Introduction
Upper gastrointestinal cancers are among the leading
causes of cancer-associated mortality worldwide. Approximately 1.5
million people are diagnosed with gastric and esophageal cancer
each year (1,2). Despite improvements in diagnosis and
therapy in the last decades, the outcome for patient with gastric
and esophageal cancers remains poor with 5-year survival rates not
exceeding 20–30% in Western societies (3–5). The
molecular mechanisms underlying carcinogenesis remain largely
elusive. Accordingly, molecular markers allowing for prediction of
the clinical course of these diseases are currently lacking. Hence,
there is a high demand for molecular markers to predict tumor
aggressiveness and response to therapy for these cancer types.
Microtubules are multifunctional cytoskeletal
proteins involved in numerous cellular processes including
maintenance of cell shape, intracellular transport and chromosome
segregation during mitosis and meiosis. Microtubules are composed
of polymers of α- and β-tubulin heterodimers. Class III β-tubulin
(TUBB3) is typically expressed in cells of neuronal origin, where
it contributes to the formation of dynamic microtubules essential
for neurite formation and maintenance (6). Several lines of evidence suggest that
TUBB3 also has an important role in tumor development. In fact,
overexpression of TUBB3 has been linked to poor clinical outcome in
numerous epithelium-derived tumor types, including non-small cell
lung (7), bladder (8), breast (9),
ovarian (10) and prostate cancer
(11). Several studies analyzing
gastric and/or esophageal cancer specimens (n=29-149) have also
suggested clinically relevant roles of TUBB3 expression levels in
upper gastrointestinal cancer (12–14). Of
note, elevated levels of TUBB3 expression have been associated with
a reduced response to taxane-based microtubule-targeting cancer
therapy (7,10–12,15).
Here we tested retrospectively TUBB3 expression in
upper gastrointestinal cancers from 230 gastric and 594 esophageal
cancers on tissue microarrays (TMA) and report the clinical follow
up from 189 gastric and 428 esophageal cancers.
Patients and methods
Patients
The 230 patients [mean age (± SD), 67 years (±12);
female/male-ratio, 0.51] with gastric and 594 patients [mean age (±
SD), 62 years (±10); female/male-ratio, 0.25] with esophageal
cancer received surgical treatment at the Department of General,
Visceral and Thoracic Surgery, University Medical Center
Hamburg-Eppendorf (Hamburg, Germany) between June 1994 and October
2006, and between January 1992 and December 2014, respectively.
TUBB3 staining and follow-up data was available for 93 patients
with gastric cancer with a median time of 13 months and for 393
esophageal cancer patients with a median time of 41 months. Tumors
were staged according to the sixth edition of the
tumor-nodes-metastasis classification, graded and histologically
subtyped according to the recommendations of the International
Union Against Cancer (UICC) (16).
Data on neoadjuvant or adjuvant cytotoxic therapy regimens or
response to treatment were unavailable. The TMA manufacturing was
performed as described in previous studies (17,18). Each
TMA block contained internal controls of normal esophageal and
gastric tissue taken from the same patient cohort.
The Ethics Committee of the Ärztekammer Hamburg
approved the present study (no. WF-049/09). According to local laws
(HmbKHG §12a), informed consent was not required. Patient
records/information were anonymized prior to analysis. All work was
performed in compliance with the Helsinki Declaration.
Immunohistochemistry
TUBB3 staining and scoring was performed as
described in a previous study (9).
