Introduction
It has been established that tumor growth depends on
angiogenesis, a process of continued expansion of endothelial cells
from pre-existing blood vessels (1,2). Tumors
in situ, which are <3 mm in diameter, exist in a
pre-vascular state and are limited in their ability to grow without
perfusion from the blood supply (1).
The recruitment of novel blood vessels increases the availability
of oxygen and metabolites to tumors. In addition, this newly formed
vasculature facilitates the escape of tumor cells to distant
regions of the body (3). The
inhibition of tumor angiogenesis, therefore, is an important
potential therapy for solid tumors. Therapies designed to inhibit
novel blood vessel formation have advantages, in that they target
the genetically stable endothelial cells with resistance to
anti-angiogenesis therapy (4).
Multiple endogenous angiogenic inhibitors, including
thrombospondin, interferon α, platelet factor 4, PEX-the C-terminal
fragment of matrix metalloproteinase 2-angiostatin and endostatin,
have been characterized and demonstrated to elicit antitumor
effects (5,6). Among these, endostatin, a 20 kDa
C-terminal proteolytic fragment of collagen XVIII, has received the
greatest attention. Not only was identified to be a potent
inhibitor of angiogenesis in vitro, but also has been
suggested to have significant antitumor effects in a variety of
preclinical tumor models (7).
Externally administered endostatin was progressed quickly into
clinical trials; however, clinical development was halted due to
limited efficacy and problems with protein formulation and
application (8). Due to this, gene
therapy applying endostatin is attractive. Anti-angiogenic gene
therapies involving non-viral methods, including plasmid or naked
DNA, and viral strategies, including adenoviruses, adeno-associated
viruses, retro-oncoviruses and lentiviruses, have been employed in
numerous rodent tumor models (9–11).
Recombinant adeno-associated vector (rAAV) is a
replication-deficient, non-pathogenic vector belonging to the group
of human parvoviruses (12). These
vectors have great potential for cancer gene therapy, as they
transduce dividing and non-dividing cells, are less immunogenic
compared with other vectors, and are able to integrate into the
host genome, allowing long-term transgene expression (13). However, only a small number of
previous studies have used rAAVs to deliver anti-angiogenic genes
for cancer therapy: Davidoff et al (14) demonstrated that the portal vein
injection of an rAAV encoding soluble fetal liver kinase 1 (Flk-1)
resulted in FLK-1 protein expression from liver for up to 6 months,
and that this vector was effective in reducing tumor vessel density
and the subsequent size of SK-NEP-1 tumors in subcutaneous and
orthotopic mouse models. It has also been demonstrated that a
single injection of an rAAV encoding endostatin provides long-term
expression of endostatin (15), and
that the rAAV may enhance the treatment efficacy of radiation in a
human colorectal tumor model (HT29) (16). Other studies demonstrated that the
systemic use of rAAVs encoding endostatin may inhibit angiogenesis,
growth and metastases in pancreatic and ovarian cancers (17–19). In
the present study, an rAAV encoding human endostatin was developed,
and its antitumor activity in a xenograft renal tumor model was
evaluated.
Materials and methods
Plasmids and viral construction
Multiple plasmids were used, including pIRES-Endo
containing human IgG-Endostatin sequence and the AAV helper-free
system (Clontech Laboratories, Inc., Mountainview, CA, USA), for
example: pAAV-multiple cloning site (MCS), pCMV-MCS, pRC and
pHelper. The pHelper, which contains adenovirus-derived genes, was
used for viral packaging to avoid adenovirus contamination.
