Introduction
The skin is the site most frequently affected by
neoplasia, and basal cell carcinoma (BCC) is the most prevalent of
all cancers in fair-skinned individuals. Epidemiologic data reveal
that worldwide prevalence and incidence rates of non-melanoma skin
cancer (NMSC) are increasing, especially in the young population,
most likely due to a combination of ozone depletion, increased
recreational outdoor activities, and changes in clothing style
(1), therefore turning BCC into a
growing public health problem (2).
Even though BCCs are usually indolent, these tumors
may also have an aggressive evolution, infiltrating deep
structures, destroying the underlying tissues and in rare instances
metastasizing (3). BCC should
therefore be treated at the earliest possible stage. Moreover,
numerous studies were directed at identifying risk factors
associated with more aggressive phenotypes (4).
Vascular structures play a substantial role in the
pathogenesis of malignant skin tumors. Hence, investigation of
vascular structures in skin lesions using non-invasive techniques
such as dermoscopy and reflectance confocal microscopy (RCM) and
evaluation of their morphology and architectural arrangement on
histopathological examination could provide valuable clues for the
diagnosis and prognosis of BCC (5,6).
BCC is characterized by a large variety of clinical
and dermoscopic traits (7) owing to
a large number of combinations of histologic features, thus making
the diagnosis of BCC not always easy. Dermoscopy is usually helpful
in identifying BCC and, with the help of several dermoscopic
criteria, discriminating it from other skin cancers, still the
majority of dermoscopic studies have been carried out mainly on
pigmented BCCs. However, BCC lesions often prove difficult to
diagnose due to the lack of pigmented structures. Thus, detection
and quantification of lesion vasculature may provide critical
information for diagnosis and prognosis (8).
Furthermore, in the case of these ulcerated BCCs the
clinician simply lacks the means to correctly diagnose a BCC, and
is therefore driven towards other entities such as melanoma or
squamous cell carcinoma (SCC) resulting in an increased patient
psychological distress and increased burden of urgent surgical
removals (9). In these cases, other
in vivo imaging techniques, such as RCM, could provide
additional information regarding the tumor vascular pattern,
increasing diagnostic accuracy and reducing moral and financial
burdens.
BCCs ability of local invasion but rarely metastasis
may be connected to its microvasculature, suggesting that
histopathological and immunohistochemical studies of microvessels
counts and angiogenic factors expression could provide a more
detailed account of the vascular mechanisms supporting its
evolution.
This review article plans to take you on a journey
through the vascular aspects of BCCs, starting with anatomical
observations, observable with the naked eye, through dermoscopy,
in vivo RCM, and ending with the physiopathological and
histological foundations of BCC vasculature development and
evolution.
Gross anatomy of the relation between BCC
and blood vessels
Recent clinical observations have led to possible
paradigm shifting hypotheses concerning risk factors for BCC
development. Heckmann et al (10) suggest that ultraviolet radiation (UV)
exposure may not be the only factor for NMSC localization. The
authors found no correlation between BCC and areas of chronic UV
exposure alone, reporting a higher incidence of BCC in the
preauricular crest compared to helix, and in the medial orbital
quadrant compared to the lateral quadrant. Others have proposed
that localized tissue changes such as reduced dermal thickness via
disturbed cell matrix interactions may promote NMSC development in
specific regions of the face (11,12).
Altogether, these observations imply the existence of additional
NMSC risk factors, other than chronic UV exposure.
Recently, the question has been raised, whether the
facial arterial network may influence NMSC localization. Studying
NMSC arterial colocalization in the fronto-temporal area by means
of echo-Doppler ultrasonography and histopathology, Kuonen et
al (13) found BCC arterial
colocalization in 59% of tumors, a significantly higher proportion
than that of random arterial colocalization in adjacently
distributed 175 mm2 surface areas (32%). Combining both
echo-Doppler and microscopic analyses revealed that 82% of tumors
colocalized with an arterial branch. The authors reported similar
rates of colocalization for the frontal versus temporal regions (78
vs. 85%) as well as in BCC versus SCC (83 vs. 80%). Taken together,
these findings suggest that BCCs of the fronto-temporal area are
preferentially localized in the close proximity of the facial
arterial blood vessels (13).
However, the study only took into account high-caliber arterial
vessels of the cutaneous and subcutaneous layers of the skin
detected by echo-Doppler ordefined by a diameter greater than 300
µm on histopathology, which is an arbitrary restriction and may
very well underestimate the actual tumor-artery
colocalizations.
Angiogenesis and BCC
The skins vascular supply is provided through a deep
dermal plexus and a superficial, subpapillary plexus. Dermal blood
vessel growth primarily occurs during embryogenesis and, as
discovered since the 1980s, is regulated by several soluble factors
of angiogenesis and antiangiogenesis (14). Among these, vascular endothelial
growth factor (VEGF) is recognized as the main proangiogenic
factor, and thrombospondin 1 and 2 as main antiangiogenic factors.
Blood vessel shape and size remain constant as long as there is a
balance between pro- and anti-angiogenic stimuli (15). Angiogenesis, a process stimulated by
hypoxia and inflammation (16,17), has
been thought of as essential for tumor growth since 1971 (18).
There is a great body evidence highlighting the
importance of aberrant angiogenesis in the pathogenesis of cancer.
Growing tumors feed on newly formed capillaries, and continuity
between the tumor and the hosts vascular system is dependent on
this microvascular bed (19).
Without angiogenesis, a tumor cannot grow beyond a size of ~1–2
mm3 and cannot metastasize (14,20,21).
Moreover, it has been shown that the angiogenic
phenotype sets the boundary line between hyperplasia and neoplasia
(20), and that even though vascular
patterns vary significantly in solid tumors, there is a certain
relationship between tumor growth and the degree of vascularization
(22).
The term ‘angiogenic switch’ refers to the
predominance of proangiogenic factors, resulting in formation of
new blood vessels (20,23). Higher microvessel densities (MVD)
measured in histologic specimens of tumors and in areas adjacent to
the tumor-stroma interface, have been linked to adverse prognosis
in a variety of tumor entities (21,22,24–35).
Expression of VEGF, the main proangiogenic factor,
has been found increased in BCCs when compared to normal skin,
although to a lesser degree than in cutaneous SCC (36). Studies suggest that VEGF in BCCs is
correlated to MVD (37).
Angiogenesis must, in turn, be supported by extracellular matrix
remodeling (38). Proteomic studies
have identified biomarkers such as COX-2, matrix metallopeptidase-9
(MMP-9), and Maspin to play important roles in the promotion of
angiogenesis and neovascularization in BCCs (39,40).
Moreover, a correlation between COX-2 overexpression and increased
levels of vascular endothelial growth factor-A, regulators of
apoptosis Mcl-1 and Bcl-2, and CD31 positive vessels has been
suggested by previous studies (41).
In contrast, tissue inhibitors of metalloproteinases (TIMPs) exert
anti-angiogenic activities through inhibition of MMP-dependent
angiogenesis (42).
There is sufficient evidence suggesting that
angiogenesis is crucial for the metastasis of vascular tumors. The
immaturity of new microvessels promotes the local shedding of
neoplastic cells into the tumoral venous stream (30,43),
thereby consolidating the expansion of metastatic colonies
(23).
What about tumors that can invade but do not
metastasize? It is hypothesized that differences in the
microvasculature associated with various types of epidermal-derived
tumors might account for these differences in tumor behavior. BCCs
are a perfect example of epidermal-derived tumors that have
potential for local invasion but in which metastatic spread is
rare, with incidences ranging from 0.0028 to 0.55% (44,45).