The recombinant rabbit monoclonal anti-TUBB3 antibody clone
EPR1568Y was used at a dilution 1:150 of (cat. no. ab68193; Abcam,
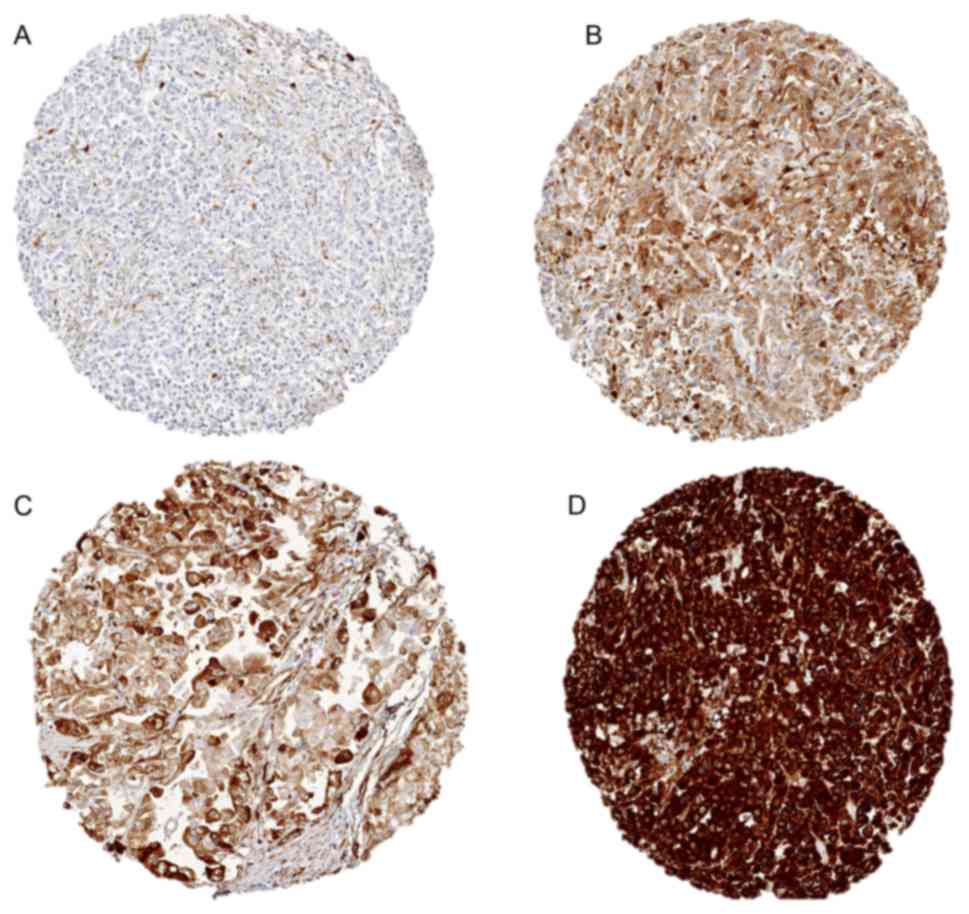
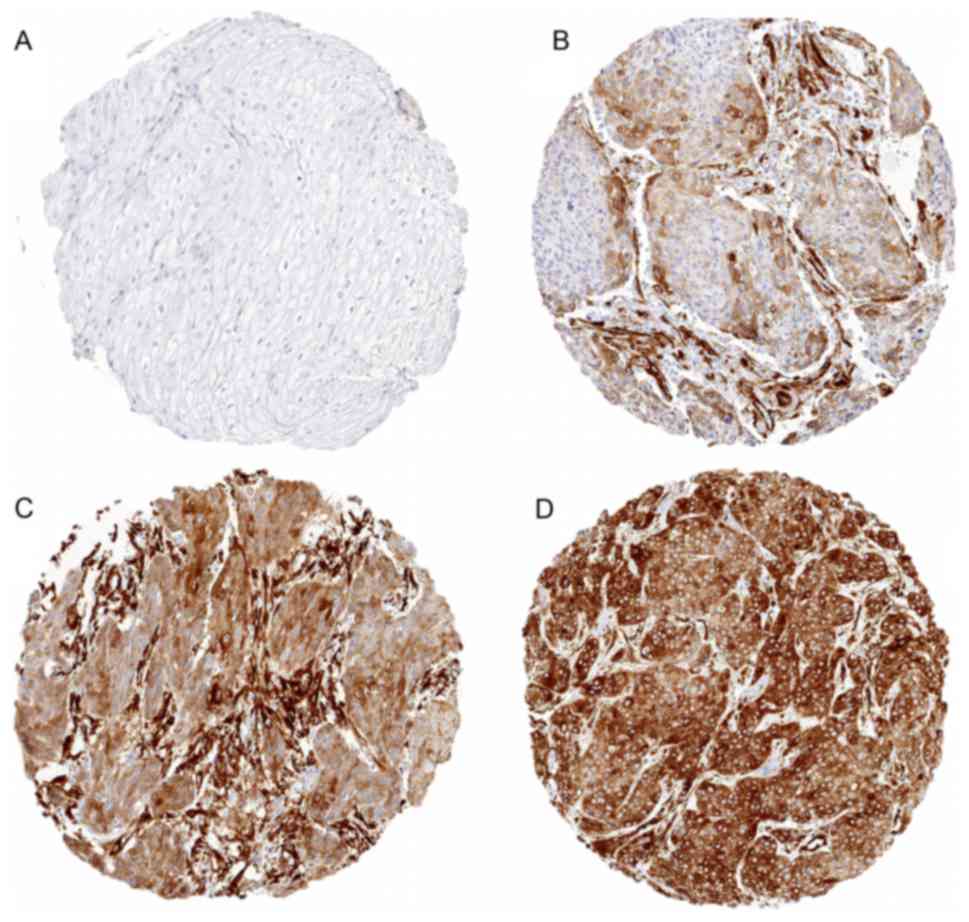
Cambridge, UK). Staining was observed in the cytoplasm of
TUBB3-expressing cells and scored as ‘negative’ (0), ‘weak’ (1+ in
≤70% of tumor cells or 2+ in ≤30% of tumor cells), ‘moderate’ (1+
in >70% of tumor cells, or 2+ in 31–70% of tumor cells, or 3+ in
≤30% of tumor cells) or ‘strong’ (2+ in >70% of tumor cells or
3+ in >30% of tumor cells) (Figs.