Plasmids were prepared by standard alkaline lysis (0.2 N NaOH/1.0%
sodium dodecyl sulfate) procedure followed by ethanol precipitation
(2.5 volumes) using the Plasmid Isolation (Alkaline Lysis) kit
(cat. no. BE-310; G-Biosciences, St. Louis, MO, USA) according to
the manufacturer's protocol. The IgG-Endostatin was amplified using
polymerase chain reaction from the pIRES-Endo plasmid containing
human IgG-Endostatin (a gift from Professor Xiao-Yan Wen,
University of Toronto, Toronto, Canada) using a Taq PCR kit (cat.
no. E5000S; New England BioLabs, Inc., Ipswich, MA, USA). The
sequence of the forward primer was GACATCGATATGAAATGCAGCTGGGTTATC
(ClaI site underlined) and the reverse primer was
TATGGATCCCTACTTGGAGGCAGTCATG (BamHI site underlined). The PCR
conditions were designed as follows: Initial denaturation at 95°C
for 30 sec, 30 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C
for 1 min followed by final extension at 72°C for 3 min. Subsequent
to sequencing, the target segment was cloned into pCMV-MCS to
construct the pCMV-Endo plasmid. To avoid inverted terminal repeats
rearrangement, pCMV-Endo was digested by NotI and the expression
cassette containing IgG-Endostatin was sub-cloned into pAAV-MCS to
construct the pAAV-Endo vector plasmid.
Preparation of rAAV-endostatin
The 293 cells (American Type Culture Collection
Manassas, VA, USA) were grown in Dulbecco's modified Eagle's medium
(DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 5% heat-inactivated fetal bovine serum (FBS;
Japan Bioserum Co., Ltd., Hiroshima, Japan) to ~80% confluence,
then digested using 0.25% trypsin (Thermo Fisher Scientific, Inc.)
and counted using a hemocytometer and a light microscope
(magnification, ×20), and then co-transfected with pAAV-Endo, pRC
and pHelper to package rAAV-Endostatin (0.3 µg/each in 25 µl DMEM
medium) using Lipofectamine™ transfection reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. All
cell lines used in the present study were cultured in a humidified
incubator with 5% CO2 at 37°C. The parameter of the gene
pulser used for electroporation was 960 µF/245V, for a cuvette with
a gap of 0.4 cm. A total of 72 h later, cells were frozen and
thawed at −20°C and 37°C for 3 cycles, and then 100 µl viral
solution was added to fresh 293 cells to prepare the second viral
generation. After 72 h, the procedure was repeated to prepare the
third viral generation. The viral solution was centrifuged at 1,000
× g in room temperature for 30 min followed by 8,000 × g at 4°C for
1 h. Following purification using the Adenovirus Purification kit
(cat. no. 631533; Clontech Laboratories, Inc.), the virus was
verified using transmission electron microscopy as previously
described (20) (magnification,
×30,000) and assayed using the human adeno-associated virus ELISA
kit (cat. no. MBS260177; MyBioSource, San Diego, CA, USA).
The human RCC OS-RC-2 cell line (American Type
Culture Collection) was maintained in the Tianjin Institute of
Urology, and cultured in DMEM supplemented with 10%
heat-inactivated FBS (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Cultured OS-RC-2 cells (~80% confluence) were
infected with rAAV-Endostatin (MOI=105) at 4°C. After 24
h, 1.0×106 OS-RC-2 cells were plated in 6-well dishes
for 24 h at 37°C. The supernatant was then collected by
centrifuging at 300 × g for 7 min at room temperature and assayed
for endostatin secretion by ELISA as aforementioned. Human
umbilical vein endothelial cells (HUVEC) (American Type Culture
Collection) chemotactic movement was analyzed by Transwell assay as
previously described (21) to
evaluate recombinant endostatin activity.
