Introduction
A previous study has demonstrated that chronic
low-grade inflammation is associated with aging and contributes to
age-associated diseases (1). For
example, cancer frequently occurs in the elderly, which is
associated with their inflammatory background (2). Recent advances have demonstrated that
the balance of cluster of differentiation (CD)4+ T cell
subsets and their cytokines serves an important role in maintaining
inflammatory-immune homeostasis and tumorigenesis (3). In 2009, Eyerich et al (4) reported a new CD4+ T cell
subset, T-helper 22 (Th22) cells, which express C-C chemokine
receptor 4 (CCR4), CCR6 and CCR10. Th22 cells mainly secrete
interleukin 22 (IL-22), IL-13, IL-26 and tumor necrosis factor-α
(TNF-α) (5). Th22 cell function is
mainly exerted through the binding of IL-22 to its receptor
(IL-10R2/IL-22R1) (6). Since IL-22R1
is selectively expressed by non-immune cells, particularly
keratinocytes, gastrointestinal and respiratory epithelial cells
(7), Th22 cells are considered as key
cells which transmit the inflammatory signal to the surrounding
tissues. Increased IL-22 levels in the circulation and tumor
microenvironment have been identified in liver, gastric, colon and
pancreatic cancer and are associated with tumor initiation and
progression (8). Nevertheless, the
exact underlying molecular mechanism of how IL-22 influences
tumorigenesis remains unclear.
Th22 cells are similar to and have synergistic
effects with Th17 cells in a variety of inflammatory-immune
diseases (9). Th22 and Th17 cells
express skin-homing receptors CCR4 and CCR10, and are regulated by
IL-6, IL-1 and IL-23 (10,11). We have previously demonstrated that
the proportion of Th17 cells significantly increases with age and
progression of colorectal cancer (12). In addition, the inflammatory aging
factors IL-6 and TNF-α are able to stimulate the transformation of
naïve CD4+ T cells into Th22 cells (13,14). These
results suggest that Th22 cells may participate in aging and
tumorigenesis.
Chronic inflammation and immune-suppression are
often complementary (15). It has
been suggested that accumulation of immune suppressor cells is one
of the causes of immune system dysfunction in the elderly (16). Myeloid-derived suppressor cells
(MDSCs) are an important type of immune suppressor cells (17). Various inflammation-associated factors
including IL-6, TNF-α and IL-22 are able to induce MDSCs (18). Furthermore, MDSCs express IL-6, TNF-α
and other cytokines to regulate inflammation and CD4+ T
cell differentiation (19). However,
the role for MDSCs in aging and tumorigenesis, and their
association with Th22 cells, remains to be determined.
Gastric cancer is an inflammation-associated type of
cancer and is the third most common cause of cancer-related
mortality worldwide. Furthermore, gastric cancer is also associated
with age-related immunodeficiency (20). To explore the role of Th22 cells,
IL-22 and MDSCs in aging and tumorigenesis, in the present study
the levels of peripheral Th22, Th17 and Th1 cells, and MDSC, and
plasma levels of IL-22, IL-6 and TNF-α were determined in elderly
patients with gastric cancer (EGC), elderly healthy controls (HE)
and young healthy controls (HY).
Materials and methods
Subjects and samples
Fresh peripheral blood was obtained from EGC (n=39;
median age, 73 years; range, 65–91 years; male/female, 24:15) from
The First Affiliated Hospital of Dalian Medical University, Dalian,
China, between October 2014 and December 2015. The patients were
histologically diagnosed. Patients receiving radiotherapy,
chemotherapy or immunotherapy were excluded from the present study.
Tumors were classified and staged according to the
tumor-node-metastasis (TNM) classification system of the
International Cancer Control/American Joint Committee on Cancer
(Edition 7) (21). Of the 39
patients, 13 were classified as early stage (Stage I/II) and 26
were at advanced stage (Stage III/IV). In addition, fresh
peripheral blood of HE (n=32; median age, 76 years; range, 65–90
years; male/female, 16:16) and HY (n=31; median age, 27 years;
range, 24–35 years; male/female, 14:17) were recruited from the
Health Check-Up Center of The First Affiliated Hospital during the
same period. Paraffin sections (3-µm) from tumor tissues and
non-tumor tissues (>5 cm from the tumor lesion) were collected
post-operatively from another 40 patients with gastric cancer (age
range, 65–78 years). The clinical characteristics of the patients
are summarized in Table I. The
present study has been approved by the Ethics Committee of The
First Affiliated Hospital of Dalian Medical University (Dalian,
China), and written informed consent was obtained from each
subject.
 | Table I.Association of IL-22 protein
expression with clinicopathological data from elderly patients with
gastric cancer. |
Table I.
