Introduction
According to U.S. cancer statistics, colorectal
cancer (CRC) is the third most common cancer and the second leading
cause of cancer-associated mortality (1). The Westernized lifestyle, particularly
increases in the prevalence of obesity and physical inactivity, has
likely impacted the recently observed rise in CRC incidence rates
in China (2,3). Furthermore, 50–60% of patients diagnosed
with CRC will develop distant metastases, which is the most common
reason for CRC mortality. The most prominent CRC metastasis is that
of the liver, with 80–90% of these patients having unresectable
metastatic liver disease (4–7). Currently, hepatic resection of isolated
CRC liver metastasis (CRLM) remains the only potential curative
option for patients with this disease. Although resection is
combined with modern adjuvant systemic regimens, the curative rate
of CRLM is only 20%, with 70% of these patients developing
recurrence, primarily in the liver (8–10).
Therefore, early prevention of CRLM is important. Current CRC
therapeutic approaches include surgery, radiotherapy and
chemotherapy. However, no effective treatments for CRLM exist, and
the effects of available metastatic CRC drugs are frequently
limited by their toxicity and side effects (11). By contrast, since it selectively
infects tumor cells and forms syncytia, oncolytic herpes simplex
virus type 2 (oHSV2) may induce antitumor immune responses
(12).
HSV-2 is a common genital pathogen and prevalent
sexually transmitted DNA virus (13)
that has been employed as an oncolytic agent, and certain
indications suggest that HSV-2 has a higher oncolytic activity
compared with HSV-1. Zhao et al (14) constructed a novel oHSV2 agent that was
able to slow tumor growth without inducing weight loss. This novel
virus was demonstrated to increase the number of natural killer
(NK) cells and mildly decrease the number of regulatory T cells
(Tregs) in the spleen. The oHSV2 FusOn-H2 was shown to have a
higher oncolytic activity compared with that of HSV-1. Furthermore,
HSV-2 is able to induce strong T-cell responses and inhibit primary
breast cancer metastasis (15). HSV-2
provides a more effective treatment for disseminated tumors in the
peritoneal cavity and for metastatic human ovarian cancer compared
with HSV-1 (16). In another previous
study based on infected cell protein 0 (ICP0) mutations within
HSV-1 and HSV-2, ICP0-defective HSV-2 (HSV-2 dICP0) exhibited more
potent antitumor activity at a lower viral burst size and induced
higher levels of cytopathic effects (CPE) compared with HSV-1 dICP0
(17). The HSV-2-based oncolytic
virus ΔPK inhibited melanoma cells from secreting the
immunosuppressive cytokine interlukin-10 and inhibited the
expression of the negative immune checkpoint regulator cytotoxic T
lymphocyte antigen 4, which significantly increased its oncolytic
activity (18). The ICP10 and ΔPK
HSV-2 agents displayed oncolytic activities against melanoma via
virus-induced programmed cell death pathways (19).
To the best of our knowledge, no reports on the
effects of oHSV2 on an in vivo mouse CRLM model exist in the
literature. In preclinical models, oHSV2 is an effective killer of
CRC and CRC stem-like cells, a significant inhibitor of tumor
growth and a promising therapeutic approach for patients with CRC
(20). The present study aimed to
assess the potential viability of oHSV2 as a therapeutic agent for
the inhibition of CRLM. Construction of a CRLM model by
intrasplenic injection of DX-3 or PC-3-P cell lines had revealed
more marked metastatic capacity compared with the direct
intravenous injection of the aforementioned cell lines (21). Here, a CRLM model was established in
BALB/c mice by intrasplenically injecting CT-26 cells into the
splenic capsule, and CT-26 cells were also subcutaneously injected
into the right flanks of the mice. oHSV2 was administered via
intratumoral injection into subcutaneous xenograft tumors in the
CRLM model, to assess its therapeutic potential in CRLM and its
ability to induce immune responses.
Materials and methods
Cell culture and reagents
The mouse colorectal cancer cell line CT-26 was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and maintained in our laboratory. The cells were cultured in
RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin,
and 10% fetal bovine serum (FBS) (Hyclone; GE Healthcare Life
Sciences) in a 5% CO2 humidified incubator at 37°C.