RCC tumor model
15 BALB/c nude mice (Shanghai SLAC Laboratory Animal
Co. Ltd., Shanghai, China) at age of 5–6 weeks and a weight of 25 g
were housed in 12/12 h light/dark cycle specific pathogen free
conditions at 24±2°C and a humidity of 1.5% CO2 with
free access to food and water. All mice were used in the following
4 experiments: i) Prophylactic model, in which 24 nude mice were
randomly divided into 3 groups and injected with 3 doses of
1.0×1011 rAAV-Endostatin viral particles (v.p),
1.0×1011 rAAV-EYFP v.p, or RPMI-1640 medium (Thermo
Fisher Scientific, Inc.), respectively, into the right hind leg
tibialis anterior muscles at 1 week intervals. At 1 week following
the final immunization, 1.0×106 OS-RC-2 cells were
inoculated subcutaneously on the dorsal surface of mice. The
xenograft formation rate, size and weight were monitored; ii)
therapeutic model, in which BALB/c nude mice were inoculated with
1×106 OS-RC-2 cells through subcutaneous injection. A
total of 10 days subsequent to tumor growth reaching ~5 mm, 3 doses
of rAAV-Endostatin or control rAAV were administered via
intratumoral injection into the RCC tumor. The xenograft tumor was
measured every day until tumor size reached ~300 mm3.
Mice were then sacrificed by cervical dislocation and xenografts
were assayed for microvessel density (MVD) by immunohistochemical
staining. Briefly, xenografts were fixed in 4% formalin overnight
at 4°C followed by paraffin embedding. Tissues were then cut into
10 µm-thick sections in, air dried overnight at room temperature,
and fixed in acetone for 10 min at room temperature. Slides were
allowed to air dry for 1 h and washed three times for 5 min each in
phosphate-buffered saline (PBS; pH 7.4). Samples were then blocked
with PBS containing 0.1% bovine serum albumin (Thermo Fisher
Scientific, Inc.) and 3% human serum albumin (Thermo Fisher
Scientific, Inc.) for at least 30 min at room temperature.
Subsequently, all slides were incubated with rabbit polyclonal
antibody against CD31 (1:50 dilution) (cat. no. ab28364; Abcam,
Cambridge, MA, USA) at 4°C overnight. Following washing with
tris-buffered saline with Tween-20 (0.1%) three times, biotinylated
goat anti-rabbit IgG (1:1,000; Jackson Immuno Research
Laboratories, Inc., West Grove, PA, USA) was added into the
sections and incubated for 30 min at room temperature followed by
incubation with Strepavidin-horseradish peroxidase (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 30 min at room temperature. The
staining was visualized using diaminobenzidine (ScyTek
Laboratories, Inc., Logan, UT, USA) under a light microscope
(×200). iii) 24 nude mice were injected with 1.0×1011
rAAV-Endostatin v.p. A total of 3 mice were sacrificed every 10
days and the endostatin concentration in serum was assayed by ELISA
as aforementioned. The rAAV-EYFP was used as control; and iv) 8
mice were injected with 1.0×1011 rAAV-Endostatin v.p and
sacrificed after 8 weeks for heart and encephalon collection.
Histological hematoxylin (10%) and eosin (1%) (H&E) staining as
described previously (22) and
transmission electron microscopy (magnification ×30,000) as
aforementioned, were performed to determine whether rAAV-Endostatin
caused ischemia or other pathological changes in these organs.
Statistical analysis
The data were analyzed using one-way analysis of
variance and Tukey's tests to detect any significance. P<0.05
was considered to indicate a statistically significant difference.
All data are presented as mean ± standard error of the mean.
Statistical analyses were performed using SAS statistical software
v9.3 (SAS Institute, Cary, NC, USA).
Results
Construction of rAAV-endostatin vector
and production of rAAV virus
The pAAV-endostatin vector plasmid was constructed
by sub-cloning of the pCMV-endostatin vector, which was established
by insertion of endostatin fragment (Fig.
1A) and verified by sequencing. rAAV-Endostatin was packaged
and demonstrated the normal viral pattern under electron
microscopy. The titer of viral preparation was 1.0×1012
v.p/ml by ELISA.
