneus have
been observed to heal well with no local pain and recurrence during
a follow-up period of >5 years (18,19,21–23).
Due to the risk of recurrence associated particularly with
chondroblastomas, 6 month CT scans are recommended for the first
two postoperative years (19,25).
Osteoid osteoma (OO)
OO is a relatively common benign tumour that is more
frequently observed in the younger population, aged 7–25 years
(1,26). It has a higher incidence in the male
population, which accounts for 74% of all cases (27).
Chronic nocturnal pain and swelling are the main
complaints of patients with OO and are usually located in the
talocalcaneal joint, and may be characteristically relieved by
non-steroidal anti-inflammatory drugs (26). Physical examinations are non-specific
and may reveal joint effusion, local temperature rise, tenderness,
stiffness and muscle atrophy (26,27).
Occasionally the patient is unable to flex the plantar ankle as a
result of severe pain (28,29).
Inflammatory reactions in the blood and marker level
abnormalities are frequently observed in reported cases (26,27,30). X-ray
only analysis reveals surrounding sclerosis of the calcaneus, which
potentially results in misdiagnosis of a SBC (26). CT is a superior method for
demonstrating a well-defined nidus, which is a round or oval
smoothly marginated lytic lesion with central mineralization
(26,27). MRI reveals low signal intensity on the
T1-weighted image and a variable signal on the T2-weighted image
due to adjacent inflammation or oedema (29–32). BS is
a meaningful method for delineating symptomatic areas (33).
Histologically, the nidus consists of osteoblasts
and osteoclasts surrounded by giant cells and vascular and fibrous
connective tissue (27). A number of
studies have suggested that OO may be managed conservatively using
anti-inflammatory medications (1,28,30). However, open surgery with or without
bone grafting (Enneking stage 1–2) is a preferential option for
patients who are insensitive to conservative treatment (29). Previously, minimally invasive
techniques, including percutaneous CT-guided resection,
radiofrequency ablation and laser photocoagulation, have undergone
remarkable progress in decreasing the risk of bone architectural
changes and fractures that result from open surgical excision
(32,34–36).
Osteochondroma
Osteochondroma in the calcaneus is rare,
constituting 10% of all osteochondromas associated with hand and
foot bones (37). It is exclusively
identified in patients <20 years-old, which indicates a
malignant transformation in adults (1,37,38).
Clinically, patients may initially present with an
enlarging mass with a painless deformity (38). Noticeable symptoms arise with the
appearance of progressive pain, swelling and stiffness, which are
attributed to fractures, malignant transformation and a low
proportion of subcutaneous tissue (38–41). Upon
physical examination, a stiff, firm and painful nodular mass may be
palpated (37–39). The motion of the ankle is observed to
be normal but presents a mildly painful active flexion (40,42).
Routine laboratory test results are within the normal range
(37–42).
On radiographs, the X-ray generally reveals a
solitary bone prominence from the bone surface that is pedunculated
or sessile (41). The hyaline
cartilage cap is not apparent on X-ray, but it is present at a low
attenuation on CT (11). The
three-dimensional imaging features of CT provide accurate
information about the dimensions of the lesion (38,39). MRI
is the best technique with which to evaluate the hyaline cartilage
cap, which presents a low signal intensity in T1- and T2-weighted
images (38–40). A thickness of the hyaline cartilage
cap >1.5 cm suggests a malignant transformation of the
chondrosarcoma (38,40), and a BS allows an optimal correlation
with the degree of endochondral bone formation (39).
The pathologic anatomy of the lesion is a polypoid
soft tissue covered with a cartilage cap (43). Biopsies reveal a spongy bone covered
with a hyaline cartilage cap, in accordance with the
characteristics of osteochondroma (43).
Treatment is usually not necessary for patients with
asymptomatic lesions (38). Surgical
removal is considered for aggressive growth subsequent to skeletal
maturity, which indicates malignant transformation (39–41). A
marginal resection (Enneking stage 1–2) is adequate and indicates a
low rate of recurrence (39–41,43–45). The
key point to avoid recurrence is the complete excision of the
lesion (45).
Chondroblastoma
Chondroblastoma is a rare chondroid tumour,
accounting for 4.5% of all bone tumour types and 2.8% of all
calcaneal tumour types (46). There
is a clear male predominance with an incidence ratio of 5:1
(46).
Local pain, soft tissue swelling and tenderness are
the main complaints of patients with chondroblastoma (46,47). A
physical examination mostly involves oedema and painful palpation
of the heel in addition to joint dysfunction of the ankle (47). All routine laboratory tests produce
results that are within normal ranges (48).
Plain radiographs demonstrate an osteolytic lesion
(Fig. 1C) with a sclerotic margin or
tiny, stippled calcifications. The fracture line appears in
patients with a pathological fracture (48). CT demonstrates a well-defined
osteolytic lesion and intracortical lucency (49). MRI for the chondroblastoma reveals an
irregular, expansive lesion and fluid-fluid levels. There is a
heterogeneous, low to intermediate signal intensity on the T1- and
T2-weighted image. All lesional and perilesional regions
demonstrated diffuse enhancement following contrast administration
(49,50). Furthermore, bone marrow oedema and
soft tissue oedema may be observed in the case of a pathologic
fracture (48).
The gross appearance of the excised lesion reveals a
soft, grey-brown, multiloculated cystic lesion with bone fragments
and a haemorrhagic mass (47,50). Histologically, the tumour is composed
of proliferating, polygonal chondroblasts, small mononuclear cells,
multinucleated giant cells and calcifications (48).
Surgical treatment is the preferred choice for
chondroblastoma, including simple curettage with bone grafting in
addition to endoscopic curettage without bone grafting (Enneking
stage 2) (47,49–51). The
latter is a more effective treatment for chondroblastoma with a
secondary ABC (49). However,
recurrence is highly attributed to the pattern of simple curettage,
inadequate resection and the association with secondary ABCs
(46,48).
Intraosseous lipoma
Intraosseous lipomas are rare tumour types,
constituting 0.1% of all benign bone tumour types. Furthermore,
only 8% of these tumour types are located in the calcaneus
(52,53). The tumour is often revealed in
patients ranging in age from 30–60 years (52,53).
Of all cases, ~40% of cases are identified
incidentally. However, in symptomatic cases, pain is the most
common symptom, particularly following a strenuous walk (52,54). The
pain may be relieved with aspirin and sufficient rest (55). Upon physical examination, an antalgic
gait pattern may be present during walking (54,55). Pain
in response to palpation surrounding the lesion is consistently
observed with or without swelling (52). There are usually no unique laboratory
results (52–55).
X-ray of the intraosseous lipoma (Fig. 1D) reveals a well-circumscribed lytic
lesion with central, single or ring-like calcifications, a majority
of which are located at the base of the calcaneus neck in the
region of the Ward's triangle. CT provides a higher-grade image,
including the anatomic location, size, extent and potential
pathologic fracture. MRI demonstrates high signal intensity on the
T1-weighted and T2-weighted image, which is more accurate in terms
of the image of the bone marrow and soft tissue (52–58).
The gross appearance of the excised lesion reveals a
yellow, friable fatty tissue with fragments of bone (54). Histologic examination presents fat
cells surrounded by necrosis and calcification, which are
interspersed with areas of haemorrhage and bony trabeculae
(52,54,55).
Asymptomatic lesions (Enneking stage 1) may be
treated conservatively as they are consistently located in the
region of the Ward's triangle, which is a non-weight-bearing area
of trabecular rarefaction (58).
However, lesions (Enneking stage 2) that are symptomatic, oversized
and prone to pathological fracture are indicated for surgical
treatment (57,58). Curettage and autologous bone grafting
is an easy and effective operation for intraosseous lipoma,
followed by a long-term non-weight bearing period (52–55).
Previously, endoscopically assisted tumour curettage followed by
filling of the bone defect with α- or β-tricalcium phosphate has
been demonstrated to be a less invasive method that allows patients
to walk normally subsequent to wound healing (59,60). In a
number of reported cases, there has been no recurrence or
complications following surgical treatment (52–63).
Giant cell tumour (GCT)
GCT of the calcaneus is a benign neoplasm that makes
up 1.2% in all cases of calcaneal tumours and exhibits high
recurrence and potentially aggressive features (64–66). It is
most commonly observed in patients who are 30–40 years old
(64).