Association of IL-22 protein
expression with clinicopathological data from elderly patients with
gastric cancer.
|
| IL-22+
cells/total cells |
|
|---|
|
|
|
|
|---|
|
| Low
infiltration | High
infiltration |
|
|---|
| Characteristic | n | <1% | 1–5% | 5–10% | ≥10% | χ2 |
P-valuea |
|---|
| Total | 40 | 4 | 15 | 14 | 7 |
|
|
| Sex |
|
|
|
|
| 0.345 | 0.457 |
|
Male | 31 | 4 | 12 | 11 | 4 |
|
|
|
Female | 9 | 0 | 3 | 3 | 3 |
|
|
|
Differentiation |
|
|
|
|
| 0.150 | 0.755 |
|
Well-moderate | 16 | 2 | 5 | 6 | 3 |
|
|
|
Poor | 24 | 2 | 10 | 8 | 4 |
|
|
| Depth of
invasion |
|
|
|
|
| 6.686 | 0.017a |
|
T1+T2 | 13 | 3 | 7 | 2 | 1 |
|
|
|
T3+T4 | 27 | 1 | 8 | 12 | 6 |
|
|
| Lymph node
metastasis |
|
|
|
|
| 1.766 | 0.216 |
|
Negative | 23 | 2 | 11 | 9 | 1 |
|
|
|
Positive | 17 | 2 | 4 | 5 | 6 |
|
|
| AJCC cancer
stage |
|
|
|
|
| 8.087 | 0.009a |
|
I–II | 16 | 1 | 11 | 2 | 2 |
|
|
|
III–IV | 24 | 3 | 4 | 12 | 5 |
|
|
| Tumor size, cm |
|
|
|
|
| 0.973 | 0.360 |
| ≤5 | 18 | 1 | 6 | 8 | 3 |
|
|
|
>5 | 22 | 3 | 9 | 6 | 4 |
|
|
Flow cytometric analysis of Th1, Th17
and Th22 cells
Peripheral blood was collected from all subjects and
cultured under stimulation conditions. Heparinized peripheral whole
blood (400 µl) in an equal volume of RPMI-1640 medium (Tiangen
Biotech Co., Ltd., Beijing, China) were incubated for 4 h at 37°C
with 5% CO2 in the presence of 25 ng/ml phorbol
myristate acetate, 1 µg/ml ionomycin and 1.7 µg/ml monensin (all
from Enzo Biochem, Inc., Farmingdale, NY, USA). Following
incubation, the cells were fixed and permeabilized using FIX and
PERM Reagent (MultiSciences Co., Ltd., Hangzhou, China), followed
by staining with peridinin-chlorophyll-protein (PerCP)-cyanine (Cy)
5.5-conjugated anti-CD4 monoclonal antibody (cat. no., 45-0048-42;
dilution,1:20; eBioscience; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at room temperature in the dark for 30 min. The
cells were next stained with fluorescein isothiocyanate
(FITC)-conjugated anti-interferon (IFN)-γ (cat. no., 11-7319-82;
dilution, 1:50), phycoerythrin (PE)-conjugated anti-IL-17 (cat.
no., 12-7179-42; dilution, 1:35) and allophycocyanin
(APC)-conjugated anti-IL-22 monoclonal antibodies (cat. no.,
50-7229-42; dilution, 1:35) at room temperature in the dark for 25
min (all from eBioscience; Thermo Fisher Scientific, Inc.). Isotype
controls [by staining with PerCP/Cy5.5-conjugated rat
immunoglobulin G (IgG) 2a (cat. no. 45-4321-80; dilution,1:20),
FITC-conjugated rat IgG1 (cat. no. 11-4301-82; dilution,
1:50), PE-conjugated rat IgG1 (cat. no. 12-4301-82;
dilution, 1:35), and APC-conjugated rat IgG1 (cat. no.
17-4301-82; dilution, 1:35) (all from eBioscience; Thermo Fisher
Scientific, Inc.) at room temperature in the dark for corresponding
time respectively] were used to enable correct compensation and
confirm antibody specificity. Stained cells were analyzed by flow
cytometric analysis using a FACSCalibur cytometer equipped with
CellQuest software (version 5.2; BD Biosciences, Franklin Lakes,
CA, USA).
Flow cytometric analysis of MDSCs
EDTA-anticoagulated peripheral whole blood was
collected from all subjects. Blood (100 µl) was mixed with
FITC-conjugated anti-human leucocyte antigen-antigen D-related
(HLA-DR) (cat. no., 11-9952-41, 1:20), APC-conjugated anti-CD11b
(cat. no., 17-0118-41; dilution; 1:20) and PE-Cy5-conjugated
anti-CD33 monoclonal antibodies (cat. no., 15-0339-41; dilution,
1:20) (all from eBioscience; Thermo Fisher Scientific, Inc.), and
incubated in the dark for 15 min. Each sample was subsequently
mixed with 1 ml lysis buffer (Beyotime Institute of Biotechnology,
Jiangsu, China) and subjected to flow cytometric analysis. In each
case, staining was compared with that of the appropriately labeled
isotype control antibody.
ELISA
Plasma was collected from peripheral blood via
centrifugation at 1,500 × g at 4°C for 5 min. Plasma IL-22, IL-6
and TNF-α levels were determined using an ELISA kit (MultiSciences
Co., Ltd., Zhejiang, China), according to the manufacturer's
protocol. The minimal concentration detection limit of IL-22, IL-6
and TNF-α was 2.56, 0.37 and 0.42 pg/ml, respectively.