Following infection with rAAV-Endostatin or
rAAV-YRFP with MOI of 105, ~95% OS-RC-2 cells were
infected (Fig. 1B). To measure
endostatin production, OS-RC-2-rAAV-Endostatin or control cells
were cultured for 72 h, and supernatant were collected for ELISA
assay. OS-RC-2 cells infected with rAAV-Endostatin produced 54.09
ng/ml recombinant endostatin in the culture supernatant compared
with 0.4 ng/ml RCC-rAAV-RYFP cells (Fig.
1C).
Tumor cell proliferation and apoptosis
is not affected in rAAV-Endostatin-infected RCC cells
In order to investigate whether rAAV-Endostatin
infection directly inhibited RCC cell growth and induce tumor cell
death, RCC cells proliferation and apoptosis was analyzed. OS-RC-2
tumor cells infected with rAAV-Endostatin or control rAAV did not
exhibit significant morphological changes (Fig. 2A). Tumor cell growth indicated that
rAAV-Endostatin infection did not promote or inhibit OS-RC-2 cell
proliferation (Fig. 2B), and analysis
of cell death following rAAV-Endostatin infection indicated that
there was no significant difference in proportions of cell death
compared with the control rAAV-infected cells (Fig. 2C).
Prophylactic effect of rAAV-Endostatin
in RCC tumor development
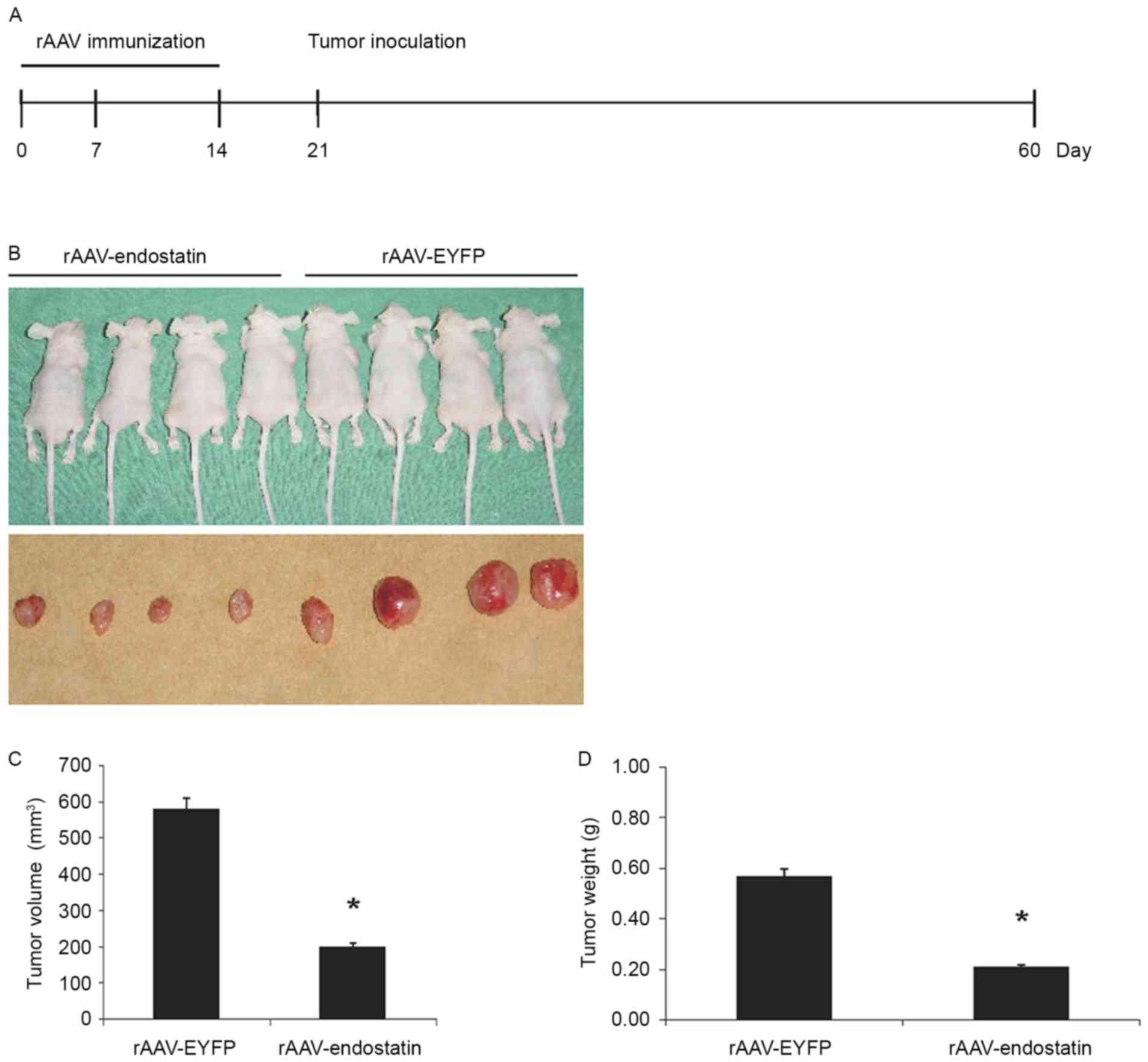
In order to investigate whether vaccination of
rAAV-Endostatin adenovirus inhibited RCC tumor growth, BALB/c nude
mice were vaccinated with 3 doses of rAAV-Endostatin
intramuscularly; 1×106 OS-RC-2 tumor cells were
subcutaneously inoculated into the BALB/c nude mice 7 days
following the third immunization (Fig.
3A). The size of tumors derived from OS-RC-2 cells infected
with rAAV-endostatin were smaller compared with those in the
controls. rAAV-Endostatin immunization led to 50% xenograft tumors
(4/8 mice) grown in nude mice whereas the control rAAV-EYFP
immunization induced 100% xenograft tumor (8/8 mice) development.
Monitoring of tumor growth demonstrated that vaccination of