The dermoscopic perspective on BCC
vasculature
The skin has a layered structure, its different
color components being determined by the presence of different
pigments. Out of all these pigments, melanin and hemoglobin are the
most dominant, the latter being responsible for the color red.
Dermoscopy is an in vivo skin examination
technique that has proven helpful in differentiating BCC from other
malignancies, such as SCC and melanoma (46–49).
Dermoscopic diagnostic criteria for BCC include leaf-like areas,
large blue-grey ovoid nests, short white streaks/chrysalis,
spoke-wheel areas and multiple blue-gray dots and globules
(7,50,51). In
addition to these pigmentary criteria, specific vascular patterns
may prove useful for BCC diagnosis, particularly when the
above-mentioned structures are missing (6,52–54).
Relatively superficial blood vessels are readily accessible to
dermoscopic investigation, thus a number of vascular patterns have
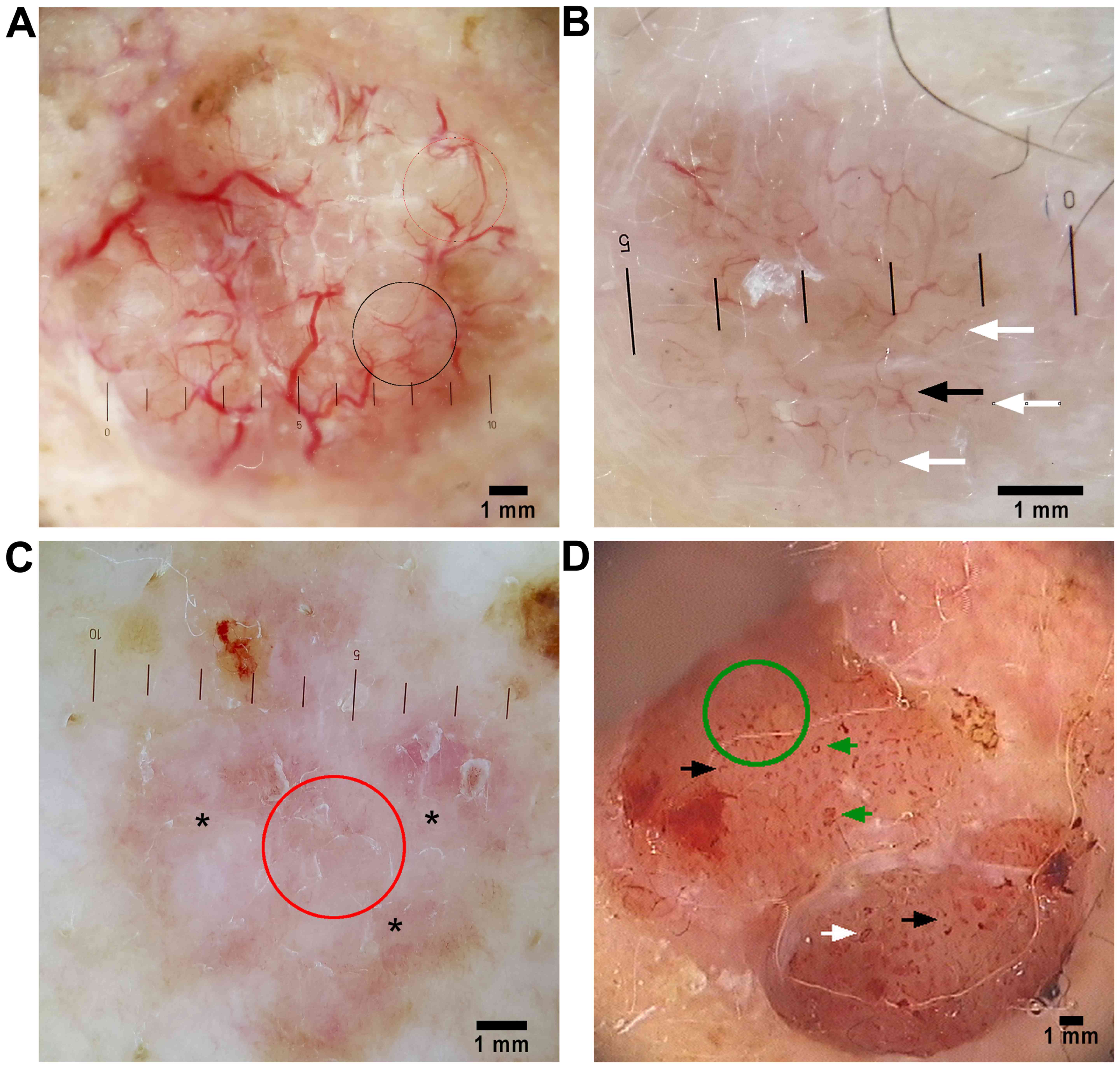
been described in BCCs (Fig. 1), all
the while keeping in mind that skin pigmentation due to melanin can
sometimes occlude the visibility of blood vessels.
Dermoscopic global vascular patterns in BCC. Global
vascular patterns helpful in the dermoscopic diagnosis of BCC have
been outlined by several authors. These patterns include either
clustered (vessels with similar morphology closely gathered
together), scattered (vessels with irregular and diffuse
distribution), homogenous (densely aligned symmetrical vessels), or
avascular (no vessels can be seen). Trigoni et al (55) found that the most consistent global
vascular pattern in all BCC subtypes is the scattered pattern
(96%), while Pan et al (56)
also found the scattered pattern to be the most frequent (97%) in
superficial BCC (sBCC). In contrast, the avascular global pattern
was noted in only 3% of nodular BCCs and 7% of sBCCs (55).
Dermoscopic local vascular patterns in
BCC
Arborizing vessels
Arborizing vessels are the most impressive vascular
pattern seen in BCC. Because in many instances clinical assessment
of telangiectasia does not account for the particular structure of
the vessels, many tumors are misdiagnosed as BCCs just because
telangiectasias are visible. As Staindl and Lametschwandtner
perfectly pointed out, blood vessels in BCC are located on the
surface, just below the epidermis, regularly traversing the whole
lesion (57). This accounts for
their perfect visibility and sharp focus, even in heavily pigmented
tumors. Furthermore, arborizing vessels in BCC have stem vessels of
>0.2 mm in diameter and wind in a strange fashion, branching
irregularly into fine capillaries. These vessels are bright red, as
opposed to normal pink vessels in the dermal plexus, which are
always slightly blurry, never being depicted as crisp (Fig. 1A). There are a few tumors (nodular
melanoma, blue nevi, and syringomas) which display similar vascular
structures at first glance. Looking closer, their vessels appear to
branch more regularly and orderly than BCC vasculature and they can
be differentiated easily by morphologic measuring techniques
(53).
Arborizing vessels have been described in all BCC
subtypes with different frequencies. Giacomel and Zalaudek
(58) report their presence in 63%
(retrospective study; n=24) of all BCC subtypes analyzed, Liebman
et al (59) place that figure
between 18.8–38.3% (retrospective study; n=149) deeming arborizing
vessels as one of the main vasculature types, as do Micantonio
et al (54) with 60.7%
presence (retrospective study; n=504), and Popadic which places
this feature as second most frequent after the milky-red background
(prospective observational study; n=151) (48). This pattern is constantly described
in nodular BCC (nBCC) and cicatricial BCC (53), whereas in superficial BCC (sBCC) they
are harder to detect (54,55), probably as a result of the greater
vascular need in the nodular type compared with sBCC. In a
retrospective observation study Pan et al (56) note that arborizing vessels were more
likely to be observed in a sample of 150 sBCC than in other
cutaneous malignancies or inflammatory skin diseases, such as
intraepidermal carcinoma or psoriasis. According to Kreusch and
Koch, the sensitivity and specificity of the arborizing vessels
pattern for BCC is very high, at 96.1 and 90.9% (n=84),
respectively (60). Thus, even
though the data comes mainly from retrospective studies, it is
clear that arborizing vessels is one of the most important
dermoscopic criteria in BCC evaluation.