Immunohistochemistry
Paraffin sections, (3-µm) from tissue specimens were
deparaffinized by immersion in xylene (15 min ×2), rehydrated using
graded ethanol (anhydrous ethanol for 5 min ×3, 90% ethanol for 5
min, 80% ethanol for 5 min and 70% ethanol for 5 min). Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide for 10
min at room temperature, followed by washes with PBS (5 min ×3) and
heated in citrate buffer (10 mM, pH 6.0) at 100°C for 3 min. This
was followed by blocking with 50 µl 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) per paraffin
section for 30 min at room temperature, followed by washes with PBS
(5 min ×3). Following blocking, the sections were incubated with
anti-IL-22 antibody (cat. no; ab18499; 1:100; Abcam, Cambridge, UK)
at room temperature for 1 h, followed by incubation with
anti-rabbit IgG secondary antibody (Boster Biological Technology,
Pleasanton, CA, USA) at 37°C for 20 min. Following a wash with PBS,
staining was visualized with 3,3′-diaminobenzidine (Beyotime
Institute of Biotechnology) as a chromogen at room temperature for
3 min. All sections were counterstained with hematoxylin at room
temperature for 1 min and viewed using a light microscope. Two
independent investigators randomly selected 10 fields at ×400
magnification and counted the proportion of stained cells. The
results were categorized into four groups on the basis of the
proportion of positive cells (<1, 1–5, 5–10 and ≥10%), with
>1% positive cells recorded as (−), 1–5% positive cells recorded
as (+), 5–10% positive cells recorded as (++), and ≥10% positive
cells recorded as (+++); (−) and (+) were defined as low
infiltration, and (++) and (+++) were defined as high infiltration.
The association between the expression of IL-22 and the patient's
sex, differentiation, depth of invasion, lymph node metastasis,
cancer stage and tumor size were analyzed.
Statistical analysis
Data were analyzed using SPSS software (version
19.0; IBM Corp., Armonk, NY, USA) or GraphPad Prism software
(version 5.0; GraphPad Software, Inc., La Jolla, CA, USA) and
expressed as the mean ± standard deviation. Comparisons between two
groups were assessed using a non-paired Student's t-test.
Comparison among the three different experimental groups was
performed using one-way analysis of variance, and differences
between two groups were determined using Newman-Keuls multiple
comparison unless the data were not normally distributed, in which
case a Kruskal-Wallis test and Nemenyi test were used. Correlation
analysis was adopted for Pearson's or Spearman's correlation
depending on data distribution. The quality of the data was
analyzed using a χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Flow cytometric analysis of Th22, Th17
and Th1 cells in patients with gastric cancer and healthy
controls
Flow cytometric analysis was used to determine the
distribution of Th22, Th17 and Th1 cells in EGC, HE and HY
(Fig. 1). Th22, Th17 and Th1 cells
were defined as
CD4+IFN-γ−IL-17−IL-22+,
CD4+IFN-γ−IL-17+IL-22−
and CD4+IFN-γ+, respectively.
 | Figure 1.Flow cytometric analysis was used to
determine the distribution of Th22, Th17 and Th1 cells in EGC, HE
and HY (Fig. 1). Flow cytometric
analysis of Th22, Th17 and Th1 cells in peripheral whole blood from
EGC (n=39), HE (n=32) and HY (n=31). (A) Lymphocytes were gated in
P1 using flow cytometry. CD4+IFN-γ−
lymphocytes were gated in P2 using flow cytometry, and
representative results of flow cytometric analyses for (B) Th1
(CD4+IFN-γ+), (C) Th22
(CD4+IFN-γ−IL-17−IL-22+)
and Th17
(CD4+IFN-γ−IL-17+IL-22−)
cells in the three groups of subjects are presented. The number of
cells stained in EGC, HE and HY in P2 were 2,654, 4,696 and 5,185,
respectively. The proportion of (D) Th22, (E) Th17 and (F) Th1
cells in the three groups of subjects. The proportion of (G) Th22
and (H) Th17 cells in peripheral whole blood derived from patients
with early (n=13) or advanced (n=26) gastric cancer. The
association between the proportion of (I) Th22 and Th17 cells, (J)
Th22 and Th1 cells, and (K) Th17 and Th1 cells, in peripheral whole
blood of all subjects. *P<0.05, **P<0.01, ***P<0.001. ns,
not significant; Th, T helper; EGC, elderly patients with gastric
cancer; HE, elderly healthy patients; HY, young healthy patients;
CD, cluster of differentiation; IFN-γ, interferon-γ; IL,
interleukin; SSC, side-scattered light; FSC, forward-scattered
light; APC, allophycocyanin; FITC, fluorescein isothiocyanate;
PerCP, peridinin-chlorophyll-protein; Cy5.5, cyanine 5.5. |
The proportion of Th22 cells was significantly
increased in HE compared with that in HY (0.79±0.27 vs. 0.36±0.21%;
P<0.001; Fig. 1C and D).
Furthermore, the proportion of Th22 cells was significantly
increased in EGC compared with that in HE (1.16±0.33 vs.
0.79±0.27%; P<0.001; Fig. 1C and
D). Similarly, Th17 cells were detected at an increased
proportion in EGC compared with that in HE (2.91±0.93 vs.
2.24±0.79%; P<0.001; Fig. 1E).
Furthermore, the proportion of Th17 cells was significantly
increased in HE compared with that in HY (2.24±0.79 vs. 1.50±0.63%;
P<0.001; Fig. 1E). However, no
significant difference was observed in the distribution of Th1
cells between EGC and HE or HY groups (7.78±1.84 vs. 7.48±1.55 or
7.65±1.60%; P>0.05; Fig. 1F). In
addition, the fraction of peripheral Th22 cells was increased in
patients with advanced gastric cancer compared with patients with
early-stage gastric cancer (1.25±0.30 vs. 0.98±0.32%; P<0.05;
Fig. 1G) and Th17 (3.20±0.90 vs.