rAAV-Endostatin adenovirus significantly suppressed tumor growth
comparing with control adenovirus vaccination (Fig. 3B-D).
Therapeutic effect of rAAV-Endostatin
in RCC tumors
To additionally investigate whether rAAV-Endostatin
had a therapeutic effect on the established RCC tumors, BALB/c nude
mice were inoculated with 1×106 OS-RC-2 cells. A total
of 10 days subsequent to tumor growth reaching ~5 mm, 3 doses of
rAAV-Endostatin or control rAAV were injected into the RCC tumor
(Fig. 4A). The tumor growth was
monitored, and the results demonstrated that the intratumoral
rAAV-Endostatin injection significantly delayed RCC tumor
development. The xenograft tumors derived from RCC cells treated
with intratumoral rAAV-Endostatin injection took longer time to
reach 300 mm3 compared with control cells (32.63±9.75
vs. 21.50±11.42 and 21.75±10.48 days, respectively; Fig. 4B). At day 60, all mice were
sacrificed. The average weight of xenograft tumors with
rAAV-Endostatin injection was 0.60±0.21 g, whereas that of
xenograft tumors with the control rAAV-EYFP injection was 0.76±0.35
g. H&E and immunochemistry staining indicated that there was
decreased angiogenesis formation in the samples that had undergone
rAAV-Endostatin adenovirus immunization compared with the control
rAAV-EYFP injection (Fig. 4C and
D).
rAAV-Endostatin suppresses RCC tumor
growth through inhibition of angiogenesis
To identify the mechanism of rAAV-Endostatin
inhibition of tumor development, the chemotactic activity of HUVEC
cells exposed to the supernatant of rAAV-Endostatin-infected
OS-RC-2 cells was investigated in vitro. HUVEC chemotactic
movement was inhibited by OS-RC-2-rAAV-Endostatin cell supernatant
in Transwell assay (22.40±2.97) compared with the control rAAV-EYFP
injection group (35.81±6.22 cells), and the inhibitory rate was
37.45% (Fig. 5A). The results
indicated that endostatin was sufficient to suppress angiogenesis.