Virus
The oHSV2 used in this study is a
replication-competent, genetically stable attenuated HSV-2 whose
construction was previously described in detail (14). This virus was derived from the
wild-type HSV-2 strain HG52 and has copy deletions of ICP34.5 and
ICP47, which increases tumor selectivity and decreases
virulence.
Cytotoxicity analysis of oHSV2 in
CT-26 cells in vitro
CT-26 cells were plated on 6-well flat-bottom plates
at 3.0×105 cells/well and grown overnight. Subsequently,
the cells were infected with oHSV2 at multiplicities of infection
(MOIs) of 0.1, 1 and 3 and incubated at 37°C in a 5% CO2
humidified incubator. Photomicrographs of the cells were captured
using an Olympus confocal microscope (Olympus Corporation, Tokyo,
Japan) equipped with a Leica camera (Leica Microsystems GmbH,
Wetzlar, Germany) with a ×100 magnification after an additional 24,
48 and 72 h of culture.
Cell Counting kit 8 (CCK8) cell
viability assay
The CCK8 assay (Dojindo Molecular Technologies,
Inc., Kumamoto Japan) was utilized to measure cell viability.
Briefly, CT-26 cells were seeded in 96-well plates at
1.0×104 cells/well in a total volume of 100 µl; each
sample was analyzed in triplicate. To test the effect of MOI on
cell viability, cells were infected with oHSV2 at MOIs of 0.1, 1
and 3. Following culturing for 24, 48, and 72 h, the culture medium
was replaced with 100 µl of a solution containing 10% CCK8, and the
mixture was incubated for 2 h at 37°C. Absorbance was measured
using a Model 550 microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 450 nm with a reference of 655 nm. The
untreated control was set to 100%, and the treated samples were
normalized to this value according to the following equation:
Survival rate (%)=(optical density (OD) of the treated cells-OD of
blank control/OD of negative control-OD of blank control) ×100.
Animals and treatment
A total of 72 6-week-old female BALB/c mice (Animal
Center of the Chinese Academy of Medical Science, Beijing, China),
with a mean weight of 16–18 g, were fed chow and water ad
libitum, and housed under specific pathogen-free conditions.
The mice were acclimated to the housing conditions (mean
temperature of 24±2°C and mean humidity of 52±8%) for 7 days. The
study protocol was approved by the Ethics of Animal Experiments
Committee of the Chinese Academy of Medical Sciences and Peking
Union Medical College (Beijing, China).
Liver metastasis animal model
The experimental CRLM model was established via
intrasplenic and subcutaneous inoculation of CT-26 cells; tumor
cell viability during the exponential growth phase was >95%.
CT-26 cells (3×105) were suspended in 100 µl RPMI-1640
serum-free medium (SFM) and subcutaneously inoculated into the
right flanks of the mice. The mice were anesthetized via an
intraperitoneal injection of 5% chloral hydrate (400 mg/kg body
weight) and fixed in the prone position. An abdominal incision ~1.0
cm in length was made between the dorsal central and left axillary
midlines to expose the spleen. Equal numbers and volumes of CT-26
tumor cells were inoculated into the splenic tip using a 4-gauge
needle to produce a visible pale wheal, followed by hemostasis for
5 min with alcohol-soaked cotton balls and wound closure. The
abdominal wound was closed if no significant bleeding or
extravasation was encountered.
Animal experiments
When the subcutaneous xenograft tumors reached 3 mm
in diameter, tumor volumes were calculated according to the
following formula: Tumor volume (mm3)=(length ×
width2)/2. The mice were randomly divided into two
groups as follows: i) Treatment with oHSV2 alone on days 1, 3, 5, 7
and 9; and ii) control treatment with RPMI-1640 SFM on days 1, 3,
5, 7 and 9. oHSV2 (1×106 PFU in 100 µl) was applied by
intratumoral injection into the right flank subcutaneous xenograft
tumors. The tumor size and body weight of each mouse were measured
every other day, and mouse survival was monitored daily during the
experimental period. The tumor volumes were determined by caliper
measurement at the indicated times after treatment. A total of 7
days after the last treatment, the mice were sacrificed by cervical
dislocation and then subjected to gross dissection. Their livers
and spleens were removed and weighed, and the numbers of visible
tumor nodules on the liver surfaces were counted.