2.33±0.72%; P<0.01; Fig. 1H).
Further analysis of IL-22+ T cell subsets
identified that there was a positive correlation between the
proportion of Th22 and Th17 cells (R2=0.4885;
P<0.0001; Fig. 1I). In contrast,
no correlation between the proportion of Th1 cells and the
proportion of Th22 cells (R2=0.0028; P=0.5942; Fig. 1J) or Th17 cells (R2=0.0012;
P=0.7271; Fig. 1K) was
identified.
Flow cytometric analysis of MDSCs in
patients with gastric cancer and healthy controls
Additionally, the distribution of MDSCs was assessed
using flow cytometric analysis. MDSCs were defined as
HLA-DR− CD33+CD11b+. A
significantly increased proportion of MDSCs was detected in EGC
(3.06±1.01%; P<0.001) compared with HE (1.59±0.98) and HY
(1.53±0.88%; Fig. 2A-D). Furthermore,
the proportion of MDSCs was increased in patients with advanced
disease relative to those with early-stage gastric cancer
(3.30±0.94 vs. 2.58±1.02%; P<0.05; Fig. 2E). No significant difference was
observed in the distribution of MDSCs between HE and HY. Positive
correlations were identified in the proportion of MDSCs and Th22
cells (R2=0.2295; P=0.002; Fig. 2F), and Th17 cells
(R2=0.1766; P=0.0077; Fig.
2G). However, no correlation was identified between the
proportion of MDSCs and that of Th1 cells (R2=0.0067;
P=0.6197; Fig. 2H).
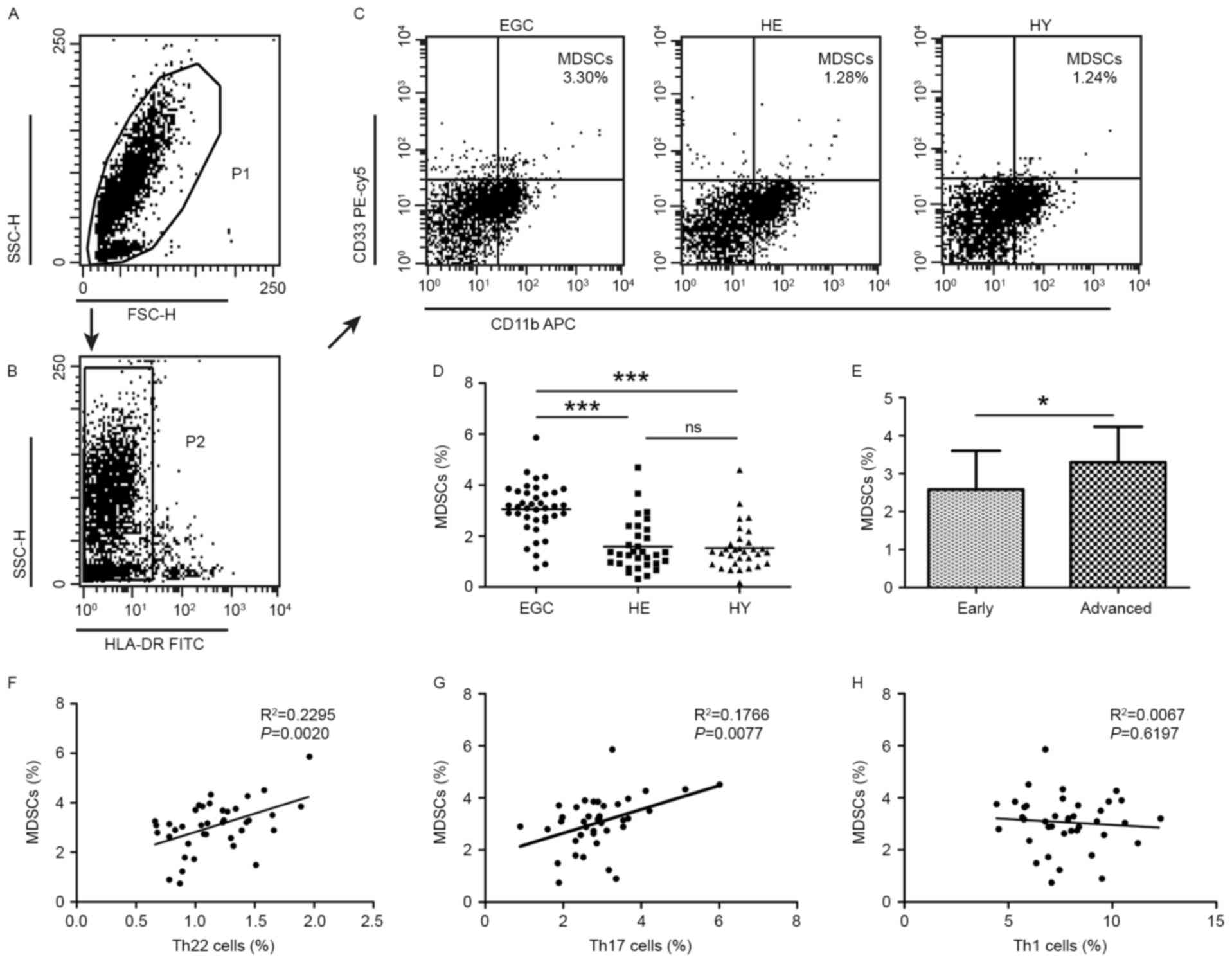 | Figure 2.Flow cytometric analysis of MDSCs in
peripheral whole blood from EGC (n=39), HE (n=32) and HY (n=31).