1 and 2).
Statistical analysis
JMP 12.0 software (SAS Institute Inc., Carey, NC,
USA) was used to calculate contingency tables and P-values with the
chi-squared (likelihood) test. Kaplan-Meier curves were drawn and
significant differences between groups were assessed by the
log-rank method. Cox regression analysis was used to compare hazard
ratios in univariate and multivariate models. P≤0.05 was considered
to indicate a statistically significant difference.
Results
TUBB3-staining
The results of the TMA analysis were interpretable
for a total of 189/230 (82%) of gastric and 431/594 (73%) of
esophageal tumor samples. In the non-informative TMA spots (18% for
gastric cancer and 27% for esophageal cancer), the tissue sample
was lacking or no unequivocal cancer tissue was observed. Normal
gastric and esophageal tissues exhibited no staining under the
selected experimental conditions. Fig.
1 shows representative images of normal gastric and esophageal
tissue.
TUBB3-expression in gastric
cancer
In gastric cancer, positive staining for TUBB3 was
detected in 118 of 189 analyzable spots (62.4%) and was rated weak
in 11.1%, moderate in 18% and strong in 33.3% of these samples.
Representative images of TUBB3 staining in gastric cancers are
given in Fig. 2. TUBB3 expression was
unrelated to tumor stage, UICC stage, Lauren classification, WHO
grading, and presence of lymph node or distant metastasis
(P>0.05 each; Table I). TUBB3
expression varied from 53.8 to 83.0% with the tumor localization
(P=0.0012; Table I).
 | Table I.Association between TUBB3 expression
and gastric cancer phenotype. |
Table I.
Association between TUBB3 expression
and gastric cancer phenotype.
|
|
| TUBB3 (%) |
|
|---|
|
|
|
|
|
|---|
| Parameter | No. evaluable | Negative | Weak | Moderate | Strong | P-value |
|---|
| All cancers | 189 | 37.6 | 11.1 | 18.0 | 33.3 |
|
| Tumor
stagea |
|
|
|
|
|
|
|
pT1+2 | 125 | 36.8 | 12.0 | 19.2 | 32.0 | 0.7753 |
|
pT3+4 | 62 | 37.1 | 9.7 | 16.1 | 37.1 |
|
|
UICC-classification |
|
|
|
|
|
|
| I | 31 | 32.3 | 9.7 | 22.6 | 35.5 | 0.8227 |
| II | 28 | 35.7 | 21.4 | 14.3 | 28.6 |
|
|
III | 86 | 41.9 | 8.1 | 18.6 | 31.4 |
|
| IV | 44 | 34.1 | 11.4 | 15.9 | 38.6 |
|
| Laurén
classificationa |
|
|
|
|
|
|
|
Diffuse | 61 | 52.5 | 13.1 | 14.8 | 19.7 | 0.0484 |
|
Mixed | 14 | 42.9 | 7.1 | 21.4 | 28.6 |
|
|
Intestinal | 109 | 28.4 | 11.0 | 20.2 | 40.4 |
|
| WHO
gradinga |
|
|
|
|
|
|
| G1 | 2 | 50.0 | 0.0 | 0.0 | 50.0 | 0.2345 |
| G2 | 58 | 25.9 | 8.6 | 22.4 | 43.1 |
|
| G3 | 126 | 42.1 | 12.7 | 15.9 | 29.4 |
|
| Tumor
localizationa |
|
|
|
|
|
|
|
Antrum | 13 | 23.1 | 38.5 | 30.8 | 7.7 | 0.0012b |
|
Corpus | 7 | 42.9 | 0.0 | 28.6 | 28.6 |
|
|
Cardia | 47 | 17.0 | 19.1 | 12.8 | 51.1 |
|
|
Other/not further
specified | 93 | 46.2 | 7.5 | 14.0 | 32.3 |
|
| Lymph node
metastasisa |
|
|
|
|
|
|
| N0 | 53 | 34.0 | 17.0 | 18.9 | 30.2 | 0.4896 |
| N1 | 133 | 37.6 | 9.0 | 18.0 | 35.3 |
|
| Distant
metastasisa |
|
|
|
|
|
|
| M0 | 129 | 38.8 | 10.9 | 16.3 | 34.1 | 0.4076 |
| M1 | 22 | 22.7 | 13.6 | 13.6 | 50.0 |
|
TUBB3-expression in esophageal
cancer
In esophageal cancer, cytoplasmic TUBB3 staining was
detected in 345 of 428 analyzable tumors (80.7%), including 233
adenocarcinomas and 195 squamous cell cancers. TUBB3 staining in
adenocarcinomas (squamous cell cancers) was considered weak in
18.0% (11.8%), moderate in 19.7% (19.0%) and strong in 36.1%
(57.9%) of these samples. Representative images of TUBB3 staining
in esophageal cancers are given in Fig.