Short fine telangiectasia
With a presence of 92% (n=24), one retrospective
study (58) found short, fine
telangiectasias (SFTs) to be a dermoscopic hallmark of sBCC
(Fig. 1B and C). This finding is
supported by a larger retrospective study, Liebman et al
(59) documenting the presence of
SFTs in 73.8–82.6% (n=149) of all nodular, superficial, and
infiltrative BCCs. On the other hand, Popadic (48), in her prospective observational
study, mentions a much lower frequency of SFTs in BCC (19.9%)
(n=151), although accompanied by a positive predictive value (PPV)
of 100%, thus suggesting that the presence of this feature is
highly evocative of the diagnosis.
Arborizing microvessels
Arborizing microvessels, seen at routine ten-fold
magnification as short, linear straight, linear serpentine vessels
of small diameter and relatively few branches (Fig. 1A and B), are more likely observed in
sBCC than in nBCC, cystic BCC (6).
According to Popadics prospective observational study with a sample
of 151 tumors, arborizing microvessels were more frequent than
large vessels (49 vs. 35%) in BCCs, but had a slightly lower
diagnostic value (PPV=97 vs. 98%) (50).
Milky-pink background
Milky-pink background, often described as a
white-red, translucent to opaque structureless area (Fig. 1C) has been found by many authors,
with various degrees of frequency ranging from 41.6 to 100%, in
superficial BCCs (55,56,58,59,61).
Popadic found milky-red background, out of all studied vascular
patterns, to have the highest sensitivity value for BCC diagnosis.
However, because this dermoscopic feature simply correlates to
lesion vascularization and is also present in several non-BCC
lesions such as actinic keratoses, SCC, Bowens disease, seborrheic
keratoses, and angiomas, it has a low specificity (48%) and does
not have significant diagnostic value for BCC (50).
Comma vessels
Comma vessels (Fig.
1D) are reported by some authors as minor vascular patterns
found in less than 10% (retrospective study; n=504) of BCC lesions
(54) while others have observed
them in 45% of all BCC subtypes, reaching 93% presence in
superficial BCCs (retrospective study; n=138) (55).
Glomerular vessels
While glomerular vessels, defined as frequently
clustered, tortuous capillaries (Fig.
1D), are archetypical of Bowens disease (52,53,62),
they have been described as a minor vascular pattern (frequency of
0–2.7% for all subtypes) of BCC in non-polarized dermoscopic
studies (n=531 and n=150, respectively) (52,56).
Loop vessels
The next two patterns, pinpoint or dotted vessels
and hairpin vessels are merely variants of the same vascular
structure, loop vessels. Vascular loops are seen in many lesions,
primarily in keratinizing tumors and melanoma. In order for these
patterns to be useful in discriminating keratinizing tumors from
melanoma, the criteria of ‘keratinization’ have been introduced by
Kreusch (53).
In thin tumors, the short capillary loops appear
dermoscopically as small red dots, of 0.01–0.02 mm in diameter
(Fig. 1D). When observed at higher
magnifications (30-fold or more) it becomes obvious that these dots
are the tips of short capillary loops. Dotted vessels are to be
considered tumoral vessels only when supplying a solid tumor whose
borders can be recognized on clinical inspection.
Although dotted vessels may be observed in nevi,
melanoma (52,53) and many keratinizing tumors with low
vertical diameters such as warts, actinic and seborrheic keratoses,
Bowens disease (59), and SCC
(52,53), they have been reported, to some
extent, in every BCC subtype (59).
Some authors have found these subtle, sparse, and focally
distributed vessels in >50% of lesions (n=149) across all
dermoscopic BCC types (59) while
larger studies report them as a minor vascular pattern, found in
<10% of lesions (n=504) (54).
In thicker tumors, vascular loops are longer and can
be seen as such, sometimes twisting and bending. However, their
diameter of ~0.01–0.03 mm remains constant along the entire course
of the vessel. This pattern in frequently encountered in the same
types of lesions as mentioned for dotted vessels, but only in
lesions of greater vertical thickness. Several studies mention the
presence of hairpin vessels in BCCs as a minor vascular feature,
with a wide range of frequency, between 2.6 and 18% (46,52,56,58,63).
As stated by Kreusch, blood vessels in all
keratinizing tumors are surrounded by a whitish halo representing
vital keratinocytes supplied by that particular vessel. This white
area gradually merges into a zone of yellow keratin. The amount of
keratin is dependent on the degree of cell differentiation as it
can be missing in poorly differentiated SCC or as a consequence of
scratching or keratolytic treatment of a lesion. This whitish halo
is absent in all melanocytic lesions, which can show pinpoint or
hairpin vessels, depending on their vertical diameter, in various
arrangement patterns (53).
It now becomes obvious how helpful global and local
vascular patterns can be to the clinician during the dermoscopic
examination of a skin lesion suspected to be a BCC. While not all
patterns have high diagnostic values and there is great variability
in reported sensitivities and specificities, the presence of
arborizing vessels or short fine telangiectasia can be of
significant value in the differential diagnosis. If vascular
patterns are easily observed through the dermoscope in intact
lesions, the situation changes with ulcerated or completely
ulcerated and heavily pigmented lesions.
Vascular dermoscopic patterns in particular
BCC subtypes
Ulcerated versus non-ulcerated BCC
In some cases, BCCs clinically present as ulcerated
nodules or papules, and therefore their clinical differential
diagnosis is difficult. In these instances, the dermoscopic
appearance of vascular patterns can significantly improve
diagnostic accuracy.
In a very recent retrospective blinded study
including 139 lesions, Arpaia et al (9) addressed the issue of vascular
dermoscopic patterns in ulcerated BCCs. The authors found that
ulcerated BCCs presented with annular, peri-ulcerous, distributed
telangiectatic vessels and hypopigmentation, both of which had high
diagnostic values (annular telangiectasias, PPV=95%; annular
hypo-pigmentation, PPV=100%). In the ulcerated areas, the comma
vascular pattern was significantly associated with the presence of
pigmentation (P=0.001) and blue-grey ovoid nests (P=0.027). In the
non-ulcerated areas, milky-pink background was associated with
leaf-like areas, and blue-white veil. However, the absence of
blue-white veil was strongly associated with the presence of
arborizing vessels. An inverse association was also observed
between the presence of hairpin vessels and the presence of at
least one of the classic BCC patterns. Through logistic regression
analysis, their analysis shows that the vascular features
influencing the correct diagnosis the most were: the presence of
arborizing vessels in ulcerated areas (100%), absence of the dotted
pattern in non-ulcerated portions, and absence of hairpin and
glomerular patterns in the ulcerated areas, the latter two being
more commonly associated with either melanoma or SCC.
Regarding completely ulcerated lesions, authors
found dotted and linear-irregular dermoscopic patterns to be the
most frequent, with a presence of 88.2 and 70.6%, respectively.
Comma and polymorph patterns occurred in ~35% of lesions and other
patterns (hairpin and glomerular) were rare (9).