Gating routine in (A) P1 and (B) P2 successively for MDSCs
(HLA-DR−CD33+CD11b+) subsets and
(C) representative results of flow cytometric analyses for MDSCs in
the three groups of subjects. The number of cells in EGC, HE and HY
in P2 were 8,394, 8,004 and 8,224, respectively. (D) The proportion
of MDSCs in the three groups of subjects. (E) The proportion of
MDSCs in peripheral whole blood derived from patients with early
(n=13) or advanced (n=26) gastric cancer. The association between
the proportion of MDSCs and (F) Th22, (G) Th17 and (H) Th1 cells in
peripheral whole blood of elderly patients with cancer. *P<0.05,
***P<0.001. ns, not significant; MDSC, myeloid-derived
suppressor cells; EGC, elderly patients with gastric cancer; HE,
elderly healthy patients; HY, young healthy patients; HLA-DR, human
leukocyte antigen-antigen D-related; CD, cluster of
differentiation; Th, T helper; SSC, side-scattered light; FSC,
forward-scattered light; PerCP, peridinin-chlorophyll-protein;
Cy5.5, cyanine 5.5; APC, allophycocyanin; FITC, fluorescein
isothiocyanate. |
Plasma levels of IL-22, IL-6 and TNF-α
in patients with gastric cancer and healthy controls
Next, the plasma levels of IL-22, IL-6 and TNF-α
were examined in patients with gastric cancer and control subjects
using ELISA. The plasma IL-22 level was significantly increased in
EGC (151.45±33.56 pg/ml) compared with HE (123.31±22.80 pg/ml;
P<0.001) and HY (109.25±28.50 pg/ml; P<0.001; Fig. 3A). No difference in IL-22 levels was
observed between HE and HY. In EGC, HE and HY, plasma IL-6 levels
were 45.90±8.60, 26.68±7.23 and 18.17±7.95 pg/ml, respectively
(P<0.001; Fig. 3B). Plasma TNF-α
levels were increased in HE compared with HY (38.66±12.94 vs.
27.40±11.97 pg/ml; P<0.01) and were further increased in EGC
(59.70±17.38 pg/ml; P<0.001; Fig.
3C). In addition, a significant difference in IL-22 plasma
levels was observed between patients with advanced-stage and
early-stage gastric cancer (168.87±41.44 vs. 131.92±33.93 pg/ml;
P<0.01; Fig. 3D). No significant
differences between patients with Stage I–II and Stage III–IV
gastric cancer regarding IL-6 levels (44.62±10.44 vs. 46.54±7.67
pg/ml; Fig. 3E) or TNF-α levels
(54.81±17.32 vs. 62.16±17.23 pg/ml; Fig.
3F) were observed. Positive correlations between proportions of
Th22 cells and plasma IL-22 (R2=0.4039; P<0.0001;
Fig. 3G), IL-6 (R2=0.6360;
P<0.0001; Fig. 3H), and TNF-α
(R2=0.5094; P<0.0001; Fig.
3I) levels were observed.
 | Figure 3.Plasma IL-22, IL-6 and TNF-α levels
from EGC (n=39), HE (n=32) and HY (n=31) were evaluated using
ELISA. Plasma levels of (A) IL-22, (B) IL-6 and (C) TNF-α in the
three groups. Plasma levels of (D) IL-22, (E) IL-6 and (F) TNF-α in
patients with early (n=13) or advanced (n=26) stages of the
disease. The association between the plasma (G) IL-22, (H) IL-6 and
(I) TNF-α levels and the proportion of Th22 cells in peripheral
whole blood of all subjects. **P<0.01, ***P<0.001. ns, not
significant; IL, interleukin; TNF-α, tumor necrosis factor-α; EGC,
elderly patients with gastric cancer; HE, elderly healthy patients;
HY, young healthy patients; Th, T helper. |
Expression of IL-22 protein in tumor
tissues
In order to determine whether IL-22 accumulates in
tumor tissues, IL-22 protein expression in tumor sections obtained
from 40 patients with gastric cancer was measured using
immunohistochemical analysis. IL-22-positive cells were present at
a significantly increased proportion within the tumor tissue and
infiltrating lymphocytes around the tumor (Fig. 4A) when compared with normal gastric
tissue. Consistently, normal gastric tissues contained no or only a
small number of IL-22-positive cells (Fig. 4B). Furthermore, IL-22 protein
expression was increased in Stage III–IV tumors compared with Stage
I–II tumors (Fig. 4C and D). The
IL-22 expression was further examined with respect to other
clinicopathological characteristics of patients with gastric
cancer. The presence of IL-22-positive cells was significantly
associated with the depth of invasion and AJCC cancer stage
(P<0.05), but not with sex, differentiation, lymph node
metastasis or tumor size (Table
I).