3. In esophageal adenocarcinomas, no association between TUBB3
and UICC stage, WHO grading, or the presence of lymph node or
distant metastasis was identified (P>0.05 each; Table II). Only the tumor stage was
significantly associated with TUBB3 expression (P=0.0289; Table II). In esophageal squamous cell
carcinomas, only the resection margin was significantly associated
with TUBB3 (P<0.05; Table III).
For the association of TUBB3 with the tumor stage a similar trend
as in the adenocarcinomas was observed.
 | Table II.Association between TUBB3 expression
and esophageal adenocarcinoma phenotype. |
Table II.
Association between TUBB3 expression
and esophageal adenocarcinoma phenotype.
|
|
| TUBB3 (%) |
|
|---|
|
|
|
|
|
|---|
| Parameter | No. evaluable | Negative | Weak | Moderate | Strong | P-value |
|---|
| All cancers | 233 | 26.2 | 18.0 | 19.7 | 36.1 |
|
| Tumor
stagea |
|
|
|
|
|
|
|
pT1a-b | 44 | 29.5 | 29.5 | 27.3 | 13.6 | 0.0289b |
|
pT2 | 25 | 32.0 | 16.0 | 24.0 | 28.0 |
|
|
pT3 | 143 | 23.1 | 15.4 | 18.9 | 42.7 |
|
|
pT4a-b | 17 | 35.3 | 17.6 | 5.9 | 41.2 |
|
|
UICC-classificationa |
|
|
|
|
|
|
| I | 43 | 32.6 | 23.3 | 25.6 | 18.6 | 0.0534 |
| II | 26 | 19.2 | 11.5 | 38.5 | 30.8 |
|
|
III | 134 | 23.9 | 19.4 | 15.7 | 41.0 |
|
| IV | 25 | 36.0 | 8.0 | 16.0 | 40.0 |
|
| WHO
gradinga |
|
|
|
|
|
|
| G1 | 9 | 22.2 | 22.2 | 22.2 | 33.3 | 0.8693 |
| G2 | 85 | 24.7 | 20.0 | 21.2 | 34.1 |
|
| G3 | 130 | 26.9 | 16.2 | 20.0 | 36.9 |
|
| G4 | 5 | 40.0 | 40.0 | 0.0 | 20.0 |
|
| Resection
margina |
|
|
|
|
|
|
| 0 | 162 | 26.5 | 18.5 | 23.5 | 31.5 | 0.0961 |
| 1 | 63 | 27.0 | 17.5 | 12.7 | 42.9 |
|
| 2 | 3 | 0.0 | 0.0 | 0.0 | 100.0 |
|
| Lymph node
metastasisa |
|
|
|
|
|
|
|
pN0 | 61 | 29.5 | 18.0 | 26.2 | 26.2 | 0.4443 |
|
pN1 | 42 | 16.7 | 23.8 | 23.8 | 35.7 |
|
|
pN2 | 57 | 28.1 | 15.8 | 15.8 | 40.4 |
|
|
pN3 | 64 | 29.7 | 15.6 | 14.1 | 40.6 |
|
| Distant
metastasisa |
|
|
|
|
|
|
| 0 | 2 | 0.0 | 50.0 | 0.0 | 50.0 | 0.2737 |
| 1 | 26 | 38.5 | 7.7 | 15.4 | 38.5 |
|
 | Table III.Association between TUBB3 expression
and esophageal squamous cell cancer phenotype. |
Table III.
Association between TUBB3 expression
and esophageal squamous cell cancer phenotype.