These observations support the earlier findings of
Popadic (48), which have found
arborizing vessels (Sn=57.1%), arborizing microvessels (Sn=66.7%),
annular hypopigmentation (Sn=66.7%), milky-red background
(Sn=76.2%), and annular distribution of telangiectatic vessels
(Sn=90.5%) to be the most frequent vascular structures in their
sample of 21 ulcerated BCCs. Out of all these, arborizing vessels,
annular distribution of telangiectatic vessels, and annular
hypopigmentation were found to be highly significant (P<0.001)
for ulcerated BCC, while arborizing microvessels and milky-red
background were only significantly (P<0.05) present in these
lesions.
Also of importance, translucency was absent in
ulcerated BCCs, whereas it commonly appears in combination with
other dermoscopic features in non-ulcerated BCCs (48).
Pigmented versus non-pigmented BCC
As stated before, dermoscopy has been primarily used
in the study of pigmented skin lesions, therefore, in our opinion,
a comparison between vascular patterns in pigmented versus
non-pigmented BCC merits attention.
After being initially described in 52% (n=142) of
pigmented BCCs (63) and in 57.1-82%
of BCCs in subsequent studies (n=609) (46,64) that
included both nBCC and sBCC, in a much smaller study (n=42)
arborizing vessels have been identified in 14.3% of superficial
pigmented and non-pigmented BCCs (65). It is possible that the BCC subtypes
(nodular and/or superficial) included in different studies may
account for the great variability in percentages reported so far
(54).
Four dermoscopic features regarding vessel
morphology, have been identified as the most valuable in the
differentiation between non-pigmented and pigmented BCCs. These
criteria include telangiectasias, arborizing vessels, red dots and
globules. In their statistical analysis Trigoni et al
(55) reveal that arborizing vessels
were more often found in pigmented (74%) rather than non-pigmented
(31%) BCCs (n=138; P<0.0001), suggesting that this criterion may
prove of value in differential diagnosis. This observation had
already been mentioned in the literature, Micantonio et al
reporting a highly significant difference in arborizing vessels
frequency between superficial pigmented versus superficial
non-pigmented BCCs, while no difference was found in pigmented vs.
non-pigmented nBCC lesions (n=504) (54).
In contrast to other investigators, Altamura et
al (46) noted a high frequency
of arborizing vessels in non-pigmented BCCs. This inconsistency
might very well be the result of the subdivision of lesions into
several groups, based on the degree of observable pigmentation.
Opposed to other studies (46,63,66,67),
Trigoni et al (55) found
atypical vessels to be more common in all categories of BCCs.
Furthermore, the authors (55)
reported telangiectasias to be less frequent compared to the high
percentages reported by Micantonio et al (54), which subdivided telangiectatic
vessels into arborizing telangiectasia and SFTs. Further regarding
SFTs, Giacomel and Zalaudek (58) found SFTs in 92.0% (n=24) of
exclusively non-pigmented sBCCs, and Scalvenzi et al
(65) described SFTs in 66.6% (n=42)
of superficial pigmented and non-pigmented BCCs. This great
variability in reported percentages might be attributed to lesion
selection in these cases.
Fibroepithelioma of Pinkus
Zalaudek et al (68) reported fine arborizing vessels,
either alone or associated with dotted vessels were predominant in
fibroepithelial BCC (fibroepithelioma of Pinkus). It appears that
even though the vessels seen in fibroepithelioma of Pinkus are
arborized and in sharp focus, they differ from the arborizing
telangiectasias of nodular BCC, lacking stem vessels of large
diameter and having fewer ramifications (6).
Taken together, these data show that it is
significantly more difficult to diagnose a BCC solely on clinical
appearance or dermoscopy if the tumor is completely ulcerated or
very heavily pigmented. In such instances, BCCs are often mistaken
for SCCs or melanomas, leading to more aggressive treatments and,
as a consequence, an increased healthcare burden.
Dermoscopy of aggressive BCC
Few studies investigate the dermoscopic vascular
features of individual BCC subtypes and, while superficial
(56,58,65) and
nodular BCC dermoscopic vascular features have widely been
reported, aggressive BCC subtypes have been relatively neglected.
These so-called ‘aggressive-growth’ subtypes are distinguished by a
poorly circumscribed infiltrative growth pattern with a tendency
towards perineural and perivascular invasion, leading to difficult
surgical excision and consequent high recurrence rates (69). A recent study that investigated the
correlation of dermoscopic criteria with different BCC histotypes
showed that dermoscopy had a low sensitivity to identify risk of
recurrence (70).
In everyday practice, it is pink in the lesion area
of BCC that commonly attracts attention during clinical
examination. Superficial BCCs have been observed to display pink in
more than half of the tumor area in 84.9% of cases, a useful clue
to identifying this tumor (71). In
contrast to this, the same authors have shown (71) that aggressive BCC subtypes display
absent or less pink in the tumor area than other subtypes. Pink
represents increased localized vascular perfusion and is more
conspicuous in polarized non-contact dermoscopy (59). The former authors (71) postulate that prominent collagen in
the tumoral stroma of infiltrating and morphoeic BCC may correlate
with the reduced pink areas. Although not formally analyzed in the
study, the non-pink tumor areas where observed to be dominated by
white structureless areas, seen more frequently in BCC through
contact dermoscopy compared to polarized non-contact dermoscopy
(59). It is yet unknown to what
extent these pink areas relate to the associated lichenoid response
in the papillary dermis, or to more specific tumor factors
(71).
While reduced or absent vessels in the central tumor
area of aggressive subtypes has been observed, where 1 in 3
aggressive BCCs showed no central vessels (71), in aggressive BCC of the lower limbs,
glomerular (81.0%), dotted (71.4%) and hairpin (66.7%) vessels were
all more frequently observed than serpentine (57.1%) or branching
(47.6%) vessels. In all BCC subtypes, at all other sites, branching
and serpentine vessels predominate over glomerular, dotted and
hairpin vessels. Therefore, the authors concluded that there is a
shift towards SCC in the aggressive BCC vessel morphology profile
(71).
Due to the fact that aggressive BCC is commonly
found in combination with nBCC, the reported association between
large diameter vessels and aggressive BCC may be result of the
additional presence of nBCC in the same lesion (71).
A recent study focused on the dermoscopic features
of aggressive BCC reported arborizing microvessels and milky-red
background (100%), followed by SFTs, and white structureless areas
(75%) to be the most frequently detected features in morphoeic BCC
(72). The same study detected
arborizing vessels and microvessels and white structureless areas
in 66.7% of infiltrative BCCs, a finding supported by previous
studies (70). Truncated vessels and
globules have been reported as the main dermoscopic finding in
micronodular BCC (70).
Regarding sclerodermiform BCC, branching vessels
present in this variant tend to be finer, more scattered, and tend
to show less branching compared to the classic arborizing vessels
of nodular or cystic BCC. Moreover, the vessels of sclerodermiform
BCC commonly occur on a whitish background with poorly defined
borders, while the arborizing vessels of nodular or cystic BCC
typically envelope a relatively well defined and translucent pink
tumor (6).
These findings confirm that although dermoscopy is
extremely useful in BCC detection, it has limited impact in
discriminating aggressive histotypes from the non-aggressive
ones.
Automatic BCC vessel detection and
segmentation in dermoscopy images
Visual inspection, either clinical or through the
dermoscope, suffers from subjectivity and a lack of precision.