Discussion
Th22 cells, a subset of CD4+ T cells, are
implicated in inflammatory-immune diseases including rheumatoid
arthritis (22), Behcet disease
(23), systemic sclerosis (24) and various types of cancer (25–27). The
results of the present study identified that the fraction of
peripheral Th22 and Th17 cells in HE increased when compared with
HY, suggesting that the balance of Th22 and Th17 cells may alter
during aging. Furthermore, the fraction of Th22 and Th17 cells was
increased in EGC when compared with either HE or HY. In addition,
the proportion of Th22 and Th17 cells was increased in patients
with advanced gastric cancer when compared with patients with
early-stage tumors. Indeed, these results are consistent with those
of our previous study performed in colorectal cancer (12). IL-6, IL-1 and IL-23 are Th22 as well
as Th17 cell polarization-inducing factors (10,11), which
may explain the observed positive correlation between Th22 cells
and Th17 cells. Collectively, the results from the present study
suggested that Th22 and Th17 cells may serve a role in the
progression of gastric cancer in the elderly.
IL-22 is the primary functional factor of Th22 cells
and is upregulated in several types of cancer (28,29). In
the present study, it was identified that IL-22 was increased in
EGC compared with HE and HY. In addition, IL-22 levels were
identified to be associated with the progression of gastric cancer
as demonstrated by the significant differences in IL-22 expression
observed between distinct gastric cancer stages. These results
imply that IL-22 may serve a role in gastric cancer progression in
the aging and elderly. To further examine the possible association
of IL-22 with other clinicopathological characteristics of elderly
patients with gastric cancer, immunohistochemical analysis of IL-22
protein expression was performed in tumors and the corresponding
normal tissue. The results identified that IL-22 was only expressed
by intratumoral and infiltrating lymphocytes around the tumor,
whereas IL-22 expression was rarely detected in normal tissue.
These results suggest an autocrine mode of IL-22 expression in
gastric cancer tissue. Additionally, these results support the
previously established idea that tissue inflammatory and immune
status may create a specific microenvironment that is able to
promote the development of tumors (30). Furthermore, there was no significant
difference in IL-22 plasma levels between HE and HY. A possible
reason for this result is that Th22 cells also secrete other
cytokines and therefore IL-22 expression levels do not fully
reflect the role of Th22 cells in the regulation of inflammatory
senescence (4–6).
A variety of cytokines and chemokines are able to
cause an increase in the fraction of Th22 cells (31). Studies have demonstrated that
increased levels of serum IL-6 and TNF-α in elderly people are
associated with cognitive discord and brain atrophy, leading to the
proposal that these cytokines are aging inflammatory cytokines
(32,33). In the present study, plasma levels of
IL-6 and TNF-α were examined in EGC in comparison with HY and HE.
Although the difference in IL-6 and TNF-α levels among the three
groups was statistically significant, no difference was observed
when these cytokine levels were compared with patients with early-
and advanced-stage gastric cancer. These results are not in
agreement with those of previous studies (34,35). One
of the possible explanations for this inconsistency may be the fact
that gastric cancer progression is a result of a number of factors
and a relatively small number of gastric cancer cases were analyzed
in the present study. Furthermore, a positive correlation between
IL-22, IL-6, and TNF-α levels and Th22 cells was identified in all
three examined groups. These results are consistent with the fact
that IL-22 is mainly secreted from Th22 cells, and that plasma IL-6
and TNF-α levels are dependent on Th22 cells (14). Furthermore, TNF-α promotes the
production of Th22 cells, which in turn secrete TNF-α (5,6).
Therefore, this positive feedback may lead to continuously
persistent chronic inflammation.
MDSCs are immunosuppressive cells that secrete
several inflammatory factors under various physiological and
pathophysiological states including cancer, chronic infection,
trauma and graft-vs.-host reactions (36). Chronic inflammation and immune
inhibition are mutually supportive (15). In the present study, the presence of
peripheral MDSCs was analyzed in EGC, HE and HY, and it was
identified that the proportion of peripheral MDSCs was
significantly increased in EGC compared with HE. Furthermore, the
proportion of MDSCs was increased in patients at a more advanced
cancer stage. Nevertheless, no significant difference in MDSCs was
observed between HE and HY. These results suggest that the increase
in MDSCs is associated with the development and the progression of
gastric cancer, but not with aging. A previous study has
demonstrated that MDSCs were able to promote tumorigenesis via high
expression of arginase-1, induction of nitric oxide synthase, and
release of nitric oxide, free radicals and other reactive oxygen
species to suppress the immune activity of T cells (37). On the other hand, MDSCs are able to
promote expression of certain angiogenic factors, including
vascular endothelial growth factor (38) and matrix metalloproteinases (39). It was identified that that MDSCs were
positively correlated with Th22 and Th17 cells in elderly patients
with gastric cancer and this regulatory axis was stronger for Th22
cells than for Th17 cells, indicating that both Th22 and Th17 cells
are involved in the regulation of MDSCs. The potential underlying
molecular mechanism may be that Th22 cells secrete cytokines,
chemokines, acute reactive protein and other inflammatory factors
together with several antimicrobial peptides including β-defensins,
S100 family proteins and Reg family proteins (40). Furthermore, it will be important to
exclude cells including CD3+ CD19+ and
CD56+ cells from these analyses. In addition, since
natural killer (NK) and natural killer T (NKT) cells may also
produce IFN-γ, further studies are required to distinguish
lymphocytes from NK and NKT cells.