Subsequent to injection of rAAV-Endostatin (1×1011 v.p),
the serum concentration of endostatin rose continuously, peaked at
day 60 and was maintained at a sustained level of 30–40 ng/ml
thereafter (Fig. 5B).
Furthermore, the MVD of xenograft tumors from the
rAAV-Endostatin immunization group was 8.30±3.14/0.739
mm2, whereas those of the control groups were
13.87±4.09/0.739 (control rAAV-EYFP) and 13.76±3.50/0.739
mm2 (RPMI-1640), respectively (Fig. 5C). This observation suggested that the
growth inhibition of xenograft tumors by rAAV-Endostatin may be
through the inhibition of angiogenesis. Heart and encephalon
tissues and other vital organs (brain, liver and kidney) did not
exhibit any pathological changes under microscopy or transmission
electron microscopy following rAAV-Endostatin injection (Fig. 6).
 | Figure 6.Transmission electron microscopy and
H&E assay of mouse vital organ following rAAV-endostatin
inoculation. A total of 8 weeks following injection of
1.0×1011 v.p rAAV-endostatin, the heart and encephalon
tissues were collected for safety examination (to ensure there were
no off-target effects of the virus infection on other tissues), and
no adverse effect was identified in either (A) heart tissue
(magnification, ×4,000; white scale bar=2 µm) or (B) encephalon
tissue (magnification, ×4,000; white scale bar=2 µm) under
electronic microscope. (C) H&E staining (magnification, ×200)
of heart, brain, liver and kidney tissues did not exhibit
significant changes in morphology. H&E, hematoxylin and
eosin. |
Discussion
Endostatin has been studied for a number of years,
due to its potent anti-angiogenesis effect (7). Although the majority of therapeutic
investigations have managed to utilize the purified endostatin
protein, there are several limitations associated with its use:
Previously, recombinant human-endostatin for clinical use was
produced primarily from Escherichia coli or yeast, and
therefore incapable of expressing its complete activity as in body;
also, the protein purification process may denature endostatin and
the purification yield is often low (23). In addition, the requirement to deliver
angiogenesis inhibitors including endostatin for an extended period
of time may lead to practical difficulties in clinical settings. As
a protein drug, endostatin has a short half-life time in
vivo and therefore requires repeated application to maintain
the required therapeutic serum levels (23). As viral-based delivery systems have
advantages for the durable expression of a transgene following a
single dose (24), viral delivery of
endostatin may be a promising strategy for tumor treatment.
The optimal vector for gene therapy would deliver
therapeutic amount of transgene for a long time. In addition, the
vector should be safe and poorly immunogenic, to allow multiple
applications as required. Furthermore, the vector should be
appropriate for the effective delivery of a transgene in a defined
type of tissues. For these reasons, an adeno-associated viral
vector was applied in the present study. As renal cancer growth is
angiogenesis-dependent, and may also secret basic fibroblast growth
factor that facilitates rAAV infection (25,26), renal
cancer serves as a suitable model for antiangiogenic gene
therapy.
As endostatin inhibits tumor-induced vascularization
only in the extracellular matrix (27), the present study adopted the IgG
signal peptide to mediate endostatin secretion. For viral
packaging, a co-transfection method with pAAV-Endo, pRC and pHelper
vectors was applied. By using a pHelper vector that contained adeno
VA, E4 and E2A regions, adenoviral contamination was avoided.
Subsequent to infection of the RCC cells with rAAV-Endostatin, the
IgG-Endostatin sequence was inserted into the cell genome.
Endostatin was then expressed and secreted. According to the
results, endothelial cell chemotactic movement was inhibited
effectively, suggesting that the endostatin concentration was
sufficient to suppress angiogenesis. In fact, a previous study
demonstrated that even a low concentration of endostatin (0.1
µg/ml) was able to achieve an inhibitory effect (28). In the prophylactic and therapeutic RCC
models, inoculation of rAAV-Endostatin inhibited xenograft tumor
formation. These observations suggested that increased endostatin
secretion in tumor environment may suppress angiogenesis, leading
to the inhibition of tumor growth. It was also observed that
rAAV-Endostatin injection led to a high serum level of endostatin.