Clinically, heel pain and swelling are the primary
complaints in patients with calcaneal GCT (64,65). On
physical examination, a tender and prominent hard swelling is
palpated. The range of ankle joint motion is normal (64–67). Serum
biochemistry is within normal limits, barring other unassociated
systemic conditions (68).
Radiographically, there is an expansile or eccentric
lytic lesion with a non-sclerotic margin, which may extend near the
articular surface (65,68). A CT scan may confirm the marked
expansion of the calcaneus (67).
However, aggressive features may additionally be identified,
including cortical expansion or destruction of the soft tissue
component (65). MRI reveals low
signal intensity on the T1-weighted image and a high signal
intensity on the T2-weighted image (69). The histological features consist of
numerous multinucleated giant cells. Spindle cells and ABC features
may be present in certain areas (65,69).
Traditionally, curettage is the primary mode of
treatment, accompanied by phenol, alcohol or cement to decrease
recurrence (Enneking stage 2) (66).
In cases of recurrence and aggressive GCTs (Enneking stage 3), limb
salvage surgery consisting of a wide excision of total
calcaneoctomy, bone allograft reconstruction and soft tissue
coverage with a sural flap, are considered superior to amputation
(65).
Chondromyxoid fibroma (CMF)
CMF of the calcaneus is a rare benign primary bone
tumour composed of immature myxoid mesenchymal and cartilaginous
tissue, making up ~7% of all cases (70–73).
The slow-growing CMF often results in a delay in
diagnosis, particularly in patients which lack symptoms (70,71). The
chief complaint in patients is chronic heel pain with a
slow-growing mass, with a limp and limited range of ankle motion
(71,72). Physical examination observations are
unremarkable, except for a palpable, hard and non-mobile protrusion
from the lesion of the calcaneus (71). Laboratory investigations do not reveal
any abnormal results (71–73).
The classic radiographic evaluation of the calcaneus
(Fig. 1E) is a well-defined,
geographic lesion with thin, sclerotic margins associated with
septation and lobulation. CT may be used to clearly define the
expanded or thin cortex in addition to the cortical integrity
(71,72). MRI is useful to characterize the
tumour and to assess the tumour extent for preoperative planning
(71). It reveals lesions as a low
signal intensity on the T1-weighted image and a prominent high
signal intensity on the T2-weighted image owing to the chondroid
and myxoid composition (72–74). Peritumoral oedema of the marrow is
seldom present, indicating a more aggressive tumour (71).
The characteristic gross appearance of the lesion is
a grey or white multilobulated mass composed of a fibro-gelatinous
substance with pieces of cartilaginous material (71,72).
Intralesional haemorrhage and cystic degeneration may additionally
be identified in a number of cases (71). Histologic examination indicates
variable proportions of cartilaginous, myxoid and fibroid tissue.
The immunohistochemical stain for S-100 is focally positive
(71,72).
Certain patients with CMF (Enneking stage 2) may be
treated using simple curettage, however, this technique has been
reported to retain tumour cells and is responsible for recurrence
(71,73). Thus, the combination of simple
curettage, curettage with dental bur augmentation, block resection
or even amputation have been demonstrated to be effective (71,73,74).
Patients should be followed up for a minimum of 3 years to monitor
signs of recurrence (71). Routinely,
a short leg cast for 6 weeks in addition to physical therapy are
essential (72).
Other benign calcaneal tumour
types
Other reported benign calcaneal tumour types
include desmoplastic fibroma, osteoblastoma, ganglion, benign
fibrous histiocytoma, primary xanthoma and intraosseous spindle
cell haemangioma (75–87). Additional details cannot be obtained
due to a limited number of case reports. Known details are provided
in Table I.
 | Table I.Characteristics of other reviewed
primary calcaneus tumour types. |
Table I.
Characteristics of other reviewed
primary calcaneus tumour types.
| First author | Type | Symptoms | Symptoms | Clinical
examination | Laboratory
test | Imaging | Gross | Pathology | Treatment | (Refs.) |
|---|
| Raatikainen | Desmoplastic
fibroma | Pain on weight
bearing | No history of
trauma motion | Mild swelling,
limited subtalar | Normal | X-ray: osteolytic
process without matrix calcification, prominent trabeculation or
septation; CT: several incomplete ridges of bone within the lesion,
full extent of the intraosseous mass; MRI: lobulated soft tissue
mass, hypo-intensity on both T1- and T2-weighted image | Grayish-white in
color, rubbery consistency | Elongated
fibroblast-like cells. with small, uniform nuclei, a dense matrix
of collagen | Wide excision
(Enneking stage 3) | (75) |
| Yu |
|
|
|
|
|
|
|
|
| (76) |
| Miyayama | Osteoblastoma | Pain while
walking | – | Swelling,
tenderness, atrophied calf muscles | Normal | X-ray: a well
circumscribed sclerotic lesion, a destructive moth-eaten or
permeative appearance | Well defined,
reddish brown and friable excised lesion with a plump | Compact
proliferation of large round epithelioid cells | Curettage and bone
graft (Enneking stage 2), wide excision (Enneking stage 3) nucleus
and abundant cytoplasm | (77) |
| Schujman |
|
|
|
|
|
|
|
|
| (78) |
| Mestdagh | Ganglion | Progressive
talaglia with swelling | – | – | – | X-ray: a
multilobular radiolucent cyst outlined by a rim of sclerotic bone;
CT: fluid attenuation | Gelatinous
consistency, mucoid centers with a yellowish color | – | Curettage with bone
graft (Enneking stage 2) | (79) |
| Murff |
|
|
|
|
|
|
|
|
| (80) |
| Tadashi | Benign fibrous | Heel pain, a
limping | No history of
antecedent trauma, penetrating injury | Tenderness over the
heel plantar | Normal | X-ray: a
well-defined, slightly radiolucent expanded lesion; CT: a cystic
lesion with mild expansion and thinning of the cortex without
soft-tissue involvement | Gray-brown colored,
irregular lesion with areas of overt hemorrhage | Spindle-shaped
fibroblastic cells arranged in storiform growth pattern in addition
to multinucleated giant cells | Curettage with bone
graft (Enneking stage 1) | (81) |
| Keskinbora |
|
|
|
|
|
|
|
|
| (82) |
| Kinberg | Primary
xanthoma | Heel pain | – | Mild uniform
swelling, moderate pain | Elaveted ACP | X-ray: well-defined
osteolytic lesion; MRI: well demarcated expansile lesion
hypo-intensity on T1-weighted image and hyper-intensity on
T2-weighted BS: increased isotope uptake | Tan brown fragment
of soft and bony tissues. Yellow, soft and granular tumorous
tissues | Mononuclear
macrophage-like cells, abundant foam cells, multinucleated giant
cells with cholesterol cleft and hemorrhages | Curettage with bone
graft (Enneking stage 2) | (83) |
| Ahmed |
|
|
|
|
|
|
|
|
| (84) |
| Yamamoto |
|
|
|
|
|
|
|
|
| (85) |
| Kapukaya |
|
|
|
|
|
|
|
|
| (86) |
| Hakozaki | Intraosseous
spindle cell hemangioma | Pain on weight
bearing | – | – | Elavated ACP | X-ray: an
osteolytic lesion with soap bubble-like multilocular appearance;
MRI: low intensity on T1-weighted image, mixed high and
intermediate intensity on T2-weighted image; marginal enhancement
on gadolinium enhanced T1-weighted fatsuppression image | Dark-red mass with
hematoma | Large cavernous
blood vessels, intermingled spindled tumor cells. Cluster of
differentiation 31 (+), α-smooth muscle actin (+). | Curettage (Enneking
stage 1) | (87) |
| Demiralp | Malignant fibrous
histiocytoma | Progressively
worsening pian, age >40 | – | Moderate difussion
and tenderness | Normal | X-ray: an
osteolytic and eccentric lesion; CT: a purellytic and poorely
marginated lesion with cortical destruction; Periosteal reactions
and endosteal scalloping; MRI: hypo-intensity on T1-weighted image,
hyperintensity in T2-weighted image with fat suppression;
Scinrigraphic examination increased uptake | – | Atypical cells with
a fibroblastic appearance, stpriform pattern | Radial resection or
amputation with chemotherapy (Enneking stage IIB) | (113) |
| Daibata | Multiple
myeloma | Pain and
swelling | Plasmacytoma of
skull and femoral neck | – | Elaveted IgG | X-ray: rarefaction
of the calcaneus;- MRI: a low-intensity signal on T1-weighted
image, a high-intensity signal on T2-weighted image; Gallium
scintigraph: strong accumulation |
| Plasma cells with
eccentric nuclei and peripherally clumped chromatin | Combination
chemotherapy with external beam irradiation (Enneking stage
III) | (114) |
| Rong |
Hemangioendo-thelioma | Severe pain | High fever,
anemia | Increased local
heat and swelling of the heel with exquisite tenderness | Elaveted ACP, WBC,
IgG, IgM, decreased albumin, coagulation abnormalities | X-ray: an
osteolytic lesion with occasional sclerosis, no periosteal
reaction; MRI: low-to-moderate signal intensity on T1-weighted
image, heterogenous high signal intensity on T2-weighted image | Hemorrhagic and
variegated features typical of angiosarcoma | Round of polygonal
cells with an abundant eosinophilic cytoplasm complex and irregular
branching vascular channels. VIII-related antigen (+) | Amputation with
chemotherapy (stage III) | (115) |
| Goldenberg | Fibrosarcoma | Pain | History of
injury | Atrophy of the
calf, enlargement of the heel with tenderness, limited plantar
flexion | Normal | X-ray: a poorly
defined lytic lesion, expanded and irregular cortex | White and rather
firm tumor with a somewhat collagenous appearance in the central
portion | Elongated and
spindle-shaped fibroblasts with collagen fibers arranged in whorls
and interlacing patterns | Wide excision or
amputation (Enneking stage IIB) | (116) |
| Gahlot | Malignant
peripheral nerve sheath tumor | Pain, swelling and
redness of the heel | – | A tender swelling
with a small superficial uncler in the heel | – | X-ray: a geographic
lytic destruction of the calcaneus with intact cortex; MRI: a
hypointensity on T1-weighted image, a hyper-intensity on
T2-weighted image with fat supression | – | Spindle cells in a
fascicular growth with hemangioperi-cytoma-like vascular pattern,
pleomorphic, mitotically active cells with hyperchromatic nuclei
and pale cytoplasm. Vimentin (+), S-100 (+) pan-cytokeratin (+).