Histopathological analysis
Following the aforementioned procedures, the livers
and spleens were collected and fixed in 10% paraformaldehyde for
12–24 h at room temperature then paraffin-embedded. Sections of 4
µM were stained with hematoxylin and eosin at room temperature (3–5
min for hematoxylin and 5–10 sec for eosin). Samples were
permeabilized with 0.1% Triton X-100, and nonspecific binding was
blocked with 5% normal goat serum (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The pathological sections were
observed with an confocal microscope (magnification, ×400; Olympus
Corporation, Tokyo, Japan) equipped with a Leica camera (Leica
Microsystems GmbH, Wetzlar, Germany).
Characterization of CD4+ T,
CD8+ T and natural killer (NK) cells in peripheral blood
using flow cytometric analysis
A total of 2 days after the last oHSV2 treatment,
CD4+ T, CD8+ T and NK cells were quantified
in mouse peripheral blood (n=3). Peripheral blood lymphocytes were
isolated by centrifugation at 250 × g at 4°C for 10 min in a
gradient lymphocyte isolation solution for mice (Tianjin Hao Yang
Biological Manufacture Co., Ltd., China) at room temperature and
washed twice with PBS. The cell suspensions were then stained for
30 min at 4°C using the following monoclonal antibodies:
Fluorescein isothiocyanate (FITC) anti-mouse CD8a (cat. no. 100708;
dilution, 1:200), Percp/cy5.5 anti-mouse CD4 (cat. no. 100406;
dilution, 1:80), Allophycocyanin anti-mouse CD3 (cat. no. 100236;
dilution, 1:40), and FITC anti-mouse 49b (cat. no. 103503;
dilution, 1:200; all obtained from BioLegend, Inc., San Diego, CA,
USA). Following washing with PBS, the cells were fixed with 4%
paraformaldehyde at room temperature for 20 min, and the
CD4+ T, CD8+ T and NK cell frequencies were
determined using flow cytometry (FACSCalibur; BD Biosciences,
Franklin Lakes, NJ, USA) and analyzed by FlowJo 7.6.1 software
(TreeStar, Inc., Ashland, OR, USA). The results were expressed as
the relative fluorescence index by dividing the fluorescence value
of the experimental groups by that of the control group.
Statistical analysis
All data were statistically analyzed in this study
were performed using GraphPad Prism 6.0 (GraphPad Software, Inc.,
La Jolla, CA, USA). All data are expressed as the mean ± standard
error of the mean from at least three independent experiments.
Independent sample t-tests were used to analyze the significance of
differences between two groups. Survival curves were calculated
using the Kaplan-Meier method and compared with the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
oHSV2 has cytotoxic effects on CT-26
cells in vitro
To investigate the effects of oHSV2 on CT-26 cells
in vitro, cells were infected with oHSV2 at different MOIs.
As presented in Fig. 1A, as the oHSV2
MOI increased, the cellular CPE became more obvious, and the
distance between the cells gradually increased. This result
suggested that the oncolytic effect of oHSV2 on CT-26 cells may be
dependent on the MOI.
To further examine the effects of oHSV2 on CT-26
cells in vitro, CT-26 cells were infected with virus at
various MOIs (MOI=0.1, 1 and 3) for 24, 48 and 72 h and subjected
to the CCK8 assay to investigate alterations in cell viability. The
viral treatment induced a concentration-dependent reduction in
CT-26 cell viability. As presented in Fig. 1B, cells treated with oHSV2 at MOI=0.1
for 72 h had a viability of 72.79%, whilst the viability decreased
to 67.09% with MOI=1 and to 35.20% with MOI=3.