|
|
| TUBB3 (%) |
|
|---|
|
|
|
|
|
|---|
| Parameter | No. evaluable | Negative | Weak | Moderate | Strong | P-value |
|---|
| All cancers | 195 | 11.3 | 11.8 | 19.0 | 57.9 |
|
| Tumor stage |
|
|
|
|
|
|
|
pT1a-b | 31 | 19.4 | 12.9 | 32.3 | 35.5 | 0.1715 |
|
pT2 | 43 | 11.6 | 16.3 | 16.3 | 55.8 |
|
|
pT3 | 109 | 9.2 | 11.0 | 16.5 | 63.3 |
|
|
pT4a-b | 12 | 8.3 | 0.0 | 16.7 | 75.0 |
|
|
UICC-classificationa |
|
|
|
|
|
|
| I | 46 | 13.0 | 8.7 | 26.1 | 52.2 | 0.5155 |
| II | 47 | 6.4 | 17.0 | 17.0 | 59.6 |
|
|
III | 62 | 12.9 | 6.5 | 19.4 | 61.3 |
|
| IV | 39 | 10.3 | 17.9 | 12.8 | 59.0 |
|
| WHO grading |
|
|
|
|
|
|
| G1 | 3 | 33.3 | 0.0 | 0.0 | 66.7 | 0.7412 |
| G2 | 124 | 10.5 | 11.3 | 21.0 | 57.3 |
|
| G3 | 68 | 11.8 | 13.2 | 16.2 | 58.8 |
|
| Resection
margina |
|
|
|
|
|
|
| 0 | 148 | 14.2 | 11.5 | 18.2 | 56.1 | 0.0461b |
| 1 | 38 | 0.0 | 13.2 | 23.7 | 63.2 |
|
| 2 | 8 | 12.5 | 0.0 | 12.5 | 75.0 |
|
| Lymph node
metastasisa |
|
|
|
|
|
|
|
pN0 | 91 | 11.0 | 9.9 | 18.7 | 60.4 | 0.9046 |
|
pN1 | 41 | 9.8 | 14.6 | 17.1 | 58.5 |
|
|
pN2 | 37 | 8.1 | 16.2 | 24.3 | 51.4 |
|
|
pN3 | 21 | 14.3 | 9.5 | 9.5 | 66.7 |
|
| Distant
metastasisa |
|
|
|
|
|
|
| 0 | 1 | 100.0 | 0.0 | 0.0 | 0.0 | 0.1828 |
| 1 | 39 | 7.7 | 17.9 | 12.8 | 61.5 |
|
| 1 | 26 | 38.5 | 7.7 | 15.4 | 38.5 |
|
Kaplan-meier analysis
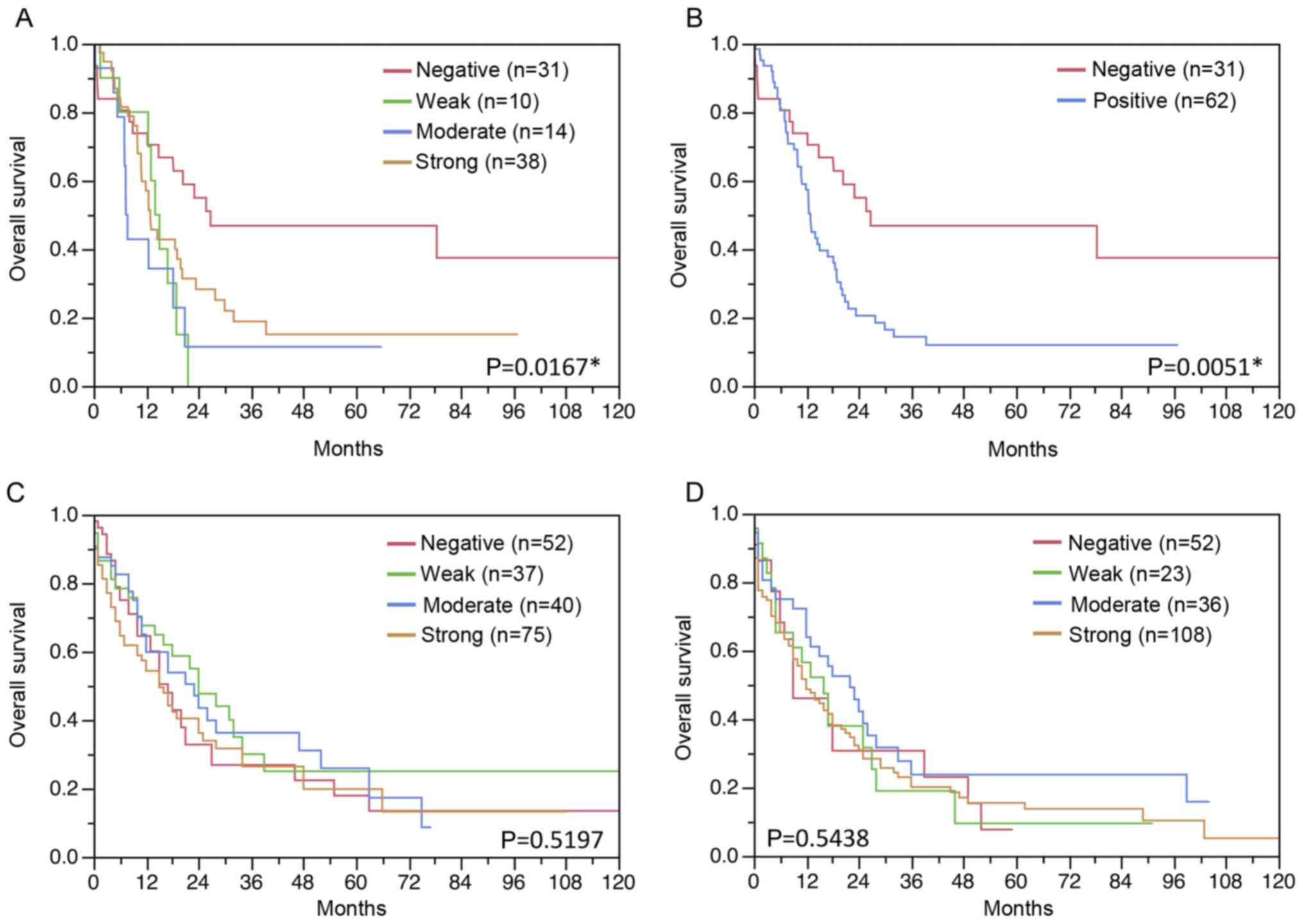
Follow-up data were available from 93 patients with
gastric cancer and 393 patients with esophageal cancer (204
adenocarcinomas and 189 squamous cell cancers) with interpretable
TUBB3 staining on the TMA. While in gastric cancer TUBB3 expression
was associated with shorter overall survival (Fig. 4A and B), TUBB3 expression had no
impact on the survival of esophageal cancer patients (P>0.05;
Fig. 4C and D).