K-i67 labeling index was ~6% | Radical excision
with high-dose radiation therapy (Enneking stage IIA) | (117) |
| Balaji | Primary epithelioid
angiosarcoma | Dull pain and
swelling | – | Diffuse swelling
and tenderness | Slightly elevated
ACP | X-ray: an
osteolytic lesion with loculations; CT: an expansile lytic lesion
with multiple loculations, hyper-dense fluid levels; BS: increase
tracer uptake | – | Epithelioid tumor
cells with moderately pleomorphic nuclei. CD31(+) | Extended curettage
and autologous bone grafting (Enneking stage IIA) | (118) |
| Madhuri | Primitive
neuroectodermal therapy tumor | Pain and
swelling | – | A warm tender
swelling, dilated skin veins | – | X-ray: multiple
lytic lesions with cortical erosion and sclerotic patchy densities;
MRI after chemotherapy: considerable reduction in the size of the
tumor, a hyper-intensity on T2-weighted image | – | Small round
monomorphic cells with round nuclei and scanty glycogen rich
cytoplasm | Wide excision with
chemotherapy (Enneking stage IIB) | (119) |
| Chandrasekar | Adamantinoma | Pain and difficulty
in weight bearing | – | – | Normal | X-ray:an eccentric
lytic and expansile lesion, involving the cortex and medullary
cavity; MRI: a hypo-intensity on T2-weighted images; BS: increased
uptake | – | The definitive
diagnosis of adamantinoma was eventually confirmed after 4
attempts | Below knee
amputation with chemotherapy (Enneking stage IB) | (120) |
Malignant calcaneal tumour types
Osteosarcoma
Osteosarcoma is the most common non-haemopoietic
primary malignant neoplasm of bone (88). The calcaneal location of osteosarcoma
is rare, accounting for only 1% of all osteosarcoma (89). Younger people (between 10 and 30 years
old) are typically affected, with a peak in the second decade of
life (89,90).
Progressively worsening pain and swelling in the
affected ankle joint are major complaints in patients with
osteosarcoma in the calcaneus (88,90,91).
During physical examination, the swelling is tender, immobile and
the mobility of the joint is restricted (88,90). A
prior history of trauma has been noted in multiple cases (89,92).
In the case of a small cell osteosarcoma in the
calcaneus, laboratory tests reveal an increase in the erythrocyte
sedimentation rate (ESR) and in C-reactive protein (CRP) levels
(91). Radiograph and CT of the
calcaneus reveal a sunburst appearance with a dense sclerotic
lesion, cortical destruction and extraosseous expansion (88,90,93). A
majority of cases consist of a mixture of lysis, sclerosis and
rarefaction (88). CT reveals soft
tissue extension and a large osteolytic lesion with cortical
thinning/breakthrough (90). MRI was
performed for preoperative staging in addition to confirming the
presence of surrounding soft tissue infiltration, presenting an
intermediate intensity on the T1-weighted image due to sclerotic
bone formation and a high signal intensity on the T2-weighted image
(88). CT of the chest, abdomen and
pelvis ought to be performed to rule out metastatic disease at the
time of diagnosis (88).
The lesion was observed as a tan-white, yellow,
heterogeneous, smooth and firm mass located in the body of the
calcaneus (88,91). The pathological image presented a
diffuse proliferation of the spindle and atypical cells
characterized by nests or cords encompassed by abundant osteoid
deposits (88,90,93,94).
Sheets of pleomorphic osteoblasts, mitotic features and osteoid
formation may additionally be observed (88,90).
Prior to and following surgery, all patients have
been reported to accept chemotherapy (88–93). The
optional surgery of the calcaneal osteosarcoma varies depending on
the tumour stage (89). Patients
(Enneking stage IIB) who have undergone below-knee amputation have
a satisfactory form of treatment, with no evidence of local
recurrence in those who survive >2 years (89). Furthermore, a calcaneal prosthetic
replacement, which is beneficial for functional consequences, may
be an appropriate treatment for special intramedullary tumours
(stage IIA) that respond well to preoperative chemotherapy
(91,93). Two patients presented with metastatic
disease (88,90).
Primary osseous lymphoma
The calcaneus is a rare site of primary osseous
lymphoma that represents ~1.2% of 246 cases in clinical research
(95).
The symptoms vary from mild non-specific pain to
soft tissue swelling, often with a lack of constitutional symptoms
(96–98). A number of cases may be accompanied by
a pathological fracture (95,96,98). A
physical examination of the affected hind foot may reveal
non-pitting oedema, reddening and tenderness (97,99).
Laboratory tests all produce results that are within normal limits
(100).
Radiographs reveal a mixture of osteolysis and
sclerotic lesions of the calcaneus, which produce a ‘moth-eaten’
and patchy appearance (96–98,101).
Periosteal reaction, pathologic fracture and soft tissue
involvement may additionally be observed (99,100). The
MRI examination presents a large, lobulated, expansile lesion
arising from the calcaneus with a low signal intensity on the
T1-weighted image, raising suspicion of neoplastic disease
(97,99,100). On
a gallium scan, increased radiotracer uptake has been noted in the
location of the lesion (98).
Intraoperative observation reveals a suet-like
tissue in a patient with a T-cell lymphoma (97). Histological examination most often
reveals a large number of lymphoid elements with hyperchromatic
large nuclei, abundant cytoplasm, and prominent nucleoli (98). Immunophenotyping and histochemical
stains for cluster of differentiation (CD)20 and L26 (a pan-B-cell
marker) are positive in cases of B-cell lymphoma (98,99).
At present, complete excision of the lesion alone
is usually not curative (88,89). In cases without metastasis (Enneking
stage IIB), the patient is treated with a multiple chemotherapy
regime. Although patients experience anaemia and fatigue with
chemotherapy, it is effective to be disease free for >5 years
(98). Radiation therapy is
recommended following chemotherapy (96–98,101).
Primary osseous lymphoma has provided a good prognosis, except in
one patient (Enneking stage III) with a recurrent lesion in the
contralateral distal tibia (100). A
majority of patients respond well to the treatment and present
without local or metastatic disease during long-term follow-up
(96–98,101).
Chondrosarcoma
Chondrosarcoma is one of the most common malignant
tumour types following osteosarcoma and myeloma, with ~3% of all
chondrosarcoma present in the foot (1). In a report of 75 cases of
chondrosarcomas in the foot, 28% were present in the calcaneus
(102).