Intratumoral oHSV2 injection inhibits
subcutaneous CT-26 cell xenograft tumor growth with no systemic
side effects
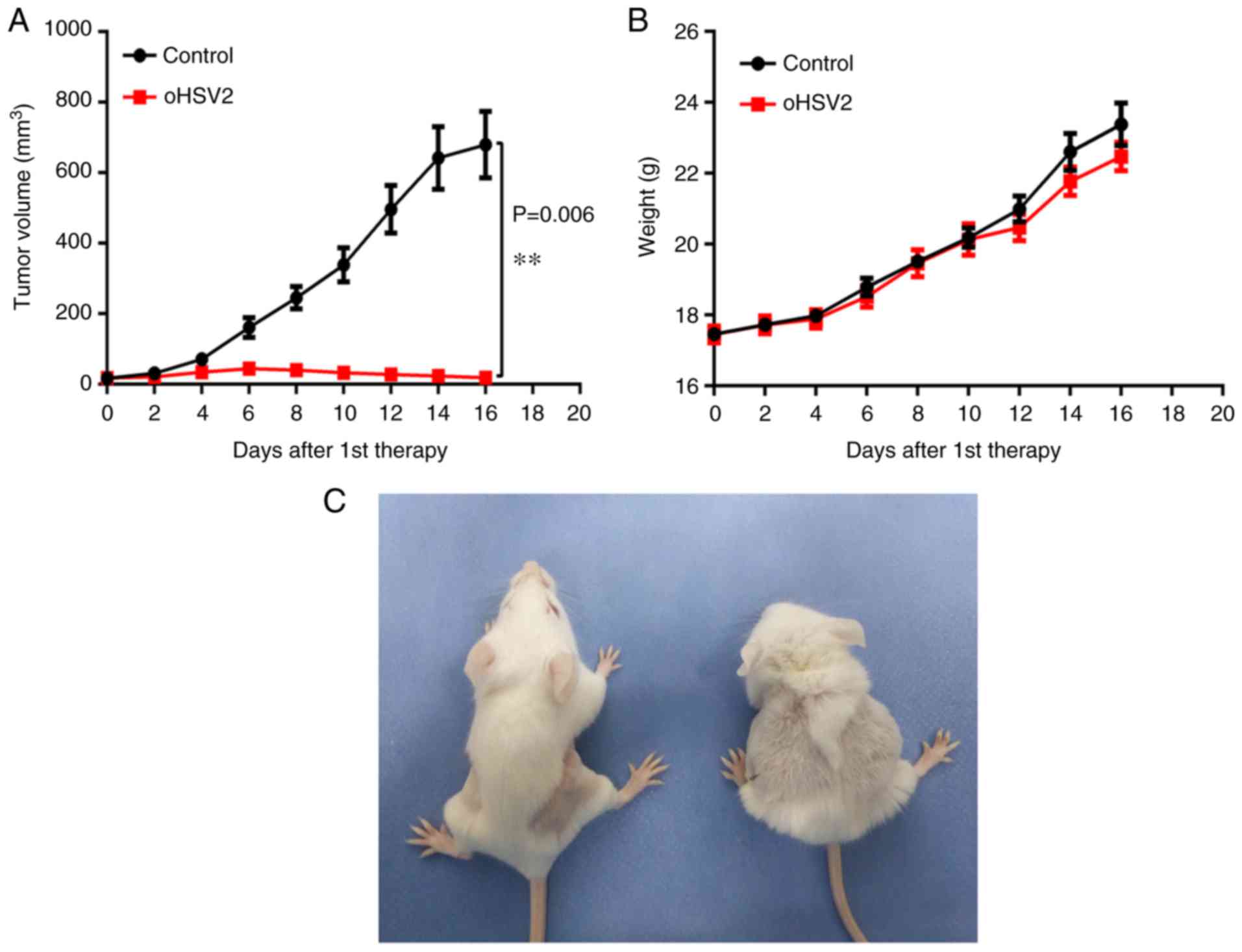
As expected, a single oHSV2 treatment application
induced significant growth inhibition compared with that induced by
the control. As presented in Fig. 2A,
in the oHSV2 treatment group the subcutaneous xenograft tumor
volume remained approximately the same, whereas the tumor volume
was significantly increased in the control group. Notably, tumor
volume in the oHSV2 treatment group increased slightly during the
first 6 days and began to decrease on day 8. However, tumor volume
in the control group remained higher compared with that in the
oHSV2 treatment group throughout the experiment. Furthermore,
alterations in the body weights of mice treated with oHSV2 compared
with the control were not statistically significant (Fig. 2B), and no necrosis or skin ulcerations
were observed in the oHSV2 treatment group. Two days after the last
treatment, mice in the oHSV2 treatment group had glossy, healthy
fur and exhibited normal behavior. In contrast, the behavior of the
mice in the control group were apathetic and dull, and their fur
was disordered (Fig. 2C). These
results demonstrated that oHSV2 exerted significant antitumor
effects and did not influence mouse body weight.