Multivariate analysis
Hazard ratios for overall survival were calculated.
In gastric cancer, TUBB3 expression was an independent risk factor
for shorter survival (P<0.05; Table
IV).
 | Table IV.Hazard ratio for overall survival of
established prognostic parameter and TUBB3 expression in gastric
cancer types. |
Table IV.
Hazard ratio for overall survival of
established prognostic parameter and TUBB3 expression in gastric
cancer types.
| Variable | Univariate
analysis | Multivariate
analysis |
|---|
| Tumor stage |
|
|
| pT3+4
vs. pT1+2 | 2.67
(1.66–4.30)c | 1.67
(1.00–2.77) |
| WHO grading |
|
|
| G3 vs.
G1+2 | 1.65
(1.00–2.83)a | 2.22
(1.29–3.95)a |
| Lymph node
metastasis |
|
|
|
Positive vs. negative | 4.43
(2.25–10.1)c | 3.11
(1.54–7.20)b |
| TUBB3
expression |
|
Positive vs. negative | 2.23
(1.28–4.08)a | 2.18
(1.22–4.12)a |
Discussion
The results of the present study demonstrate that
TUBB3 is frequently expressed in upper gastrointestinal cancer
types associated with patient prognosis only in gastric cancer, but
not in esophageal adenocarcinoma and esophageal squamous cell
cancer.
TUBB3 expression was identified in 62.4% of the 189
gastric cancer tissues, in 73.8% of the 233 esophageal
adenocarcinoma tissues and 88.7% of the 195 esophageal squamous
cell cancer tissues in the present study, but was undetectable in
the respective normal tissue samples. In principle, these
immunohistochemical results are compatible with earlier studies on
these tumor types. This particularly applies to gastric tumors,
where two earlier studies on gastric cancer tissues (n=115 and 146)
reported comparable data, namely detectable TUBB3 expression in 36
and 53% of tumor samples (12,19). The
results of two earlier studies on esophageal squamous cell cancers
were more conflicting, reporting TUBB3-positive rates of 7 and 95%,
respectively (14,20). The striking discrepancy of these data
is typical for studies using ‘homemade’ immunohistochemical
protocols. It is known, that the use of different antibodies,
immunohistochemistry protocols and scoring criteria can result in
discrepant data (21).
The important function of TUBB3 in the maintenance
of the dynamic plasticity of microtubules (22,23) -a
prerequisite for cell motility, invasive growth, mitotic spindle
orientation, and cell cycle progression-would be consistent with a
significant role for TUBB3 in tumor development and progression.
The high frequency of detectable TUBB3 staining in early gastric
cancer in combination with the lack of a further elevation in
frequency with the tumor stage increasing, may suggest that up
regulation of TUBB3 is an event in carcinogenesis of gastric cancer
and has a relevance in cancer development rather than cancer
progression. Other studies have failed to identify an association
between TUBB3 expression and clinico-pathological parameters or
patient prognosis in gastric or esophageal carcinomas (12,19,20). In
the present study, analysis of a much larger number of tumors did
reveal a significant association with patient outcome in gastric
cancer providing some arguments for TUBB3 testing. This is in line
to the results on the predictive value of TUBB3 in a variety of
other cancer types. Using the same staining protocol, another
recent study by our group identified the prognostic value of TUBB3
in prostate cancer, which was independent of established pre- and
post-operatively available prognostic features (24). Others studies have reported TUBB3
overexpression is linked to late tumor stage and poor prognosis in
breast (25), lung (26,27), colon
(28), ovarian (10,29,30),
prostate (11,24) and several neurological cancers
(28).