Chondrosarcoma has an indolent natural history,
ranging from 8 months to 36 years (102). Clinically, chondrosarcoma is
characterized by insidious, gradual and progressive pain (103). Upon physical examination, the
swelling is diffuse, mildly tender to palpation, and occasionally
accompanied by a local increase in temperature (103,104).
Common radiographic features (Fig. 1F) include deep endosteal scalloping
with bone expansion, cortical destruction, endosteal erosion,
matrix calcification, periosteal reaction and pathologic fracture
(102–107). CT of the calcaneus has been more
accurate in the detection of soft tissue extension, calcification
and cortical involvement (105).
Using MRI, a well-defined lobulated mass with a low signal
intensity may be observed on the T1-weighted image and a high
signal intensity on the T2-weighted image with multiple septations,
reflecting the high water content of this cartilaginous neoplasm
(106,107). In the contrast-enhanced image, rings
and arcs may be observed as a typical lobulated growth pattern
(107). On BSs, there is increased
tracer uptake in the lesion. BS and chest X-ray/CT must be
performed routinely to rule out metastasis even in the absence of
clinical symptoms (105).
Macroscopically, the tumour tissue presents with a
straw-yellow colour (103).
Microscopy may reveal numerous pleomorphic chondrocytes with
hypercellularity and cytologic atypia. An abundant foamy to clear
cytoplasm is a feature of clear cell chondrosarcoma (105). With the use of immunohistochemistry,
the tumour cells are positive for S-100 proteins and negative for
cytokeratin and CD68 (104–106).
Surgery ought to be a top priority for the
treatment of chondrosarcoma. Wide surgical resection or amputation
remains regarded as a mainstay method, while simple curettage or
local excision is not recommended due to recurrence (102–106).
If the tumour (Enneking stage IIA) is restricted to a bone that may
be completely removed with an appropriate amount of soft tissue, a
wide excision is recommended and usually requires at least a 5 cm
safety margin (102,103,106,107).
For patients with a stage IIB tumour, amputation is recommended and
believed to result in an improved disease-free survival rate
(102–107). Although chemotherapy and
radiotherapy have no effect in chondrosarcoma, they are
administered as a palliative therapy in the case (stage III) of
metastatic and inoperable disease (103,105).
Recurrence and metastasis of chondrosarcoma
constituted nearly 50% of all patients in one previous study
(102). Metastasis typically occurs
to lungs and proximal bones (103–105).
Owing to the propensity for recurrence and metastasis, all patients
with chondrosarcoma of the calcaneus require long-term follow-up
(102–104).
Ewing's sarcoma
Ewing's sarcoma of the calcaneus is a primary,
aggressive malignant tumour, of which >50% of all cases occur in
the foot (108).
Local pain and swelling of the ankle are the
primary symptoms of patients, in addition to a limp foot and
erythema (108,109). Constitutional symptoms, including
fever and weight loss are observed in a number of cases (108). Upon examination, the swelling is
always extensive around the heel, resulting in stretched and shiny
skin in addition to visibly dilated subcutaneous veins (108,110).
Blood laboratory tests revealed elevated ESR, acid
phosphatase (ACP) and leucocytosis levels accompanied by mild or
moderate anaemia (108–110). X-ray revealed a ‘moth-eaten’ type of
destruction with an aggressive periosteal reaction and cortical
violation in the calcaneus (109).
CT visibly demonstrated a classical ‘sun-ray’ or ‘onion-peel’
appearance of the periosteal reaction, which may additionally be
parallel, speculated or mixed (109–111).
MRI presented a low signal intensity on the T1-weighted image and a
high signal intensity on the T2-weighted image, which is unable to
adequately distinguish the tumour from necrosis. Therefore, the
enhancement pattern following administration of contrast medium is
competent for the differentiation between oedema and haemorrhage
(111). BSs reveal an increased area
of uptake in the region of the tumour. Chest radiography/CT is
necessary in order to detect bone metastases (110–112).
A biopsy of the tumour with histopathological
analysis revealed a malignant small round-cell tumour with little
or no intercellular stroma (109–112).
CD99 and periodic acid-Schiff were immunohistochemically positive
(109,112).
A combination of surgical resection, multidrug
chemotherapy and radiotherapy are particularly applicable in
comparison with surgery alone (108–112).
Multidrug chemotherapy increases the overall survival rate of
patients (Enneking stage IIB to III) with Ewing's sarcoma by
reducing the risk of metastases, whereas surgery combined with
preoperative chemotherapy reduces the risk of local recurrence
(108). The prognosis of this tumour
is the worst among any primary sarcoma known to metastasize to the
lungs and other bones including the tibia, fibula or tarsal bone
(109–111). Patients with localized Ewing's
sarcoma have a higher probability of disease-free prognosis
(108).
Other malignant calcaneal tumour
types
Other sporadic malignant cases that have been ever
reported, including malignant fibrous histiocytoma, multiple
myeloma, haemangioendothelioma, fibrosarcoma, malignant peripheral
nerve sheath tumour, primary epithelioid angiosarcoma, primitive
neuroectodermal tumour, and adamantinoma, are summarized in
Table I (113–120).
Discussion
The calcaneus is the largest tarsal bone of the
foot in humans. It develops from an ossification similar to that of
the epiphysis of a long bone, during the 4th-7th week of foetal
development (1). Therefore, the
calcaneus is a potential location for an osseous tumour, occupying
the second most common site in the foot following metatarsals
(1). Previous studies on calcaneal
tumour types are limited and dominated by case reports and reviews.
Consequently, a substantial number of clinicians are unfamiliar
with calcaneal tumour types, resulting in a delay in diagnosis or
unnecessary treatment (2,61). Thus, early diagnosis and management
are essential to improve outcomes, particularly in high-grade
malignancies.
A majority of benign tumour types of the calcaneus
are asymptomatic, including a SBC, intraosseous lipoma and
osteochondroma (4,44,45). They
are diagnosed incidentally as a result of minor trauma to the foot
or ankle (3,22). The most common complaint of these
symptomatic patients is an intermittent or constant heel pain over
the duration of a few months to years (22,55,57). For
benign tumour types, the pain often occurs at night and may be
relieved temporarily by nonsteroidal anti-inflammatory drugs with a
weakening effect due to tolerance (61,64,65). A
rapidly growing pain may result from a malignant tumour (2). Pathological fracture following minor
trauma consistently results in sudden pain due to substantial
cortical disruption of the calcaneus (1,57).
Swelling is a common secondary symptom of active and aggressive
lesions (4,5,89,101).
Upon physical examination, patients who suffer from
severe pain and swelling may experience difficulty in weight
bearing, presenting an antalgic gait pattern (64,65).
Motion of the subtalar, midtarsal and ankle joints may be limited
or painful only if the tumour is in close proximity to the joint
(121). Certain symptoms including
atrophy of the calf, enlargement of the heel and visible dilated
subcutaneous veins may additionally be observed (1,2,10). Upon palpation, a soft or hard mass may
be detected in the region surrounding the lesion. Furthermore,
local redness, effusion, heat or even superficial ulcers is a
specific sign of a malignant and aggressive tumour (10,121,122). A
vibratory exam may elicit pain, particularly if the tumour involves
cortical or subchondral bone (22).
In general, symptoms and physical examinations are nonspecific,
potentially resulting in misdiagnosis including plantar fasciitis,
chronic ankle sprain, os-trigonum syndrome, calcaneal apophysitis,
retained foreign bodies and fractures (2,26,30,32,64,121).
Therefore, a comprehensive history and physical examination should
be completed for any patient with pain and swelling of the foot to
obviate the delay in diagnosis and allow an early and accurate
diagnosis. It is additionally important to identify whether any
other sites are involved (1,2).
Laboratory tests of calcaneal tumours remain
insufficient in previous studies (1,2). The main
tests conducted include testing the levels of ESR, CRP, ACP,
leucocytosis, coagulation tests and protein electrophoresis
(3,4,18,19). Although the results are usually within
normal limits in a majority of cases, an elevated ACP may be
observed in tumours including Ewing's sarcoma, primary xanthoma and
intraosseous spindle cell haemangioma (87,108–110).
An increased level of immunoglobulin may be detected in multiple
myeloma (114). However,
differential diagnoses cannot depend exclusively on laboratory
tests (1).