CRLM in BALB/c mice
Mice were sacrificed by cervical dislocation at 19
days post-tumor cell inoculation. Their livers were dissected, and
different-sized nodules were distributed on the liver surfaces of
all mice (Fig. 3). Significantly more
nodules of diameter >1 mm were observed in the control group
compared with the oHSV2 treatment group. As presented in Fig. 3, histopathological analysis of tissue
sections confirmed that the liver nodules were poorly
differentiated adenocarcinomas, demonstrating successful
establishment of the CRLM model. The results revealed that the
intrasplenic injection was effective in building the CRLM model in
BALB/c mice.
oHSV2 treatment inhibits CRLM in
vivo
As described above, the livers and spleens of the
mice were separated and weighed, and the numbers of visible tumor
nodules on the liver surfaces were counted in the two groups 7 days
after the last treatment. As presented in Fig. 3, numerous tumor nodules formed on the
livers of the control group mice, while few tumor nodules formed on
the livers of the oHSV2 mice. Statistical analysis revealed that
the number of nodules formed in the control group livers was
significantly higher compared with those of the oHSV2 treatment
group (Fig. 4A). As presented in
Fig. 4B, liver weights in the control
group were increased compared with those in the oHSV2 treatment
group (P<0.05). However, the spleen weights in the control and
oHSV2 treatment groups were not significantly different (Fig. 4C, P=0.116). With the exception of the
mice used in the aforementioned experiments, mouse survival was
assessed every day until the end of the experiment, revealing that
the overall survival (OS) of mice in the oHSV2 treatment group was
prolonged compared with that of control group mice (Fig. 4D, P<0.05). These data illustrated
that intratumoral injection of oHSV2 into the right flanks of mice
with established liver metastasis nodules significantly inhibited
CRLM and prolonged OS.
oHSV2 treatment increases the
percentages of CD4+ T, CD8+ T and NK cells in murine peripheral
blood
To examine the mechanisms underlying the suppression
of CRLM by treatment with oHSV2 in immunocompetent BALB/c mice
bearing the CT-26 cell CRLM model, the effects of oHSV2 on inducing
immunological alterations in the peripheral blood were
investigated. The percentages of CD4+ T, CD8+
T and NK cells in mouse peripheral blood were assessed by flow
cytometry. As presented in Fig. 5,
treatment with oHSV2 increased the peripheral blood percentages of
CD4+ T, CD8+ T and NK cells compared with
those in the control group (P=0.0250, 0.0025 and 0.0166,
respectively), suggesting that oHSV2 may upregulate specific
antitumor immune responses in BALB/c mice bearing the CT-26 cell
CRLM model.
Discussion
It has previously demonstrated that oHSV2 may
cytolytically destroy tumor cells in vitro and inhibit
xenograft tumor growth by increasing the immune response in
immunocompetent mice in vivo (14,22–24).
Oncolytic viruses are able to specifically target and kill tumor
cells and provide an in situ cancer immunotherapy vaccine.
Investigators have demonstrated that HSV vectors may inhibit the
growth of established tumors at distant sites (25), although the potential inhibition of
CRLM by oncolytic viruses has not been examined.
Despite recent improvements in comprehensive CRC
therapies, the 5-year survival rate for metastatic CRC (mCRC)
remains <30% (26). To date,
treatment with all of the available drugs in combination or in
sequence until progression or unacceptable toxicity occurs has been
the standard therapy for mCRC (27).
Viruses are currently the most promising therapeutic approach to
treating tumors (28,29). In 2015, the US Food and Drug
Administration approved talimogene laherparepvec (T-VEC) for use in
melanoma patients with injectable, nonresectable skin and lymph
node lesions (30).
Oncolytic viruses specifically infect, replicate in,
and kill cancer cells while leaving normal cells unharmed (31,32), and
simultaneously release tumor-specific antigens that are recognized
by CD8+ T lymphocytes (33). oHSV2 agents are armed with
granulocyte-macrophage colony-stimulating factor, a hematopoietic
growth factor and pro-inflammatory cytokine that promotes the
activation and tissue accumulation of monocytes, macrophages, and
granulocytes (14,34,35). The
majority of solid tumors are infiltrated by a large variety of
immune cells, including CD3+ T cells (CD4+
helper and CD8+ cytotoxic T cells) and NK cells
(36–38).