The present study was a retrospective study. Thus it
remains to be seen whether the prognostic value of TUBB3 expression
in gastric cancer can be validated in a prospective study.
In summary, the results of the present study
demonstrate that TUBB3 is frequently expressed in upper
gastrointestinal cancer types, including esophageal and gastric
tumors. For gastric cancer, TUBB3 expression might be a prognostic
factor.
Acknowledgements
The authors would like to thank Mrs. Janett Lütgens,
Mrs. Sünje Seekamp and Mrs. Inge Brandt (Institute of Pathology,
University Medical Center Hamburg-Eppendorf) for excellent
technical assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DH, FJ, RS and GS designed the study and drafted the
manuscript. EÖ, CS and JRI participated in study design. EN, CG,
MCH, CF, KM, MA, MF and AH performed immunohistochemical analysis
and scoring. CL, VR, SW and MN participated in pathology data
analysis. CH-M, NCB and RS performed statistical analysis. MB, DP,
and DSL participated in data interpretation and helped to draft the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Ärztekammer Hamburg
approved the study protocol (WF-049/09). According to local laws
(HmbKHG §12a), patient informed consent was not required. Patient
records/information were anonymized and de-identified prior to
analysis. All procedures have been performed in compliance with the
principles outlined in the Helsinki Declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TUBB3
|
Class III β tubulin
|
|
TMA
|
tissue microarray
|
|
UICC
|
International Union Against Cancer
|
References
|
1
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pasechnikov V, Chukov S, Fedorov E,
Kikuste I and Leja M: Gastric cancer: Prevention, screening and
early diagnosis. World J Gastroenterol. 20:13842–13862. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
CancerGenome Atlas Research Network;
AnalysisWorking Group: Asan University; BC Cancer Agency; Brigham
and Women's Hospital; Broad Institute; Brown University; Case
Western Reserve University; Dana-Farber Cancer Institute; Duke
University, et al, . Integrated genomic characterization of
oesophageal carcinoma. Nature. 541:169–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moro K, Nagahashi M, Naito T, Nagai Y,
Katada T, Minagawa M, Hasegawa J, Tani T, Shimakage N, Usuda H, et
al: Gastric adenosquamous carcinoma producing granulocyte-colony
stimulating factor: A case of a rare malignancy. Surg Case Rep.
3:672017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuda T and Saika K: The 5-year relative
survival rate of stomach cancer in the USA, Europe and Japan. Jpn J
Clin Oncol. 43:1157–1158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewis SA, Lee MG and Cowan NJ: Five mouse
tubulin isotypes and their regulated expression during development.
J Cell Biol. 101:852–861. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YL, Luo XP and Xian L: The prognostic
role of the class III β-tubulin in non-small cell lung cancer
(NSCLC) patients receiving the taxane/vinorebine-based
chemotherapy: A meta-analysis. PLoS One. 9:e939972014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hinsch A, Chaker A, Burdelski C, Koop C,
Tsourlakis MC, Steurer S, Rink M, Eichenauer TS, Wilczak W, Wittmer
C, et al: βIII-tubulin overexpression is linked to aggressive tumor
features and genetic instability in urinary bladder cancer. Hum
Pathol. 61:210–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lebok P, Öztürk M, Heilenkotter U,
Jaenicke F, Müller V, Paluchowski P, Geist S, Wilke C, Burandt E,
Lebeau A, et al: High levels of class III β-tubulin expression are
associated with aggressive tumor features in breast cancer. Oncol
Lett. 11:1987–1994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kavallaris M, Kuo DY, Burkhart CA, Regl
DL, Norris MD, Haber M and Horwitz SB: Taxol-resistant epithelial
ovarian tumors are associated with altered expression of specific
beta-tubulin isotypes. J Clin Invest. 100:1282–1293. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ranganathan S, Benetatos CA, Colarusso PJ,
Dexter DW and Hudes GR: Altered beta-tubulin isotype expression in
paclitaxel-resistant human prostate carcinoma cells. Br J Cancer.