X-ray is the first choice for the detection of the
lesion and to make a tentative diagnosis (57). Although the calcaneus differs from
other long bones in terms of shape, dimension and trabecular bone
architecture, radiographs of the calcaneal tumour are similar to
those of long bones in terms of imaging characteristics (1,2). It is
essential to carefully examine imaging changes, including the
extent, location, calcification, sclerosis cortical violation,
permeative destruction, periosteal reaction, pathological fracture
and soft tissue mass of the tumour (10,57). A
well-defined osteolytic area with a sharp and sclerotic margin is
mainly presented in benign tumour types (121). For malignant and aggressive tumour
types, a ‘moth-eaten’ (multiple erosions with coalescence into
larger lesions) or permeative (multiple, small lytic areas) pattern
are typical (100,102). Slow-growing, low-grade tumours cause
reactive thickening of the cortex, whilst high-grade neoplasms that
are more aggressive destroy the cortex. Radiographs retain the
initial imaging modality, providing valuable information regarding
the morphology and biological behaviour of calcaneal tumours
(1,57). However, when compared with CT and MRI,
there remains lack of information for formulating a reasonable
differential diagnosis (45). CT is
an excellent auxiliary method for providing determinations of
morphological modifications (27,34). In
particular, MRI has a unique advantage for defining the soft tissue
extent and stage of the tumour and helping to implement surgical
resection in addition to gauging the risk of fracture (16,102,106).
Among several cases, increased uptake in the lesion may be observed
in a BS, which is useful in identifying active processes and
multiple lesions (1,2,89). CT of
the chest and abdomen is an essential prerequisite for detecting
bone metastases (97,98,100).
Histological examination is the gold standard for
the diagnosis of a majority of calcaneal tumour types, which
remains in accordance with their intrinsic pathological
characteristics (1,2). There are three types of biopsies in
reported reviews, as follows: i) For a benign calcaneal tumour, the
excisional biopsy means removing the tumour entirely followed by
the tumour being sent to the pathologist. It is not recommended for
those with any doubt about the diagnosis. ii) An open incisional
biopsy is used to remove a piece of the specimen from the tumour
and has been validated as the most accurate. iii) A needle biopsy
is safer and less traumatic compared with an open incisional
biopsy, with a reduced potential of contamination and complications
(1,122). However, there is a risk of
overlooking problematic cells, which may result in a false negative
result. If the diagnosis is uncertain, a second or multiple
biopsies are essential (21). In
fact, due to a lack of adequate clinical and radiographic
information, an incorrect diagnosis may be made by the pathologist
based only on microscopic features. For instance, an ABC may be
misdiagnosed as an osteosarcoma due to the similar histological
features (25). In this circumstance,
a combined diagnosis based on radiology, pathology and surgery,
following a thorough review of all available information, is
imperative (10,121,122).
Upon treatment of benign tumour types, conservative
treatment of asymptomatic patients (including those with SBC and
OO) is advocated due to the negligible symptoms that they produce
and the rarity of complications including pathological fractures
(3,33). The patient must be followed-up on a
regular basis to observe whether the lesion increases or decreases
in size. If persistent heel pain disturbs the patient's daily life
or there is a risk of impending fracture, surgical treatment is the
only choice (4,10). In the present study, the Enneking
system was used in order to formulate a relatively safe and
reasonable operation plan (Table II)
(1,10,12).
Curettage with bone grafting and resection are major surgical
treatment options for patients with a benign tumour of the
calcaneus (123). In comparison with
open curettage with more soft tissue dissection, there has been
considerable development of endoscopic resection, which may
diminish the risk of iatrogenic sural nerve damage and accelerate
superficial wound healing (124).
For malignant tumours of the calcaneus, radical resection
(including limb salvaging) and amputation should be thoroughly
assessed in terms of local tumour appearance, histological grade
and physical disabilities (including antalgic gait pattern)
(88–90,97,98,107–110).
Although amputation is considered the most common option in order
to achieve adequate local control of a primary malignant bone
tumour, phantom pain is highly prevalent among patients with lower
limb amputation due to of the loss of major nerves (125). With the development of effective
neoadjuvant chemotherapy, limb salvage surgery has become a
superior treatment for primary calcaneal malignancy (126). However, reconstruction following
radical resection remains a challenge to surgeons in terms of
preservation of function as well as quality of life (119,126).
Fortunately, biological reconstruction using pedicled
osteocutaneous fibular flaps has been confirmed to be a successful
limb salvage procedure (126).
Furthermore, a calcaneal prosthetic replacement may be appropriate
for special intramedullary tumour types that respond positively to
preoperative chemotherapy (91,93). In
all cases that result in an unstable calcaneal structure, the lower
limb is immobilized in a short leg cast for 6 weeks followed by
resumption of weight bearing (1,10,126). Physical therapy is recommended
following the radiographical appearance of signs of bone healing.
Follow-up reports on the prognosis of calcaneal tumours are scarce
(10). A majority of patients with
benign tumour types heal, excluding a few cases with a palindromia
(1,121). For malignant tumour types, the
prognosis is relatively poor, resulting disability and a high rate
of metastasis (1,98,126).
 | Table II.Summary of the classification and
treatment. |
Table II.
Summary of the classification and
treatment.
| Stage |
Characteristics | Surgery | Examples |
|---|
| 1 | Inactive | Curettage | SBC,
Osteochondroma |
| 2 | Inactive | Curettage and bone
grating | SBC,
Osteochondroma |
| 3 | Aggressive,
destructive | Marginal
resection | Giant cell tumor
ABC |
| IA | Low-grade
intracompartmental | Wide resection | – |
| IB | Low-grade
extracompartmental | Wide resection or
amputation (involvement of joint, vessel and nerve) | Adamantinoma |
| IIA | High-grade
intracompartmental | Radical resection
or calcaneal prosthetic replacement with chemotherapy | Chondrosarcoma,
Osteosarcoma |
| IIB | High-grade
extracompartmental | Below-knee
amputation with chemotherapy | Chondrosarcoma,
Osteosarcoma |
| III | Low-and High-grade
lesions with metastases respectively | Combination
chemotherapy with external beam irradiation | Multiple Myeloma
hemangioendothelioma |
Conclusion
The calcaneus is a rare location for the occurrence
and development a primary tumour (1).
Heel pain and localized swelling of the ankle are the most common
presenting symptoms in previous reports of calcaneal tumour types,
which are often misdiagnosed as soft tissue injuries. A calcaneal
tumour ought to be considered when a patient presents with
persistent or severe pain, swelling or the absence of trauma
(10). A tentative diagnosis of a
calcaneal tumour is made radiologically. Thus, an X-ray is the
first choice for detection of the lesion, followed by auxiliary CT,
MRI and BS examinations (1,2,123). A
well-defined osteolytic area with a sclerotic margin is primarily
presented in benign tumour types. For malignant tumour types, a
moth-eaten or permeative pattern is a typical characteristic
(1). Histological examination is the
gold standard for the diagnosis of a majority of calcaneal tumour
types (2). The final diagnosis
entails a combination of the symptomatology, imageology and
histopathology. It is imperative not only to exclude other
differential diagnoses but additionally to select a preferable
therapeutic regimen and prognosticate the disease outcome. The
treatment of a calcaneal tumour varies depending on the Enneking
system. Follow-up reports on the prognosis of patients with a
calcaneal tumour are scarce. Due to the insufficient availability
of studies on calcaneal tumour types, further studies should shed
light on a substantially more detailed standard for the diagnosis
and treatment of the primary calcaneal tumour.
Acknowledgements
Not applicable.
Funding
The design of the study was supported by National
Natural Science Foundation of China (grant no. 81573734).
Availability of data and materials
Not applicable.
Authors' contributions
MS and SW were responsible for the conception and
design of the study. LY was responsible for drafting, revising the
initial manuscript. JZ, JC and WW contributed to the collection,
analysis and interpretation of data. ML and XW revised and expanded
the manuscript. SW was responsible for the study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
BS
|
bone scan
|
|
ACP
|
acid phosphatase
|
|
CRP
|
C-reactive protein
|
|
ESR
|
erythrocyte sedimentation rate
|
|
SBC
|
simple bone cysts
|
|
ABC
|
aneurysmal bone cysts
|
|
CMF
|
chondromyxoid fibroma
|
References
|
1
|
Kilgore WB and Parrish WM: Calcaneal
tumors and tumor-like conditions. Foot Ankle Clin. 10:541–565.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young PS, Bell SW, MacDuff EM and Mahendra
A: Primary osseous tumors of the hindfoot: Why the delay in
diagnosis and should we be concerned? Clin Orthop Relat Res.