The MVD assay of the xenografts verified that the level of
endostatin was able to inhibit tumor angiogenesis. Therefore,
inoculation of rAAV-Endostatin may inhibit tumor neoangiogenesis
and subsequent tumor growth. No adverse effects in heart and
encephalon tissues following rAAV-Endostatin use were observed,
suggesting that rAAV-Endostatin is safe and may be considered for
use in a clinical setting. In conclusion, the biologically-active
rAAV-Endostatin has been developed and demonstrated to be effective
for the inhibition of renal tumor angiogenesis and growth. The
rAAV-Endostatin appears to be safe for general use as a gene
therapy agent against renal cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
SE and HR performed the experiments and analyzed the
data. SE and LB designed the study and co-wrote the manuscript.
Ethics and consent to participate
This study has been approved by the ethnics
committee of Tianjin Medical Hospital and written informed consent
was obtained from all participants.
Patient consent for publication
The study participants provided consent for the data
and any associated images to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Folkman J: Seminars in medicine of the
Beth Israel Hospital, Boston. Clinical applications of research on
angiogenesis. New Engl J Med. 333:1757–1763. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kerbel R and Folkman J: Clinical
translation of angiogenesis inhibitors. Nat Rev Cancer. 2:727–739.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fidler IJ and Ellis LM: The implications
of angiogenesis for the biology and therapy of cancer metastasis.
Cell. 79:185–188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Kenawi AE and El-Remessy AB:
Angiogenesis inhibitors in cancer therapy: Mechanistic perspective
on classification and treatment rationales. Brit J Pharmacol.
170:712–729. 2013. View Article : Google Scholar
|
|
5
|
Burke PA and DeNardo SJ: Antiangiogenic
agents and their promising potential in combined therapy. Crit Rev
Oncol Hemat. 39:155–171. 2001. View Article : Google Scholar
|
|
6
|
Liekens S, De Clercq E and Neyts J:
Angiogenesis: Regulators and clinical applications. Biochem
Pharmacol. 61:253–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
OReilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim J, Duong T, Lee G, Seong BL, El-Rifai
W, Ruley HE and Jo D: The effect of intracellular protein delivery
on the anti-tumor activity of recombinant human endostatin.
Biomaterials. 34:6261–6271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blezinger P, Wang JJ, Gondo M, Quezada A,
Mehrens D, French M, Singhal A, Sullivan S, Rolland A, Ralston R
and Min W: Systemic inhibition of tumor growth and tumor metastases
by intramuscular administration of the endostatin gene. Nat
Biotechnol. 17:343–348. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma HI, Lin SZ, Chiang YH, Li J, Chen SL,
Tsao YP and Xiao X: Intratumoral gene therapy of malignant brain
tumor in a rat model with angiostatin delivered by adeno-associated
viral (AAV) vector. Gene Ther. 9:2–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma HI, Guo P, Li J, Lin SZ, Chiang YH,
Xiao X and Cheng SY: Suppression of intracranial human glioma
growth after intramuscular administration of an adeno-associated
viral vector expressing angiostatin. Cancer Res. 62:756–763.
2002.PubMed/NCBI
|
|
12
|
Daya S and Berns KI: Gene therapy using
adeno-associated virus vectors. Clin Microbiol Rev. 21:583–593.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponnazhagan S, Curiel DT, Shaw DR, Alvarez
RD and Siegal GP: Adeno-associated virus for cancer gene therapy.