77:562–566. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hwang JE, Hong JY, Kim K, Kim SH, Choi WY,
Kim MJ, Jung SH, Shim HJ, Bae WK, Hwang EC, et al: Class III
β-tubulin is a predictive marker for taxane-based chemotherapy in
recurrent and metastatic gastric cancer. BMC Cancer. 13:4312013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao Y, Zhang G, Wang P, Zhou J, Gan W,
Song Y, Huang L, Zhang Y, Luo G, Gong J and Zhang L: Clinical
significance of UGT1A1 polymorphism and expression of ERCC1, BRCA1,
TYMS, RRM1, TUBB3, STMN1 and TOP2A in gastric cancer. BMC
Gastroenterol. 17:22017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Ding S, Liang Y, Zheng Y, Li W, Yang
L, Zheng X and Jiang J: Expression of ERCC1, TYMS, TUBB3, RRM1 and
TOP2A in patients with esophageal squamous cell carcinoma: A
hierarchical clustering analysis. Exp Ther Med. 7:1578–1582. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burkhart CA, Kavallaris M and Band Horwitz
S: The role of beta-tubulin isotypes in resistance to antimitotic
drugs. Biochim Biophys Acta. 1471:O1–O9. 2001.PubMed/NCBI
|
|
16
|
Brierley JD, Gospodarowicz MK and
Wittekind C: UICC TNM Classification of Malignant Tumours. 8th
edition. Wiley Blackwell; New York, NY: 2017
|
|
17
|
Kononen J, Bubendorf L, Kallioniemi A,
Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G
and Kallioniemi OP: Tissue microarrays for high-throughput
molecular profiling of tumor specimens. Nat Med. 4:844–847. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mirlacher M and Simon R: Recipient block
TMA technique. Methods Mol Biol. 664:37–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Urano N, Fujiwara Y, Doki Y, Kim SJ,
Miyoshi Y, Noguchi S, Miyata H, Takiguchi S, Yasuda T, Yano M and
Monden M: Clinical significance of class III beta-tubulin
expression and its predictive value for resistance to
docetaxel-based chemotherapy in gastric cancer. Int J Oncol.
28:375–381. 2006.PubMed/NCBI
|
|
20
|
Nair KS, Naidoo R and Chetty R:
Microsatellite analysis of the APC gene and immunoexpression of
E-cadherin, catenin and tubulin in esophageal squamous cell
carcinoma. Hum Pathol. 37:125–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schlomm T, Iwers L, Kirstein P, Jessen B,
Köllermann J, Minner S, Passow-Drolet A, Mirlacher M,
Milde-Langosch K, Graefen M, et al: Clinical significance of p53
alterations in surgically treated prostate cancers. Mod Pathol.
21:1371–1378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Panda D, Miller HP, Banerjee A, Ludueña RF
and Wilson L: Microtubule dynamics in vitro are regulated by the
tubulin isotype composition. Proc Natl Acad Sci USA.
91:11358–11362. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Falconer MM, Echeverri CJ and Brown DL:
Differential sorting of beta tubulin isotypes into
colchicine-stable microtubules during neuronal and muscle
differentiation of embryonal carcinoma cells. Cell Motil
Cytoskeleton. 21:313–325. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsourlakis MC, Weigand P, Grupp K, Kluth
M, Steurer S, Schlomm T, Graefen M, Huland H, Salomon G, Steuber T,
et al: βIII-tubulin overexpression is an independent predictor of
prostate cancer progression tightly linked to ERG fusion status and
PTEN deletion. Am J Pathol. 184:609–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horak CE, Pusztai L, Xing G, Trifan OC,
Saura C, Tseng LM, Chan S, Welcher R and Liu D: Biomarker analysis
of neoadjuvant doxorubicin/cyclophosphamide followed by ixabepilone
or Paclitaxel in early-stage breast cancer. Clin Cancer Res.
19:1587–1595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levallet G, Bergot E, Antoine M, Creveuil
C, Santos AO, Beau-Faller M, de Fraipont F, Brambilla E, Levallet
J, Morin F, et al: High TUBB3 expression, an independent prognostic
marker in patients with early non-small cell lung cancer treated by
preoperative chemotherapy, is regulated by K-Ras signaling pathway.
Mol Cancer Ther. 11:1203–1213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HL, Ruan L, Zheng LM, Whyte D, Tzeng
CM and Zhou XW: Association between class III β-tubulin expression
and response to paclitaxel/vinorebine-based chemotherapy for
non-small cell lung cancer: a meta-analysis. Lung Cancer. 77:9–15.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katsetos CD, Herman MM and Mörk SJ: Class
III beta-tubulin in human development and cancer. Cell Motil
Cytoskeleton. 55:77–96. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao S, Zhao X, Lin B, Hu Z, Yan L and Gao
J: Clinical implications of REST and TUBB3 in ovarian cancer and
its relationship to paclitaxel resistance. Tumour Biol.
33:1759–1765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carrara L, Guzzo F, Roque DM, Bellone S,
Emiliano C, Sartori E, Pecorelli S, Schwartz PE, Rutherford TJ and
Santin AD: Differential in vitro sensitivity to patupilone versus
paclitaxel in uterine and ovarian carcinosarcoma cell lines is
linked to tubulin-beta-III expression. Gynecol Oncol. 125:231–236.
2012. View Article : Google Scholar : PubMed/NCBI
|