The authors of the present study have previously
demonstrated the antitumor effects of oHSV2 (14). In the present study, a series of
experiments were performed to further assess the therapeutic
effects of oHSV2 on CRLM in mice. First, the effects of oHSV2 on
CT-26 cells were assessed with a CCK8 assay in vitro,
revealing that oHSV2 markedly inhibited CT-26 cell growth. In
addition, as the MOI of oHSV2 increased, the survival rate of CT-26
cells gradually deceased. To evaluate whether oHSV2 was effective,
a right flank subcutaneous xenograft tumor and CRLM model was
successfully established in BALB/c mice and oHSV2 was
intratumorally-injected into the xenograft tumors. Tumor volumes in
the oHSV2 treatment group were significantly lower compared with
those in the control group, and the numbers of metastasized liver
nodules exhibited a similar trend, illustrating that oHSV2 was able
to inhibit the growth and metastasis of the CT-26 cell CRLM model.
Flow cytometry was used to analyze peripheral blood samples
collected from the two groups of mice, in order to assess the
effects of immune cell numbers on the inhibition of CRLM. The
percentages of CD4+ T, CD8+ T and NK cells in
the oHSV2 treatment group were increased compared with those in the
control group, demonstrating that oHSV2 delivered intratumorally to
subcutaneous xenograft tumors induced immune responses to inhibit
CRLM.
CRLM is a dynamic process in which the immune
microenvironment serves an important role in tumor initiation,
proliferation, growth and metastasis (39). However, tumors resist clinical
treatment via the rapid progression of immune escape and
immunosuppressive mechanisms, leading to treatment failure
(40). Meanwhile, cellular immune
reactions are modified during metastasis, inducing tumor cell
senescence (41). In the context of
oncolytic HSV, the initial stages of immunogenic virus replication
leading to activation of antitumor immunity were demonstrated to be
more important compared with the persistence of the virus
replicating within the tumor (42),
illustrating the importance of controlling the tumor
microenvironment for successful tumor therapy.
To the best of our knowledge, this is the first
study which evaluates the antimetastatic effects of oHSV2 in a CRLM
model established in BALB/c mice. Herein, oHSV2 was
intratumorally-injected into right flank subcutaneous xenograft
tumors, inducing indirect inhibition of tumor growth and inhibition
of CRLM. In vitro, the growth capacity of CT-26 cells was
reduced by administration of oHSV2. In vivo, treatment with
oHSV2 effectively prolonged the overall mouse survival and also
restricted liver metastasis in the CRLM model. oHSV2 also caused an
increase in the percentages of CD4+ T, CD8+ T
and NK cells in the mouse peripheral blood, further demonstrating
the inhibitory effect of oHSV2 on CRLM. Although oHSV2 serves as a
potential novel approach for the treatment of CRLM, there remains a
need to further verify its efficacy in CRLM models in other CRC
cell lines.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81650016) and CAMS
Innovation Fund for Medical Sciences (CIFMS) (grant no.
2016-I2M-1-001).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author, on reasonable
request.
Authors' contributions
SL, WZ, QG and BL conceived and designed the
experiments. FW, WZ and XH performed the experiments. FW and WZ
analyzed the data. FW and WZ wrote the manuscript. JL and XH
assisted with histological analysis. FW, WZ, XH, and JL
participated in animal experiments and sample collection.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics of
Animal Experiments Committee of the Chinese Academy of Medical
Sciences and Peking Union Medical College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goss PE, Strasser-Weippl K, Lee-Bychkovsky
BL, Fan L, Li J, Chavarri-Guerra Y, Liedke PE, Pramesh CS,
Badovinac-Crnjevic T, Sheikine Y, et al: Challenges to effective
cancer control in China, India and Russia. Lancet Oncol.
15:489–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Varghese C and Shin HR: Strengthening
cancer control in China. Lancet Oncol. 15:484–485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adam R: Chemotherapy and surgery: New
perspectives on the treatment of unresectable liver metastases. Ann
Oncol. 14 Suppl 2:ii13–ii16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salah S, Ardissone F, Gonzalez M, Gervaz
P, Riquet M, Watanabe K, Zabaleta J, Al-Rimawi D, Toubasi S, Massad
E, et al: Pulmonary metastasectomy in colorectal cancer patients
with previously resected liver metastasis: Pooled analysis. Ann
Surg Oncol. 22:1844–1850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van den Eynde M and Hendlisz A: Treatment
of colorectal liver metastases: A review. Rev Recent Clin Trials.