Furthermore, some vascular features are quite small and normally
occluded by other structures making their detection a challenging
task. Although studies in dermoscopy all concur on the importance
and significant diagnostic value of vascular structures, very few
studies exist on the quantitative and systematic analysis of blood
vessels in dermoscopic images.
Several studies (73–75) have
sought to solve these problems through machine learning using
automatic vessel detection and segmentation algorithms. More
recently, Kharazmi et al (8)
presented a novel segmentation technique for feature extraction of
cutaneous blood vessels in dermoscopy images that accounts for both
the background color components of the skin and blood vessel shape.
Compared to previous studies, this technique promises superior
vascular feature extraction useful for BCC classification.
Reflectance confocal microscopy of BCC
vasculature
Although dermoscopy has its indisputable place in
the non-invasive diagnosis of skin lesions, even
video-dermatoscopes that can display images enlarged 30- or even
70-fold do not show blood flow and cannot resolve microscopic
details, sometimes needed if an accurate diagnosis is to be made.
RCM is a novel imaging tool which provides horizontal optical
sections of the skin at nearly histological resolution. In recent
years it has become an established technique for noninvasive
diagnosis in several skin disorders (76–78),
especially in skin oncology (79–83).
Using RCM, cutaneous vascular structures and blood
flow can be visualized in real-time. In the dermis, vessels appear
as dark spaces transitioned by small, bright structures,
representing blood cells. RCM examination of normal skin upper
dermal vascular plexus shows horizontally oriented blood vessels.
Vessels that run vertically and horizontally are seen as round or
canalicular dark spaces, respectively (84,85).
Blood flow can be observed due to fast moving bright particles
inside the vessel lumina.
Various studies in this field have proposed specific
confocal microscopic diagnostic criteria for nonpigmented skin
tumors (86–89). RCM has the advantage of real-time
observation of vascular morphology and distribution patterns, which
can provide supplemental information for the diagnosis or
monitoring of nonpigmented skin lesions. A fair number of confocal
microscopic vascular criteria for NMSC diagnosis have already been
determined by previous research (79,85,90–93).
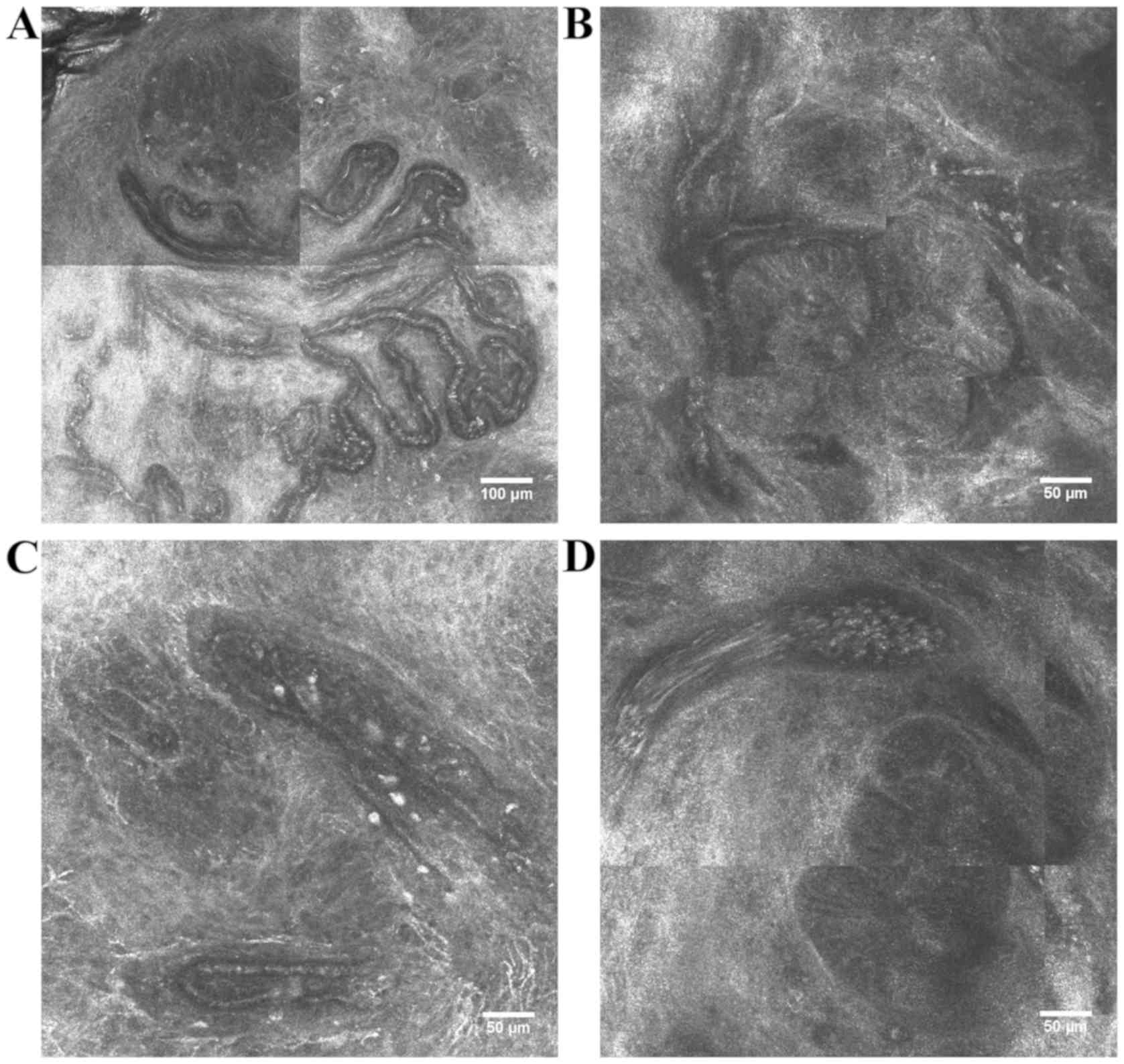
Sauermann et al found numerous, horizontal,
parallel oriented, enlarged blood vessels (diameter 10–105 vs.
10–14 µm in uninvolved skin) with very fast blood flow immediately
beneath the epidermis (35 to 50 µm beneath the surface) in the
tumoral region of BCCs. The authors found the same changes in
fibrosing BCC (94). We could
observe comparable vascular findings in RCM optical sections of
BCCs (Fig. 2) (unpublished
results).
One study conducted a comparative analysis of the
RCM vascular features between several nonpigmented tumoral lesions
which included 56 BCCs, 13 seborrheic keratoses (SKs), 11 SCCs, 8
actinic keratoses (AKs), 7 Bowens Disease, 3 keratoacanthomas, and
24 other nonpigmented tumors (95).
The authors (95) reported a strong
association between vascular polymorphism and malignancy and a
significantly (P<0.05) higher positive predictive value (72.1%)
of branching vessels for BCC lesions than any other tumor groups
analyzed. These findings are supported by others (95,96), who
also reported BCC lesions to display vascular polymorphism on RCM
examination, with at least two vascular morphologies as a result of
bizarre angiogenesis. Moreover, they found that non-branching,
high-diameter, straight linear and tubular vessels that surround
tumoral islands have higher PPV for BCC compared with other
nonpigmented lesions, a common finding in other confocal studies
(96,97). Underpinning these in vivo
observations, Grunt et al (98) histologically describe tumor cells
beds in BCC as being enveloped by basket-like capillary plexus, and
that telangiectatic but flattened capillaries run superficially,
over long distances, across tumors. Among all the tumor types, BCC
vasculature exhibited the largest vascular diameter (tubular
vessels at 53.5 µm and branching vessels at 53 µm). Branching
canalicular vessels have proved useful for differentiating BCC from
AKs and SCC, which have different vascular patterns (96).