Depending on the different organs and tissue
microenvironments, Th22 cells and IL-22 may also have a protective
role in cancer. For instance, in renal cell carcinoma, IL-22
inhibits tumor growth in a dose-dependent manner (41). In addition, IL-22 contributes to
epithelial cell defense against Helicobacter pylori
infection that is one of the major risk factors for gastric cancer
(42). Thus, it remains to be
determined whether Th22 cells and IL-22 exacerbate or ameliorate
tumorigenesis.
In summary, to the best of our knowledge, the
results of the present study demonstrate for the first time that
the peripheral Th22 and Th17 cells as well as IL-6 and TNF-α plasma
levels increase with aging. These results suggest that peripheral
Th22 and Th17 cells together with MDSCs and IL-22 levels are
important markers of gastric cancer progression in elderly
patients.
Acknowledgements
Not applicable.
Funding
The study was supported by a grant from Health and
Family Planning Commission of Liaoning Province, China for the
project of key medical specialties of Liaoning Province [No:
2015(111)].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and YW contributed to the experimental design and
implementation, performed the experiments, drafted the manuscript,
and performed data analysis. HZ conceived and designed the
experiments, and modified the manuscript. JWa and XJ helped to
collect the data. JWe and XW contributed to data analysis. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital, Dalian Medical University
(YJ-KY-FB-2014-42). Written informed consent was obtained from all
participants or their families prior to obtaining the samples.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Franceschi C and Campisi J: Chronic
inflammation (inflammaging) and its potential contribution to
age-associated diseases. J Gerontol A Biol Sci Med Sci. 69 Suppl
1:S4–S9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Izano M, Wei EK, Tai C, Swede H, Gregorich
S, Harris TB, Klepin H, Satterfield S, Murphy R, Newman AB, et al:
Chronic inflammation and risk of colorectal and other
obesity-related cancers: The health, aging and body composition
study. Int J Cancer. 138:1118–1128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diller ML, Kudchadkar RR, Delman KA,
Lawson DH and Ford ML: Balancing inflammation: The link between
Th17 and rtegulatory T cells. Mediators Inflamm. 2016:63092192016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eyerich S, Eyerich K, Pennino D, Carbone
T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann
C, Behrendt H, et al: Th22 cells represent a distinct human T cell
subset involved in epidermal immunity and remodeling. J Clin
Invest. 119:3573–3585. 2009.PubMed/NCBI
|
|
5
|
Trifari S, Kaplan CD, Tran EH, Crellin NK
and Spits H: Identification of a human helper T cell population
that has abundant production of interleukin 22 and is distinct from
T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 10:864–871. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolk K, Witte E, Witte K, Warszawska K and
Sabat R: Biology of interleukin-22. Semin Immunopathol. 32:17–31.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim C and Savan R: The role of the
IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev.
25:257–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lanfranca Perusina M, Lin Y, Fang J, Zou W
and Frankel T: Biological and pathological activities of
interleukin-22. J Mol Med (Berl). 94:523–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Wang T, Wang XQ, Du RZ, Zhang KN,
Liu XG, Ma DX, Yu S, Su GH, Li ZH, et al: Elevated frequencies of
circulating Th22 cell in addition to Th17 cell and Th17/Th1 cell in
patients with acute coronary syndrome. PLoS One. 8:e714662013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anuradha R, George PJ, Chandrasekaran V,
Kumaran PP, Nutman TB and Babu S: Interleukin 1 (IL-1)- and
IL-23-mediated expansion of filarial antigen-specific Th17 and Th22
cells in filarial lymphedema. Clin Vaccine Immunol. 21:960–965.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miossec P, Korn T and Kuchroo VK:
Interleukin-17 and type 17 helper T cells. N Engl J Med.
361:888–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Wang Y, Han C, Li P and Zhang H:
Colorectal cancer progression is associated with accumulation of
Th17 lymphocytes in tumor tissues and increased serum levels of
interleukin-6. Tohoku J Exp Med. 233:175–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu IC, Lin CC and Hsiung CA: Emerging
roles of frailty and inflammaging in risk assessment of age-related
chronic diseases in older adults: The intersection between aging
biology and personalized medicine. Biomedicine (Taipei). 5:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duhen T, Geiger R, Jarrossay D,
Lanzavecchia A and Sallusto F: Production of interleukin 22 but not
interleukin 17 by a subset of human skin-homing memory T cells. Nat
Immunol. 10:857–863. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parker KH, Beury DW and Ostrand-Rosenberg
S: Myeloid-derived suppressor cells: Critical cells driving immune
suppression in the tumor microenvironment. Adv Cancer Res.
128:95–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bueno V, Sant'Anna OA and Lord JM: Ageing
and myeloid-derived suppressor cells: possible involvement in
immunosenescence and age-related disease. Age (Dordr). 36:97292014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Solito S, Falisi E, Diaz-Montero CM, Doni
A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P,
Onicescu G, et al: A human promyelocytic-like population is
responsible for the immune suppression mediated by myeloid-derived
suppressor cells. Blood. 118:2254–2265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lechner MG, Liebertz DJ and Epstein AL:
Characterization of cytokine-induced myeloid-derived suppressor
cells from normal human peripheral blood mononuclear cells. J
Immunol. 185:2273–2284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chatterjee S, Das S, Chakraborty P, Manna
A, Chatterjee M and Choudhuri SK: Myeloid derived suppressor cells
(MDSCs) can induce the generation of Th17 response from naive CD4+
T cells. Immunobiology. 218:718–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGuire S: World cancer report 2014.