4:56–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maffione AM, Lopci E, Bluemel C,
Giammarile F, Herrmann K and Rubello D: Diagnostic accuracy and
impact on management of (18)F-FDG PET and PET/CT in colorectal
liver metastasis: A meta-analysis and systematic review. Eur J Nucl
Med Mol Imaging. 42:152–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomlinson JS, Jarnagin WR, DeMatteo RP,
Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH and
D'Angelica M: Actual 10-year survival after resection of colorectal
liver metastases defines cure. J Clin Oncol. 25:4575–4580. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fong Y, Fortner J, Sun RL, Brennan MF and
Blumgart LH: Clinical score for predicting recurrence after hepatic
resection for metastatic colorectal cancer: Analysis of 1001
consecutive cases. Ann Surg. 230:309–321. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
House MG, Kemeny NE, Gonen M, Fong Y,
Allen PJ, Paty PB, DeMatteo RP, Blumgart LH, Jarnagin WR and
D'Angelica MI: Comparison of adjuvant systemic chemotherapy with or
without hepatic arterial infusional chemotherapy after hepatic
resection for metastatic colorectal cancer. Ann Surg. 254:851–856.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobuchi S, Ito Y, Hayakawa T, Nishimura A,
Shibata N, Takada K and Sakaeda T: Pharmacokinetic-pharmacodynamic
(PK-PD) modeling and simulation of 5-fluorouracil for erythropenia
in rats. J Pharmacol Toxicol Methods. 70:134–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu X, Tao L, Cai R, Prigge J and Zhang X:
A mutant type 2 herpes simplex virus deleted for the protein kinase
domain of the ICP10 gene is a potent oncolytic virus. Mol Ther.
13:882–890. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anzivino E, Fioriti D, Mischitelli M,
Bellizzi A, Barucca V, Chiarini F and Pietropaolo V: Herpes simplex
virus infection in pregnancy and in neonate: Status of art of
epidemiology, diagnosis, therapy and prevention. Virol J. 6:402009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Q, Zhang W, Ning Z, Zhuang X, Lu H,
Liang J, Li J, Zhang Y, Dong Y, Zhang Y, et al: A novel oncolytic
herpes simplex virus type 2 has potent anti-tumor activity. PloS
One. 9:e931032014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Dutuor A, Fu X and Zhang X:
Induction of strong antitumor immunity by an HSV-2-based oncolytic
virus in a murine mammary tumor model. J Gene Med. 9:161–169. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu X, Tao L and Zhang X: An oncolytic
virus derived from type 2 herpes simplex virus has potent
therapeutic effect against metastatic ovarian cancer. Cancer Gene
Ther. 14:480–487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Workenhe ST, Simmons G, Pol JG, Lichty BD,
Halford WP and Mossman KL: Immunogenic HSV-mediated oncolysis
shapes the antitumor immune response and contributes to therapeutic
efficacy. Mol Ther. 22:123–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bollino D, Colunga A, Li B and Aurelian L:
ΔPK oncolytic activity includes modulation of the tumor cell
milieu. J Gen Virol. 97:496–508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colunga AG, Laing JM and Aurelian L: The
HSV-2 mutant DeltaPK induces melanoma oncolysis through
nonredundant death programs and associated with autophagy and
pyroptosis proteins. Gene Ther. 17:315–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang H, Peng T, Li J, Wang Y, Zhang W,
Zhang P, Peng S, Du T, Li Y, Yan Q and Liu B: Treatment of colon
cancer with oncolytic herpes simplex virus in preclinical models.
Gene Ther. 23:450–459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozlowski JM, Fidler IJ, Campbell D, Xu
ZL, Kaighn ME and Hart IR: Metastatic behavior of human tumor cell
lines grown in the nude mouse. Cancer Res. 44:3522–3529.
1984.PubMed/NCBI
|
|
22
|
Yin L, Zhao C, Han J, Li Z, Zhen Y, Xiao
R, Xu Z and Sun Y: Antitumor effects of oncolytic herpes simplex
virus type 2 against colorectal cancer in vitro and in vivo. Ther
Clin Risk Manag. 13:117–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang H, Peng T, Li J, Wang Y, Zhang W,
Zhang P, Peng S, Du T, Li Y, Yan Q and Liu B: Treatment of colon
cancer with oncolytic herpes simplex virus in preclinical models.