Abnormal blood flow is frequently observed in BCCs
as a result of neoplastic angiogenesis (88). In one study (99), increased vasculature showed a
sensitivity of 95.8% for BCC diagnosis. Although skin lesions with
blood flow in opposite directions demonstrated a significantly
higher chance of malignant potential (95), this feature was not found
statistically significant in histopathology. A slight drawback of
RCM is the fact that the technique cannot differentiate between
preexisting blood vessels and the ones resulting from
neoangiogenesis. Be that as it may, vascular polymorphism is
associated with more complicated angiogenesis, thus it is highly
suggestive of malignancy.
Histopathological view of BCC
vasculature
Histopathology allows for the evaluation of
microvessel densities, expression of various proangiogenic factors
through immunohistochemistry, and the investigation of
neovascularization heterogeneity, therefore creating a more
detailed picture of tumor aggressiveness and prognosis.
Studies attempting to predict tumor behavior by
quantifying MVD face a number of obstacles. First and foremost is
the fact that there is no perfect vascular marker (100). Yet, in order to determine MVD,
several endothelial cell index have been employed in
immunohistochemical staining methods, such as CD34 and VEGF in the
study by Loggini et al (27),
and platelet factor VIII in the study by Sari Aslani and
Aledavood (101). However, the
sensitivity and specificity of these indices are less than CD31
(102). Examining the relationship
between vascular density of recurrent and non-recurrent lesions and
mitosis, Yerebakan et al have used CD31 and Ki67 (103). Rasi et al (104) determined SCC and BCC vascular
density using CD31, and Chin et al (25) used the same index to compare MVD in
the body and stroma of BCC, SCC and trichoepitheliomas. Their
results are briefly discussed below.
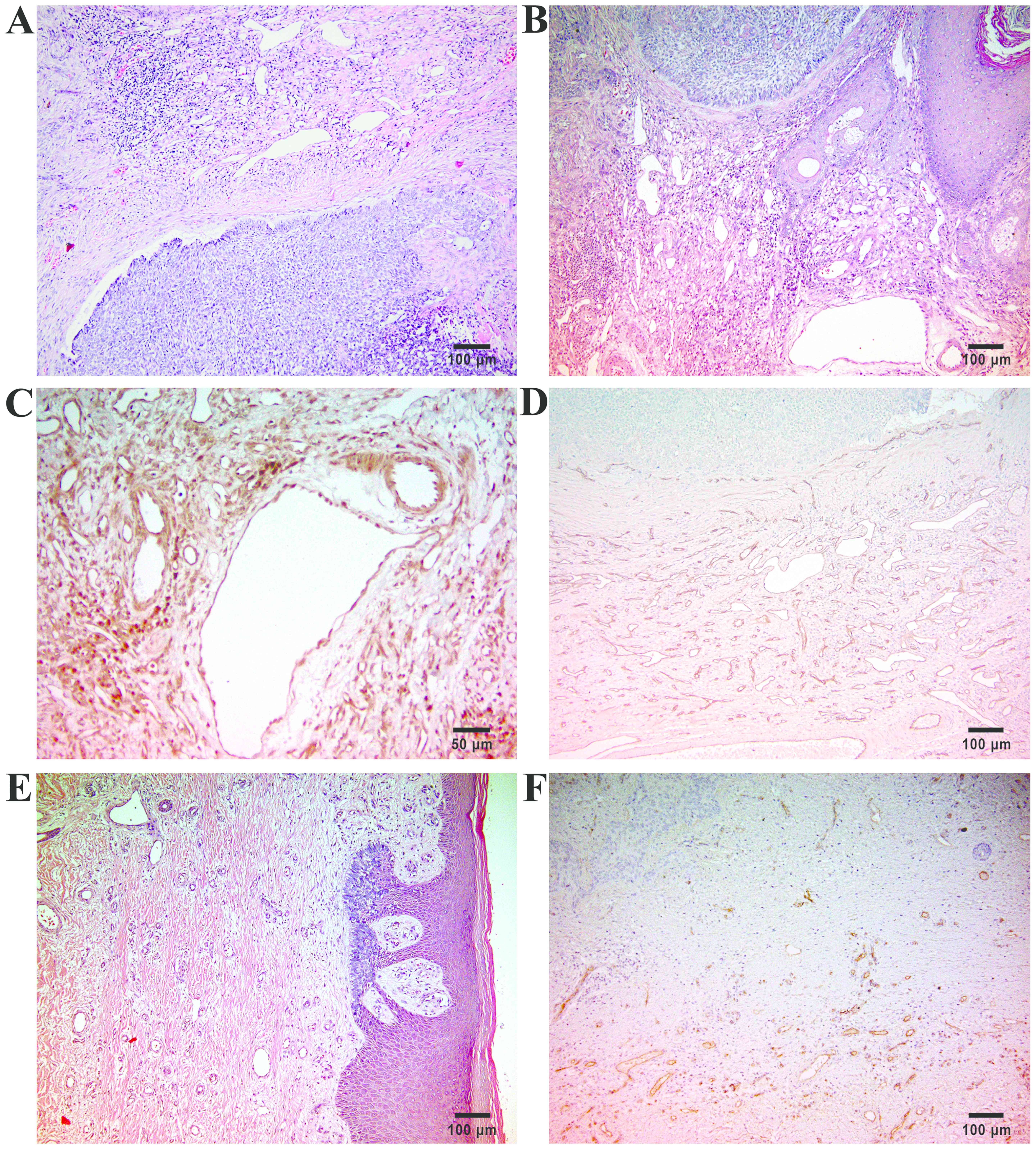
Immunohistochemical studies investigating MVD and
VEGF expression using CD31 and VEGF antibodies have revealed that
both these measures were higher in the morpheaform and nodular BCCs
(average value of 28.3 vessels/mm2) than in the
superficial specimens (average value of 17.4
vessels/mm2) (4), whereas
Winter et al (35) have found
no differences in MVD between nodular and morphoeic lesions but did
note a highly significant (P<0.0001) difference between nBCC
(24.7±6.7 peritumoral vessels/field), Pinkus tumors (19.7±6.6
vessels/field) and trichoblastomas (15.3±5.1 peritumoral
vessels/field). The latter authors noted generally low intratumoral
MVD and higher values in peritumoral stroma with a strong
correlation to tumor dignity (35),
supporting previous studies (25–27).
Therefore, both MVD and VEGF expression have been found to
gradually increase from the noninvasive to the more aggressive
lesions, defending earlier findings (28). Moreover, these results suggest that
determination of peritumoral MVD might facilitate differential
diagnosis.
A more recent study by Vuletic et al
compared MVD and VEGF expression using CD34 and VEGF antibodies in
101 lesions, in relation to BCC histotypes and demographics.
Superficial, nodular, cystic, keratinocytic, adenoid, infiltrative,
and metatypical BCCs were included in the study. Their results show
significantly higher MVD but no significant difference in VEGF
expression in the infiltrative, adenoid, metatypical and nodular
types. The lowest VEGF expression was found in superficial BCCs,
while infiltrative and metatypical subtypes presented the highest
values (105). Their results
reinforce the fact that the angiogenic potential of BCC is related
to lesion histotype. Our group could observe similar vascular
findings in histopathological sections of BCCs (Fig. 3) (unpublished results).