Cancer Res. 61:6313–6321. 2001.PubMed/NCBI
|
|
14
|
Davidoff AM, Nathwani AC, Spurbeck WW, Ng
CY, Zhou J and Vanin EF: rAAV-mediated long-term liver-generated
expression of an angiogenesis inhibitor can restrict renal tumor
growth in mice. Cancer Res. 62:3077–3083. 2002.PubMed/NCBI
|
|
15
|
Shi WY, Teschendorf C, Muzyczka N and
Siemann DW: Adeno-associated virus-mediated gene transfer of
endostatin inhibits angiogenesis and tumor growth in vivo. Cancer
Gene Ther. 9:513–521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi W, Teschendorf C, Muzyczka N and
Siemann DW: Gene therapy delivery of endostatin enhances the
treatment efficacy of radiation. Radiother Oncol. 66:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ponnazhagan S, Mahendra G, Kumar S, Shaw
DR, Stockard CR, Grizzle WE and Meleth S: Adeno-associated virus
2-mediated antiangiogenic cancer gene therapy: Long-term efficacy
of a vector encoding angiostatin and endostatin over vectors
encoding a single factor. Cancer Res. 64:1781–1787. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noro T, Miyake K, Suzuki-Miyake N,
Igarashi T, Uchida E, Misawa T, Yamazaki Y and Shimada T:
Adeno-associated viral vector-mediated expression of endostatin
inhibits tumor growth and metastasis in an orthotropic pancreatic
cancer model in hamsters. Cancer Res. 64:7486–7490. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Subramanian IV, Ghebre R and Ramakrishnan
S: Adeno-associated virus-mediated delivery of a mutant endostatin
suppresses ovarian carcinoma growth in mice. Gene Ther. 12:30–38.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H: Comparative observation of the
recombinant adeno-associated virus 2 using transmission electron
microscopy and atomic force microscopy. Microsc Microanal.
13:384–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Justus CR, Leffler N, Ruiz-Echevarria M
and Yang LV: In vitro cell migration and invasion assays. J Vis
Exp. Jun 1–2014.doi: 10.3791/51046. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puri TS, Shakaib MI, Chang A, Mathew L,
Olayinka O, Minto AW, Sarav M, Hack BK and Quigg RJ: Chronic kidney
disease induced in mice by reversible unilateral ureteral
obstruction is dependent on genetic background. Am J Physiol Renal
Physiol. 298:F1024–F1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mohajeri A, Sanaei S, Kiafar F, Fattahi A,
Khalili M and Zarghami N: The challenges of recombinant endostatin
in clinical application: Focus on the different expression systems
and molecular bioengineering. Adv Pharm Bull. 7:21–34. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katz MG, Fargnoli AS, Williams RD and
Bridges CR: Gene therapy delivery systems for enhancing viral and
nonviral vectors for cardiac diseases: Current concepts and future
applications. Hum Gene Ther. 24:914–927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
OBrien T, Cranston D, Fuggle S, Bicknell R
and Harris AL: Two mechanisms of basic fibroblast growth
factor-induced angiogenesis in bladder cancer. Cancer Res.
57:136–140. 1997.PubMed/NCBI
|
|
26
|
Nguyen JT, Wu R, Clouse ME, Hlatky L and
Terwilliger EF: Adeno-associated virus-mediated delivery of
anti-angiogenic factors as an antitumor strategy. Hum Gene Ther.
10:839. 1999.
|
|
27
|
Kim YM, Jang JW, Lee OH, Yeon J, Choi EY,
Kim KW, Lee ST and Kwon YG: Endostatin inhibits endothelial and
tumor cellular invasion by blocking the activation and catalytic
activity of matrix metalloproteinase. Cancer Res. 60:5410–5413.
2000.PubMed/NCBI
|
|
28
|
Dhanabal M, Ramchandran R, Volk R,
Stillman IE, Lombardo M, Iruela-Arispe ML, Simons M and Sukhatme
VP: Endostatin: Yeast production, mutants, and antitumor effect in
renal cell carcinoma. Cancer Res. 59:189–197. 1999.PubMed/NCBI
|