471:871–877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanna SJ, Dasic D and Floyd A: Simple bone
cysts of the calcaneus: A report of five cases and a review of the
literature. Foot Ankle Int. 25:680–684. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Polat O, Sağlik Y, Adiguzel HE, Arikan M
and Yildiz HY: Our clinical experience on calcaneal bone cysts: 36
cysts in 33 patients. Arch Orthop Trauma Surg. 129:1489–1494. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takada J, Hoshi M, Oebisu N, Ieguchi M,
Kakehashi A, Wanibuchi H and Nakamura H: A comparative study of
clinicopathological features between simple bone cysts of the
calcaneus and the long bone. Foot Ankle Int. 35:374–382. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park IH, Micic ID and Jeon IH: A study of
23 unicameral bone cysts of the calcaneus: Open chip allogeneic
bone graft versus percutaneous injection of bone powder with
autogenous bone marrow. Foot Ankle Int. 29:164–170. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lenze U, Stolberg-Stolberg J, Pohlig F,
Lenze F, von Eisenhart-Rothe R, Rechl H and Toepfer A: Unicameral
bone cyst in the calcaneus of mirror image twins. J Foot Ankle
Surg. 54:754–757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yildirim C, Akmaz I, Sahin O and Keklikci
K: Simple calcaneal bone cysts: A pilot study comparing open versus
endoscopic curettage and grafting. J Bone Joint Surg Br.
93:1626–1631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Zhang G, Li S, Wu Z, Yuan W and
Hong J: Percutaneous treatment of calcaneus fractures associated
with underlying bone cysts. Foot Ankle Int. 33:424–429. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levy DM, Gross CE and Garras DN: Treatment
of unicameral bone cysts of the calcaneus: A systematic review. J
Foot Ankle Surg. 54:652–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rhee JH, Lewis RB and Murphey MD: Primary
osseous tumors of the foot and ankle. Magn Reson Imaging Clin N Am.
16:71–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 153:106–120. 1980.
|
|
13
|
Gallagher TA, Lim-Dunham JE and Vade A: CT
findings of a unicameral calcaneal bone cyst containing a
fluid-fluid level. Comput Med Imaging Graph. 31:111–113. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dormans JP and Dormans NJ: Use of
percutaneous intramedullary decompression and medical-grade calcium
sulfate pellets for treatment of unicameral bone cysts of the
calcaneus in children. Orthopedics. 27 Suppl 1:S137–S139.
2004.PubMed/NCBI
|
|
15
|
Abdel-Wanis ME, Tsuchiya H, Uehara K and
Tomita K: Minimal curettage, multiple drilling, and continuous
decompression through a cannulated screw for treatment of calcaneal
simple bone cysts in children. J Pediatr Orthop. 22:540–543. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elias I, Zoga AC, Raikin SM, Schweitzer ME
and Morrison WB: Incidence and morphologic characteristics of
benign calcaneal cystic lesions on MRI. Foot Ankle Int. 28:707–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altermatt S, Schwöbel M and Pochon JP:
Operative treatment of solitary bone cysts with tricalcium
phosphate ceramic. Eur J Pediatr Surg. 2:180–182. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Babazadeh S, Broadhead ML, Schlicht SM,
Powell GJ and Tymms GM: Pathologic fracture of a calcaneal
aneurysmal bone cyst. J Foot Ankle Surg. 50:727–732. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaplanoğlu V, Ciliz DS, Kaplanoğlu H and
Elverici E: Aneurysmal bone cyst of the calcaneus. J Clin Imaging
Sci. 4:602014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuna S and Gudena R: ‘Soap bubble’ in the
calcaneus. CMAJ. 183:11712011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Parashari UC, Khanduri S, Upadhyay D,
Bhadury S and Singhal S: Radiologic and pathologic correlation of
aneurysmal bone cysts at unusual sites. J Cancer Res Ther.
8:103–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malik A, Deb P, Mani NS and D'Souza J:
Aneurysmal bone cyst of the calcaneum: An expansile locally
destructive lesion. J Cancer Res Ther. 6:570–572. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tequabo Y, Admasie D, Gebeyaw A and Yusuf
N: Aneurysmal bone cyst of the calcaneus. Ethiop Med J. 50:271–273.
2012.PubMed/NCBI
|
|
24
|
Carpenter B and Motley T: Bone matrix
therapy for aneurysmal bone cysts. J Am Podiatr Med Assoc.
95:394–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mohan, Susanta and Shivakumar: An unusual
case of aneurismal bone cyst of the calcaneus: A case report. J
Evolution of Medical and Dental Sciences. 3:4363–4366. 2014.
View Article : Google Scholar
|
|
26
|
Aratake M, Shigeyuki M, Atsushi H,
Takeuchi R and Saito T: Case of juxta-articular osteoid osteoma of
calcaneus mimicking arthritis. J Foot Ankle Surg. 51:237–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zouari L, Bousson V, Hamzé B, Roulot E,
Roqueplan F and Laredo JD: CT-guided percutaneous laser
photocoagulation of osteoid osteomas of the hands and feet. Eur
Radiol. 18:2635–2641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pogliacomi F and Vaienti E: Misdiagnosed
iuxta-articular osteoid osteoma of the calcaneus following an
injury. Acta Biomed. 74:144–150. 2003.PubMed/NCBI
|
|
29
|
Okuda R, Kinoshita M, Morikawa J, Jotoku
T, Shima H and Abe M: Tibialis spastic varus foot caused by osteoid
osteoma of the calcaneus. Clin Orthop Relat Res. 149–152. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lo AB, Chow AH, Wong WC, Hui JP and Yuen
MK: Osteoid osteoma of the calcaneum: A small painful lesion
causing confusing symptoms. Hong Kong Med J. 18:70–72.
2012.PubMed/NCBI
|
|
31
|
Migues A, Velan O, Solari G, Pace G,
Slullitel G and Araujo ES: Osteoid osteoma of the calcaneus:
Percutaneous radiofrequency ablation. J Foot Ankle Surg.
44:469–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sanhudo JA: Osteoid osteoma of the
calcaneus mimicking os trigonum syndrome: A case report. Foot Ankle
Int. 27:548–551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamada T, Matsubara H, Kimura H, Aikawa T,
Yoshida Y and Tsuchiya H: Intra-articular osteoid osteoma of the
calcaneus: A case report and review. Radiol Case Rep. 11:212–216.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daniilidis K, Martinelli N, Gosheger G,
Hoell S, Henrichs M, Vogt B, Hardes J and Vieth V: Percutaneous
CT-guided radio-frequency ablation of osteoid osteoma of the foot
and ankle. Arch Orthop Trauma Surg. 132:1707–1710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wallace AN, Tomasian A, Chang RO and
Jennings JW: Treatment of osteoid osteomas using a navigational
bipolar radiofrequency ablation system. Cardiovasc Intervent
Radiol. 39:768–772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hosalkar HS, Garg S, Moroz L, Pollack A
and Dormans JP: The diagnostic accuracy of MRI versus CT imaging
for osteoid osteoma in children. Clin Orthop Relat Res.
433:171–177. 2005. View Article : Google Scholar
|
|
37
|
Nogier A, De Pinieux G, Hottya G and
Anract P: Case reports: Enlargement of a calcaneal osteochondroma
after skeletal maturity. Clin Orthop Relat Res. 447:260–266. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koplay M, Toker S, Sahin L and Kilincoglu
V: A calcaneal osteochondroma with recurrence in a skeletally
mature patient: A case report. Cases J. 2:70132009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumar R, Anjana and Kundan M:
Retrocalcaneal Bursitis due to Rare Calcaneal Osteochondroma in
Adult Male: Excision and Outcome. J Orthop Case Rep. 6:16–19.
2016.PubMed/NCBI
|
|
40
|
Jung HG, Carag JA, Park JY, Bae EJ, Lim SD
and Kim HS: Osteochondroma of the calcaneus presenting as Haglund's
deformity. Foot Ankle Surg. 17:e20–e22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Blitz NM and Lopez KT: Giant solitary
osteochondroma of the inferior medial calcaneal tubercle: A case
report and review of the literature. J Foot Ankle Surg. 47:206–212.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akmaz I, Arpacioğlu MO, Pehlivan O,
Solakoglu C, Kiral A, Kaplan H and Rodop O: Calcaneal
osteochondroma. J Am Podiatr Med Assoc. 94:409–411. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sella EJ and Chrostowski JH: Calcaneal
osteochondromas. Orthopedics. 18:573–574. 1995.PubMed/NCBI
|
|
44
|
Nakanishi H, Araki N, Mukai K, Ohno H,
Matsui Y and Hosoya T: Soft-tissue osteochondroma in the calcaneal
pad: A case report. J Foot Ankle Surg. 40:396–400. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Murphey MD, Choi JJ, Kransdorf MJ,
Flemming DJ and Gannon FH: Imaging of osteochondroma: Variants and
complications with radiologic-pathologic correlation.