Geneva, Switzerland: World health organization, international
agency for research on cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International union against cancer (UICC) TNM classification of
malignant tumours. 7th edition. New York: Wiley-Liss; 2010
|
|
22
|
Zhang L, Li JM, Liu XG, Ma DX, Hu NW, Li
YG, Li W, Hu Y, Yu S, Qu X, et al: Elevated Th22 cells correlated
with Th17 cells in patients with rheumatoid arthritis. J Clin
Immunol. 31:606–614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cetin Aktas E, Cosan F, Cefle A and Deniz
G: IL-22-secreting Th22 and IFN-γ-secreting Th17 cells in Behcet's
disease. Mod Rheumatol. 24:802–807. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Truchetet ME, Brembilla NC, Montanari E,
Allanore Y and Chizzolini C: Increased frequency of circulating
Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis:
Association with interstitial lung disease. Arthritis Res Ther.
13:R1662011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang YH, Cao YF, Jiang ZY, Zhang S and
Gao F: Th22 cell accumulation is associated with colorectal cancer
development. World J Gastroenterol. 21:4216–4224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuang DM, Xiao X, Zhao Q, Chen MM, Li XF,
Liu RX, Wei Y, Ouyang FZ, Chen DP, Wu Y, et al: B7-H1-expressing
antigen-presenting cells mediate polarization of protumorigenic
Th22 subsets. J Clin Invest. 124:4657–4667. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tian T, Sun Y, Li M, He N, Yuan C, Yu S,
Wang M, Ji C and Ma D: Increased Th22 cells as well as Th17 cells
in patients with adult T-cell acute lymphoblastic leukemia. Clin
Chim Acta. 426:108–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu X, Tang Y, Guo S, Zhang Y, Tian Y, Ni B
and Wang H: Increased intratumoral interleukin 22 levels and
frequencies of interleukin 22-producing CD4+ T cells correlate with
pancreatic cancer progression. Pancreas. 43:470–477. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH,
Guo G, Chen W, Liu XF, Zhang JY, Liu T, et al: Increased
intratumoral IL-22-producing CD4(+) T cells and Th22 cells
correlate with gastric cancer progression and predict poor patient
survival. Cancer Immunol Immunother. 61:1965–1975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Axelrad JE, Lichtiger S and Yajnik V:
Inflammatory bowel disease and cancer: The role of inflammation,
immunosuppression, and cancer treatment. World J Gastroenterol.
22:4794–4801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sommer A and Fabri M: Vitamin D regulates
cytokine patterns secreted by dendritic cells to promote
differentiation of IL-22-producing T cells. PLoS One.
10:e01303952015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gorelick PB: Role of inflammation in
cognitive impairment: Results of observational epidemiological
studies and clinical trials. Ann N Y Acad Sci. 1207:155–162. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Satizabal CL, Zhu YC, Mazoyer B, Dufouil C
and Tzourio C: Circulating IL-6 and CRP are associated with MRI
findings in the elderly: The 3C-Dijon study. Neurology. 78:720–727.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao G, Zhu G, Huang Y, Zheng W, Hua J,
Yang S, Zhuang J and Ye J: IL-6 mediates the signal pathway of
JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis
in gastric cancer. Oncol Rep. 35:1787–1795. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo L, Ou JL, Zhang T, Ma L and Qu LF:
Effect of expressions of tumor necrosis factor alpha and
interleukin 1B on peritoneal metastasis of gastric cancer. Tumour
Biol. 36:8853–8860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Highfill SL, Rodriguez PC, Zhou Q, Goetz
CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody
JS, Munn DH, et al: Bone marrow myeloid-derived suppressor cells
(MDSCs) inhibit graft-vs.-host disease (GVHD) via an
arginase-1-dependent mechanism that is up-regulated by
interleukin-13. Blood. 116:5738–5747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Greten TF, Manns MP and Korangy F: Myeloid
derived suppressor cells in human diseases. Int Immunopharmacol.
11:802–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gerber HP, Olazoglu E and Grewal IS:
Targeting inflammatory cells to improve anti-VEGF therapies in
oncology. Recent Results Cancer Res. 180:185–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Melani C, Sangaletti S, Barazzetta FM,
Werb Z and Colombo MP: Amino-biphosphonate-mediated MMP-9
inhibition breaks the tumor-bone marrow axis responsible for
myeloid-derived suppressor cell expansion and macrophage
infiltration in tumor stroma. Cancer Res. 67:11438–11446. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhuang Y, Cheng P, Liu XF, Peng LS, Li BS,
Wang TT, Chen N, Li WH, Shi Y, Chen W, et al: A pro-inflammatory
role for Th22 cells in Helicobacter pylori-associated
gastritis. Gut. 64:1368–1378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang F, Shang D, Zhang Y and Tian Y:
Interleukin-22 suppresses the growth of A498 renal cell carcinoma
cells via regulation of STAT1 pathway. PLoS One. 6:e203822011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dixon BR, Radin JN, Piazuelo MB, Contreras
DC and Algood HM: IL-17a and IL-22 induce expression of
antimicrobials in gastrointestinal epithelial cells and may
contribute to epithelial cell defense against Helicobacter
pylori. PLoS One. 11:e01485142016. View Article : Google Scholar : PubMed/NCBI
|


