Gene Ther. 23:450–459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu X, Rivera A, Tao L and Zhang X: An
HSV-2 based oncolytic virus can function as an attractant to guide
migration of adoptively transferred T cells to tumor sites.
Oncotarget. 6:902–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Toda M, Rabkin SD, Kojima H and Martuza
RL: Herpes simplex virus as an in situ cancer vaccine for the
induction of specific anti-tumor immunity. Hum Gene Ther.
10:385–393. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ottolino-Perry K, Acuna SA, Angarita FA,
Sellers C, Zerhouni S, Tang N and McCart JA: Oncolytic vaccinia
virus synergizes with irinotecan in colorectal cancer. Mol Oncol.
9:1539–1552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Esin E and Yalcin S: Maintenance strategy
in metastatic colorectal cancer: A systematic review. Cancer Treat
Rev. 42:82–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Russell SJ, Peng KW and Bell JC: Oncolytic
virotherapy. Nat Biotechnol. 30:658–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sze DY, Reid TR and Rose SC: Oncolytic
virotherapy. J Vasc Interv Radiol. 24:1115–1122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pol J, Kroemer G and Galluzzi L: First
oncolytic virus approved for melanoma immunotherapy.
Oncoimmunology. 5:e11156412015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shinozaki K, Ebert O and Woo SL:
Eradication of advanced hepatocellular carcinoma in rats via
repeated hepatic arterial infusions of recombinant VSV. Hepatology.
41:196–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fong Y, Kim T, Bhargava A, Schwartz L,
Brown K, Brody L, Covey A, Karrasch M, Getrajdman G, Mescheder A,
et al: A herpes oncolytic virus can be delivered via the
vasculature to produce biologic changes in human colorectal cancer.
Mol Ther. 17:389–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toda M, Rabkin SD, Kojima H and Martuza
RL: Herpes simplex virus as an in situ cancer vaccine for the
induction of specific anti-tumor immunity. Hum Gene Ther.
10:385–393. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi Y, Liu CH, Roberts AI, Das J, Xu G,
Ren G, Zhang Y, Zhang L, Yuan ZR, Tan HS, et al:
Granulocyte-macrophage colony-stimulating factor (GM-CSF) and
T-cell responses: What we do and don't know. Cell Res. 16:126–133.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Croxford AL, Spath S and Becher B: GM-CSF
in neuroinflammation: Licensing myeloid cells for tissue damage.
Trends Immunol. 36:651–662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cerwenka A, Baron JL and Lanier LL:
Ectopic expression of retinoic acid early inducible-1 gene (RAE-1)
permits natural killer cell-mediated rejection of a MHC class
I-bearing tumor in vivo. Proc Natl Acad Sci USA. 98:11521–11526.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Diefenbach A, Jensen ER, Jamieson AM and
Raulet DH: Rae1 and H60 ligands of the NKG2D receptor stimulate
tumor immunity. Nature. 413:165–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density and location of immune cells
within human colorectal tumors predict clinical outcome. Science.
313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pancione M, Giordano G, Remo A, Febbraro
A, Sabatino L, Manfrin E, Ceccarelli M and Colantuoni V: Immune
escape mechanisms in colorectal cancer pathogenesis and liver
metastasis. J Immunol Res. 2014:6868792014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alpizar YA, Chain B, Collins MK, Greenwood
J, Katz D, Stauss HJ and Mitchison NA: Ten years of progress in
vaccination against cancer: The need to counteract cancer evasion
by dual targeting in future therapies. Cancer Immunol Immunother.
60:1127–1135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seebauer CT, Brunner S, Glockzin G, Piso
P, Ruemmele P, Schlitt HJ, Geissler EK, Fichtner-Feigl S and
Kesselring R: Peritoneal carcinomatosis of colorectal cancer is
characterized by structural and functional reorganization of the
tumor microenvironment inducing senescence and proliferation arrest
in cancer cells. Oncoimmunology. 5:e12425432016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Workenhe ST, Simmons G, Pol JG, Lichty BD,
Halford WP and Mossman KL: Immunogenic HSV-mediated oncolysis
shapes the antitumor immune response and contributes to therapeutic
efficacy. Mol Ther. 22:123–131. 2014. View Article : Google Scholar : PubMed/NCBI
|