Chin et al (25), using a multivariate model allowing
for age, sex, and depth of tumor invasion, found higher MVD in SCC
than BCCs and trichoepitheliomas (TE), although no difference in
MVD was noted between nodular and morphoeic BCCs. Even though they
found vessels in the body of SCC but not in nodular BCC or TE, the
authors could not demonstrate a correlation between MVD and the
depth of invasion in neither SCC, BCC, nor TE. Blood vessels in
direct contact with tumor cells could not even be found in
morphoeic BCC.
Contrary to Staibano et al (28) who reported neovascularization
heterogeneity in BCCs, Chin et al (25) did not reach this result, even when
staining for PDGF-Rβ, PDGF-B, Factor VIII, VEGF-receptor 2, and
smooth muscle actin. However, the former authors describe the
presence of intense inflammation which might have led to
difficulties in microvascular evaluation. There is also a problem
in methodology in Chins study, as studied tumors were located on
the head and neck and control skin was obtained from the breast,
thus controls were poorly matched for age, sex, and most
importantly, body site. Despite these limitations, these results
are similar to previous studies, proving increased MVD in both BCC
and SCC (26).
The absence of a proven correlation between
vascular density and depth of invasion in BCCs is consistent with
the existing concept of a stromal angiogenic switch that precedes
the onset of invasive behavior. Chin et al (25) easily found intra-tumoral blood
vessels in SCCs, but not in TEs or BCCs. This difference in the
microvasculature would, therefore, explain how increased MVD can
account for both invasiveness and metastatic potential. Perhaps the
best possible test for this hypothesis would be to examine those
rare instances of BCCs that have undergone metastatic spread. The
prediction would be that blood vessels would be found in the body
of such tumors. Carbone et al (4) did just that, and measured MVD in
several primary superficial BCCs in a patient with BCC lung
metastases obtaining a value of 55.5 vessels/mm2, a
value higher than both morpheaform and nodular BCCs examined in the
same study (4), again confirming the
strong relation between tumor vascularization and aggressiveness
(28,36,106).
What does the scarcity of small blood vessels
within the body of nodular BCCs compared to the abundance in the
peritumoral stroma mean biologically? Some studies link
intratumoral vessel formation to metastatic potential of SCC in
contrast to BCC (27), while others
consider the high peritumoral MVD are attributed to local
aggressiveness (25,26,28,107).
The scarcity of blood vessels in the body of nBCC provides a good
explanation for their ulcerated appearance, while the stromal
angiogenic response would explain the appearance of telangiectasia
surrounding the tumor. The lack of intra-tumoral blood vessels
would account for the absence of metastatic potential leading to
the obvious hypothesis that those BCCs that do metastasize contain
intratumoral vessels. Opposite to this, SCCs which arise from a
similar cell type, do generate an angiogenic response in the tumor
body and frequently metastasize.
The forming picture shows that the angiogenic
response takes place in the peritumoral stroma, at the edge of
these tumors. Microvessels counts have frequently been reported to
be highest at the tumor periphery (35,108)
and it is here, in the tumor periphery, that the greatest
endothelial cell proliferation occurs (109). The invasive potential of these
tumors would then be explained by the presence of stromal
angiogenesis. This course of reasoning suggests that it is the
vasculature in the body of the tumor that is involved in the
haematogenous dissemination of neoplastic cells. Usually, but not
perfectly, invasive and metastatic potentials are correlated.
Supporting these arguments, Staibano et al (28) found that the angiogenic process is
especially noticeable at the border line between tumor and stroma,
right at the invasive front of lesions, and between the sheets of
invasive neoplastic cells, even at a significant distance from the
main tumoral body. These authors also suggest that the weak
angiogenesis at the peripheral border of non-aggressive BCCs,
resembling the vascularization of preinvasive solid tumors
(22), accounts for their indolent
biological behaviour. Unfortunately, this study by Staibano et
al had a major drawback: the immunohistochemical technique used
could not distinguish between preexisting vessels and newly formed
ones, thus implying the need for studies concerning the production
of angiogenic factors by tumor cells.
Considering the hypothesis that the event granting
metastatic potential is the development of intra-tumoral
angiogenesis we may wonder why not all studies show a correlation
between MVD and prognosis. The observation that vessel density
diminishes as one progresses from the periphery towards the center
of a tumor has two possible explanations. It could be that as the
tumor grows, the blood vessels do not, resulting in a more widely
spaced vascular network. The second scenario is that blood vessel
regression occurs within the tumor. Either model implies that
angiogenesis takes place at the tumor edge and therefore, blood
vessels enter the tumoral mass by co-option, a phenomenon
thoroughly described in a rat glioma xenograft model (110). This second framework is supported
by findings in intermediate-thickness cutaneous melanoma, where
authors (22,111) have found significant higher
vascularity associated with metastasis, independent from thickness.
In the case of BCCs, where MVDs are low, only the second
possibility can hold. It is believed that, through an unknown
mechanism, there is a zone between the stromal angiogenesis and the
leading edge of the tumor where blood vessels undergo complete
regression. It is well known that tumors can generate
antiangiogenic factors. A number of such factors, including
angiostatin (112), were discovered
through the phenomenon of tumor interference, by which the presence
of one tumor implanted into an animal inhibits the growth of a
second. The antiangiogenic agent involved in BCC blood vessel
regression would have to have a short range of action compared with
proangiogenic factors (25).
Although further studies are required to more
accurately describe the mechanisms behind VEGF overexpression in
aggressive BCC subtypes, MVD and VEGF expression may prove to be
useful prognostic factors to assess the risk of tumor
invasiveness.
Discussion
The rapidly increasing incidence of NMSCs over the
last decades (113) has led to the
need for better prevention and treatment strategies, although this
can only be achieved through a better biological understanding of
NMSC development, involved tumor-promoting factors, risk factors
(13), and markers for early
diagnosis and aggressive subtype recognition.
Further studies are required to determine if the
preferential development of BCCs close to high caliber arterial
vessels reflects a mutual functional influence between tumor and
arterial blood flow. As already discussed, tumors promote vessel
formation in their close proximity, due to angiogenic factors
secreted due to hypoxia (114).
However, angiogenesis usually promotes low-caliber vessels
formation, therefore, tumor colocalization with high-caliber
arteries hardly reflects tumor induced angiogenesis alone. The
question that arises is whether arterial blood flow enhances the
development of NMSCs. Recently, Polacheck et al (115) have shown that mechanical stresses
may directly affect tumor progression. The finding of Shields et
al (116) demonstrate a similar
concept, according to which lymphatic flow could actually increase
the lymphatic dissemination of neoplastic cells. Furthermore, the
forces exercised by blood or lymphatic flow were shown to influence
nuclear translocation and activation of transcription factors in
vascular or lymphatic endothelial cells (117,118).
More interestingly, laminar or oscillatory flow shear stresses have
been shown to differently affect endothelial cells, inducing
different transcription programs. Altogether, these findings
suggest that physical blood flow impacts the tumor microenvironment
in several ways (13). If this link
were to be undeniably demonstrated, it would constitute a stepping
stone for clinicians, a sort of unrefined map of danger areas when
screening patients for skin malignancies.
Owing to the ubiquity of dermoscopy, establishment
of specific dermoscopic criteria is essential for early and
accurate diagnosis of BCC in its different variants. A great deal
of studies involving BCCs have focused around defining dermoscopic
criteria determined by melanin based structures such as blue-ovoid
nests, only a few approaching the area of dermoscopically
observable angioarchitecture. It is exactly in the most difficult
cases, for example in poorly or non-pigmented tumors, that findings
in blood vessels may provide additional information, much needed
for a correct diagnosis. Several vascular features for BCC
diagnosis have been described, yet the most reliable being
considered sharply focused, superficial, typical, telangiectatic
vessels (52,63,66,119–121).