Radiographics. 20:1407–1434. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Davila JA, Amrami KK, Sundaram M, Adkins
MC and Unni KK: Chondroblastoma of the hands and feet. Skeletal
Radiol. 33:582–587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guedes A, Barreto B, Barreto Soares LG,
Athanazio DA and Athanazio PR: Calcaneal chondroblastoma with
secondary aneurysmal bone cyst: A case report. J Foot Ankle Surg.
49:2982010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dutt L, Schade VL and Manoso MW: Calcaneal
chondroblastoma with pathologic fracture and recurrence. J Foot
Ankle Surg. 54:258–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Otsuka T, Kobayashi M, Yonezawa M,
Kamiyama F, Matsushita Y and Matsui N: Treatment of chondroblastoma
of the calcaneus with a secondary aneurysmal bone cyst using
endoscopic curettage without bone grafting. Arthroscopy.
18:430–435. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ly JQ, LaGatta LM and Beall DP: Calcaneal
chondroblastoma with secondary aneurysmal bone cyst. AJR Am J
Roentgenol. 182:1302004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu D, Xiao P, Gao Z, Xue L, Wang L, Liu Y
and Han A: Chondroblastoma with adamantinoma differentiation of the
calcaneus. Pathology. 43:174–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bertram C, Popken F and Rutt J:
Intraosseous lipoma of the calcaneus. Langenbecks Arch Surg.
386:313–317. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ulucay C, Altintas F, Ozkan NK, Inan M and
Ugutmen E: Surgical treatment for calcaneal intraosseous lipomas.
Foot (Edinb). 19:93–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Narang S and Gangopadhyay M: Calcaneal
intraosseous lipoma: A case report and review of the literature. J
Foot Ankle Surg. 50:216–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Weinfeld GD, Yu GV and Good JJ:
Intraosseous lipoma of the calcaneus: A review and report of four
cases. J Foot Ankle Surg. 41:398–411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pappas AJ, Haffner KE and Mendicino SS: An
intraosseous lipoma of the calcaneus: A case report. J Foot Ankle
Surg. 53:638–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hassani M, Gharehdaghi M, Khooei AR,
Ghodsi E and Nazarzadeh H: Bilateral intraosseous tumor of the
calcaneus with imaging-pathologic discordance a case report and
literatures review. Arch Bone Jt Surg. 2:238–242. 2014.PubMed/NCBI
|
|
58
|
Aumar DK, Dadjo YB and Chagar B:
Intraosseous lipoma of the calcaneus: Report of a case and review
of the literature. J Foot Ankle Surg. 52:360–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Futani H, Fukunaga S, Nishio S, Yagi M and
Yoshiya S: Successful treatment of bilateral calcaneal intraosseous
lipomas using endoscopically assisted tumor resection. Anticancer
Res. 27:4311–4314. 2007.PubMed/NCBI
|
|
60
|
Muramatsu K, Tominaga Y, Hashimoto T and
Taguchi T: Symptomatic intraosseous lipoma in the calcaneus.
Anticancer Res. 34:963–966. 2014.PubMed/NCBI
|
|
61
|
Karthik K and Aarthi S: Intraosseous
lipoma of the calcaneus mimicking plantar fascitis. Foot Ankle
Surg. 17:e25–e27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Efrimescu CI, Bandorf N, Maxwell N and
Niall D: A rare case of calcaneal tumour in a young adult. BMJ Case
Rep. 2012:pii: bcr0220125896. 2012.PubMed/NCBI
|
|
63
|
Murphey MD, Carroll JF, Flemming DJ, Pope
TL, Gannon FH and Kransdorf MJ: From the archives of the AFIP:
Benign musculoskeletal lipomatous lesions. Radiographics.
24:1433–1466. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Minhas MS, Khan KM and Muzzammil M: Giant
cell tumour of foot bones-25 years experience in a tertiary care
hospital. J Pak Med Assoc. 65 Suppl 3:S67–S71. 2015.PubMed/NCBI
|
|
65
|
Kamal AF, Waryudi A, Effendi Z and Kodrat
E: Management of aggressive giant cell tumor of calcaneal bone: A
case report. Int J Surg Case Rep. 28:176–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ozer D, Er T, Aycan OE, Oke R, Coskun M
and Kabukcuoglu YS: May bone cement be used to treat benign
aggressive bone tumors of the feet with confidence? Foot(Edinb).
24:1–5. 2014.PubMed/NCBI
|
|
67
|
OConnor P, Gibbon W and Stone M:
Sonographic demonstration of fluid-fluid levels in an aneurysmal
bone cyst secondary to a giant cell tumour of the calcaneus.
Clinical Radiology Extra. 59:43–47. 2004. View Article : Google Scholar
|
|
68
|
Dhillon MS, Prasad Prabhudev A, Virk MS
and Aggarwal S: Multicentric giant cell tumor involving the same
foot: A case report and review of literature. Indian J Orthop.
41:154–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yale JF and Kaplan JA: Aneurysmal bone
cyst arising from a giant cell tumor of the calcaneus. J Am Podiatr
Med Assoc. 85:708–709. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Joshi A, Shahi R, Singh S and Chand P K C
BR: Calcaneal myxoid chondrosarcoma: A rare case. JNMA J Nepal Med
Assoc. 52:130–132. 2012.PubMed/NCBI
|
|
71
|
Roberts EJ, Meier MJ, Hild G, Masadeh S,
Hardy M and Bakotic BW: Chondromyxoid fibroma of the calcaneus: Two
case reports and literature review. J Foot Ankle Surg. 52:643–649.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jamshidi K, Mazhar FN and Yahyazadeh H:
Chondromyxoid fibroma of calcaneus. Foot Ankle Surg. 19:48–52.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ebrahimzadeh MH and Dallouei SR:
Chondromyxoid fibroma of the calcaneus. J Am Podiatr Med Assoc.
97:223–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Budny AM, Ismail A and Osher L:
Chondromyxoid Fibroma. J Foot Ankle Surg. 47:153–159. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Raatikainen TK, Kaarela OI, Holmström T,
Kyllönen AP, Teerikangas HE and Waris TH: Desmoplastic fibroma of
the calcaneus treated with a microvascular bone graft case report.
Scand J Plast Reconstr Surg Hand Surg. 33:111–116. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yu JS, Lawrence S, Pathria M, Resnick D
and Haghighi P: Desmoplastic fibroma of the calcaneus. Skeletal
Radiol. 24:451–454. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Miyayama H, Sakamoto K, Ide M, Ise K,
Hirota K, Yasunaga T and Ishihara A: Aggressive osteoblastoma of
the calcaneus. Cancer. 71:346–353. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schujman O: Benign osteoblastoma of the
calcaneus. Curr Orthop Prac. 197:1977.
|
|
79
|
Mestdagh C, Forgeois P and LecomteHoucke
M: A rare entity: The calcaneus intraosseous ganglion Revue de
Chirurgie Orthopedique and Reparatrice de l'Appareil Moteur.
83:274–277. 1997.
|
|
80
|
Murff R and Ashry HR: Intraosseous ganglia
of the foot. J Foot Ankle Surg. 33:396–401. 1994.PubMed/NCBI
|
|
81
|
Tadashi H, Takanori H and Howard D:
Epithelioid fibrous histiocytoma of bone. Int J Surg Pathol.
3:43–48. 1995. View Article : Google Scholar
|
|
82
|
Keskinbora M, Köse O, Karslioglu Y,
Demiralp B and Basbozkurt M: Another cystic lesion in the
calcaneus: Benign fibrous histiocytoma of bone. J Am Podiatr Med
Assoc. 103:141–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kinberg P: Xanthoma of a calcaneus. J Foot
Ankle Surg. 37:531–534. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ahmed G, Al Dosari M, El-Mahi M and
Abolfotouh SM: Primary xanthoma of calcaneus bone: Case report. Int
J Surg Case Rep. 5:699–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yamamoto T, Kawamoto T, Marui T, Akisue T,
Hitora T, Nagira K, Yoshiya S and Kurosaka M: Multimodality imaging
features of primary xanthoma of the calcaneus. Skeletal Radiol.
32:367–370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kapukaya A, Arslan H, Ozkul E and Mizrak
B: Primary xanthofibroma in the calcaneus: A case report. Acta
Orthop Traumatol Turc. 45:203–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hakozaki M, Tajino T, Watanabe K, Yamada
H, Kikuchi S, Hojo H, Ishida T and Konno S: Intraosseous spindle
cell hemangioma of the calcaneus: A case report and review of the
literature. Ann Diagn Pathol. 16:369–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Taslakian B, Issa G, Saab R, Jabbour MN
and Khoury NJ: Calcaneal osteosarcoma: A rare cause of heel pain in
the paediatric population. BMJ Case Rep. 2013:2013. View Article : Google Scholar
|
|
89
|
Choong PF, Qureshi AA, Sim FH and Unni KK:
Osteosarcoma of the foot: A review of 52 patients at the mayo
clinic. Acta Orthop Scand. 70:361–364. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rahbar M and Mardanpour K: Calcaneal
osteosarcoma; a case report. Iranian J Med Sci. 33:121–123.
2008.
|
|
91
|
Chou LB, Malawer MM, Kollender Y and
Wellborn CC: Prosthetic replacement for intramedullary calcaneal
osteosarcoma: A case report. Foot Ankle Int. 19:411–415. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Leithner A, Bodo K, Scheipl S, Radl R,
Kastner N and Windhager R: Two cases of calcaneal osteosarcomas
presenting as aneurysmal bone cysts. Foot Ankle Int. 25:815–818.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chou LB and Malawer MM: Osteosarcoma of
the calcaneus treated with prosthetic replacement with twelve years
of followup: A case report. Foot Ankle Int. 28:841–844. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tsuji Y, Kusuzaki K, Kanemitsu K,
Matsumoto T, Ishikawa Y and Hirasawa Y: Calcaneal osteosarcoma
associated with Werner syndrome. J Bone Joint Surg Am.
82:1308–1313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mulligan ME and Kransdorf MJ: Sequestra in
primary lymphoma of bone: Prevalence and radiologic features. AJR
Am J Roentgenol. 160:1245–1248. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jamshidi K and Ahangar AF: Primary
lymphoma of the calcaneus: A Case Report. Shafa Ortho J. 3:1–4.
2016.
|
|
97
|
Niemczyk M, Babiak I, Wyzgal J, Pykalo R
and Paczek L: Primary T-cell lymphoma of the calcaneus in the
kidney transplant recipient. Nephrol Dial Transplant. 22:1475–1476.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Blume P, Charlot-Hicks F and Mohammed S:
Case report and review of primary bone diffuse large B-cell
lymphoma involving the calcaneus. J Foot Ankle Surg. 52:666–672.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
White LM, Siegel S, Shin SS, Weisman MH
and Sartoris DJ: Primary lymphoma of the calcaneus. Skeletal
Radiol. 25:775–778. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Skorman SE and Martin R: Primary lymphoma
of the calcaneus with recurrence in the distal tibia: A case
report. J Foot Ankle Surg. 38:278–282. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Singh DP, Dhillon MS, Sur RK, Sharma SC
and Radotra BD: Primary lymphoma of the bones of the foot:
Management of two cases. Foot Ankle. 11:314–316. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ogose A, Unni KK, Swee RG, May GK, Rowland
CM and Sim FH: Chondrosarcoma of small bones of the hands and feet.
Cancer. 80:50–59. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
de Moraes FB, Linhares ND, de Souza
Domingues PM, Warzocha VN and Soares JM: Calcaneal chondrosarcoma:
A case report. Rev Bras Ortop. 49:409–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Babu Suresh S, Sudhakar G, Kiran KR, Babu
Suresh TV and Sridevi MU: Calcaneal chondrosarcoma: A case report.
Foot (Edinb). 23:166–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Nagmani S, Rakesh J, Aditya A, Sandeep P,
Arjun RH and Debasis G: Clear cell chondrosarcoma calcaneum-a case
report and review of literature. Foot (Edinb). 25:36–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kwon JW, Choi JA, Kwack KS, Oh JH, Chung
JH and Kang HS: Myxoid chondrosarcoma in the calcaneus: A case
report with MR imaging findings. Skeletal Radiol. 36 Suppl
1:S82–S85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wagner A, Venbrocks RA and Fuhrmann RA:
Chondrosarcoma of the calcaneus: Amputation or resection with limb
preservation: A case report. Foot Ankle Int. 28:1090–1094. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Casadei R, Magnani M, Biagini R and
Mercuri M: Prognostic factors in ewing's sarcoma of the foot. Clin
Orthop Relat Res. 420:230–238. 2004. View Article : Google Scholar
|
|
109
|
Tiwari AK, Sisodia Y and Gautam Y: Ewing s
sarcoma of the calcaneus bone. Sri Lanka J Surg. 32:4–5. 2014.
View Article : Google Scholar
|
|
110
|
Agarwal N and Sabir AB: Ewing's sarcoma of
the calcaneus with metastases to the tibia and fibula. Acta Orthop
Belg. 74:270–272. 2008.PubMed/NCBI
|
|
111
|
Jalal H, Belhadj Z, Enneddam H, Madhar M,
Fikry T, Essadki O and Ousehal A: Contribution of magnetic
resonance imaging in the diagnosis of talus skip metastases of
Ewing's sarcoma of the calcaneus in a child: A case report. J Med
Case Rep. 5:4512011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Traki L, Rkain H, Aktaou S, Raissouni H,
Lazrak N, Benbouazza K and Hajjaj-Hassouni N: Sonography can be
useful in diagnosis of ewing sarcoma of the calcaneus. J Ultrasound
Med. 30:1439–1441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Demiralp B, Erler K, Ozturan EK, Bek D,
Ozdemir T and Kurt B: An uncommon presentation of malignant fibrous
histiocytoma of the calcaneus. J Am Podiatr Med Assoc. 97:218–222.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Daibata M, Bandobashi K and Taguchi H:
Calcaneus involvement by multiple myeloma. Am J Hematol.
80:311–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rong Y, Sato K, Yamamura S, Sugiura H,
Katagiri H and Iwata H: Malignant hemangioendothelioma of the left
calcaneus associated with fever and hematological abnormalities.
Skeletal Radiol. 26:64–66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Goldenberg RR: Well Differentiated
fibrosarcoma of the calcaneus: Report of a case treated by
resection. J Bone Joint Surg. 42:1151–1155. 1960. View Article : Google Scholar
|
|
117
|
Gahlot GP, Mridha AR, Nath D, Khan SA and
Gamanagatti S: Intraosseous primary malignant peripheral nerve
sheath tumor of the calcaneus: An unusual case and review of
literature. Indian J Pathol Microbiol. 58:220–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Balaji GG, Arockiaraj JS, Roy AC and
Deepak B: Primary epithelioid angiosarcoma of the calcaneum: A
diagnostic dilemma. J Foot Ankle Surg. 53:239–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Madhuri V, Balakumar B, Walter NM, Prakash
H, Dutt V and Chowdhurie L: Function after total calcanectomy for
malignant tumor in a child: Is complex reconstruction necessary? J
Foot Ankle Surg. 51:71–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chandrasekar CR, Mohammed R, Rafalla AA
and Grimer RJ: Adamantinoma of the calcaneum-a case report. Foot
(Edinb). 19:58–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Oommen AT, Madhuri V and Walter NM: Review
of foot tumors seen in a university tumor institute. Indian J
Cancer. 46:234–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ruggieri P, Angelini A, Jorge FD, Maraldi
M and Giannini S: Review of foot tumors seen in a university tumor
institute. J Foot Ankle Surg. 53:282–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Toepfer A, Lenze U and Harrasser N:
Calcaneal ossoscopy. Arthrosc Tech. 5:e627–e631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Toepfer A, Lenze U, Gerdesmeyer L, Pohlig
F and Harrasser N: Endoscopic resection and allografting for benign
osteolytic lesions of the calcaneus. Springerplus. 5:4272016.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Dijkstra PU, Geertzen JH, Stewart R and
van der Schans CP: Phantom pain and risk factors: A multivariate
analysis. J Pain Symptom Manage. 24:578–585. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li J and Wang Z: Surgical treatment of
malignant tumors of the calcaneus. J Am Podiatr Med Assoc.
104:71–76. 2014. View Article : Google Scholar : PubMed/NCBI
|