Still, with the help of machine learning algorithms we look forward
to seeing quick and precise methods of vascular feature extraction
and quantification, which could help in the differentiation of skin
tumors.
In RCM, the vasculature of BCCs is characterized by
relatively superficial and mainly horizontally orientated blood
vessels that are increased in diameter and number and also
irregularly shaped. Vessels parallel oriented can be observed side
by side. Comparable changes in skin vasculature have not been
described in this combination of features in other skin diseases
with known angiogenic activity. In inflammatory conditions such as
psoriasis or atopic dermatitis, the vessels are enlarged but
orientated vertically (76,77,122).
In SCC and melanoma the vascularization shows a more irregular
vessel orientation and the leukocyte rolling phenomenon is not as
marked as in BCC (79,94). Because angiogenesis might be a
regulating factor for BCC aggressiveness, real-time imaging of BCC
vasculature by RCM might also prove useful in studies on
antiangiogenic therapy.
As already postulated by Rudolf Virchow in 1863
(123), and by Folkman et al
in 1971 (18), angiogenesis is vital
for tumor growth. Blood vessel density has been found to be
correlated to tumor aggressiveness and prognosis in a variety of
human cancers (124–127). Furthermore, the study of tumor
blood vessels may help the therapeutic effort, as angiogenesis and
tumor vasculature serve as targets for novel oncologic therapy
regimens (128).
Histopathological determination of microvascular
densities has been shown to serve as an aid for differential
diagnosis. Moreover, VEGF, the main proangiogenic cytokine, has
been found to have increased expression in BCCs when compared to
normal skin, admitting to a lesser degree than in SCCs (36). Also, VEGF expression in BCCs has been
shown to correlate to microvessel density (37). While some authors attribute
intratumoral vessel development to metastatic capacity (27), peritumoral vessel density is seen as
a trait of local aggressiveness (28,107),
referring to BCCs.
Is the vascularization of BCC affected by the
well-known pathogenic pathway of Smoothened/Gli activation due to
the absence or reduction of PTCH signaling? Translocations or
mutations characteristic of particular tumors have also been
detected in stromal cells (129).
The observation of Winter et al (35) made in regressed BCCs during oral
treatment with a smo/gli pathway inhibitor that even in the absence
of tumor cells, the vessel-containing tumor bed was still present,
might disprove this theory in BCCs. The question as to whether
fibroblasts in the stroma of BCCs carry the PTCH mutation is still,
to the best of our knowledge, under investigation.
In conclusion, through the dermoscope, the
pigmented structures in many BCCs, dermal nevi, and
keratoacanthomas are visible with exceptional clearness, giving
instant access to the diagnosis (130). However, vascular features may
become decisive in poorly or non-pigmented lesions, when dermoscopy
is correctly performed. Many dermoscopic images of tumors published
in the literature show the abrupt ending of blood vessels,
indicating a compression artifact. As a result, valuable
information is lost (53). In
contrast to dermoscopy, vascular structure identification and
quantification in histopathology brings a smaller benefit for
diagnosing these tumors, as the vertical sections of the tissue do
not allow observation of the entire structure of the vasculature.
It is, therefore, overwhelming, that most studies on skin tumor
vascularization refer to statistical analyses of findings in
histologic sections, such as microvessel density (131,132).
RCM on the other hand, has the advantage of real-time observation
of vasculature and blood flow. Furthermore, RCM optical sections
are horizontal, parallel to the horizontalized blood vessels often
encountered in BCCs, thus giving the examiner access to a bigger
picture of the tumors angioarchitecture. When factoring in time
spent per lesion, RCM of a lesion takes, in our experience ~10 min
and can easily be done bedside, while histology, even frozen
sections, requires far more time, considering the time needed to
obtain a skin biopsy. In conclusion, the emerging data in the
literature have shown that while no single technique is perfect for
tumor blood vessel evaluation, their complementary use can have an
important clinical impact, despite their previously mentioned
individual limitations.
Acknowledgements
Not applicable.
Funding
This study was partially supported by a grant of
Romanian Ministry of Research and Innovation (Bucharest, Romania),
CCCDI-UEFISCDI (project no. 61PCCDI⁄2018
PN-III-P1-1.2-PCCDI-2017-0341) within PNCDI–III.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors contributions
ML, MIP and VMV contributed to references
acquisition and design, analysis and systematization of data,
manuscript drafting, and critical revision of it for important
intellectual content. CC, SZ and DB were responsible for the
analysis and systematization of data, manuscript drafting, and
critical revision of it for important intellectual content. ML, CC
and DB were involved in providing of dermoscopic and reflectance
confocal microscopic images. SZ contributed to providing of
histopathologic images. All authors read and approved the final
version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glanz K, Schoenfeld ER and Steffen A: A
randomized trial of tailored skin cancer prevention messages for
adults: Project SCAPE. Am J Public Health. 100:735–741. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papagheorghe LML, Lupu M, Pehoiu AG,
Voiculescu VM and Giurcaneanu C: Basal cell carcinoma-increasing
incidence leads to global health burden. Rom J Clin Exp Dermatol.
2:106–111. 2015.
|
|
3
|
Ionescu DN, Arida M and Jukic DM:
Metastatic basal cell carcinoma: Four case reports, review of
literature, and immunohistochemical evaluation. Arch Pathol Lab
Med. 130:45–51. 2006.PubMed/NCBI
|
|
4
|
Carbone A, Viola P, Varrati S, Angelucci
D, Tulli A and Amerio P: Microvessel density and VEGF expression
seems to correlate with invasiveness of basal cell carcinoma. Eur J
Dermatol. 21:608–609. 2011.PubMed/NCBI
|
|
5
|
Haliasos HC, Zalaudek I, Malvehy J,
Lanschuetzer C, Hinter H, Hofmann-Wellenhof R, Braun R and Marghoob
AA: Dermoscopy of benign and malignant neoplasms in the pediatric
population. Semin Cutan Med Surg. 29:218–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zalaudek I, Kreusch J, Giacomel J, Ferrara
G, Catricalà C and Argenziano G: How to diagnose nonpigmented skin
tumors: a review of vascular structures seen with dermoscopy: part
II. Nonmelanocytic skin tumors. J Am Acad Dermatol. 63:377–388.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solomon I, Lupu M, Draghici CC, Voiculescu
VM and Giurcaneanu C: Dermatoscopic pattern variability in basal
cell carcinoma-implications in diagnosis, preoperative assessment,
and tumor management. Rom J Clin Exp Dermatol. 5:36–42. 2018.
|
|
8
|
Kharazmi P, Lui H, Wang ZJ and Lee TK:
Automatic detection of basal cell carcinoma using
vascular-extracted features from dermoscopy images. Canadian
Conference on Electrical and Computer Engineering (CCECE). IEEE;
Vancouver, BC, Canada: 2016, doi: 10.1109/CCECE.2016.7726666.
|
|
9
|
Arpaia N, Filoni A, Bonamonte D, Giudice
G, Fanelli M and Vestita M: Vascular patterns in cutaneous
ulcerated basal cell carcinoma: A retrospective blinded study
including dermoscopy. Acta Derm Venereol. 97:612–616. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heckmann M, Zogelmeier F and Konz B:
Frequency of facial basal cell carcinoma does not correlate with
site-specific UV exposure. Arch Dermatol. 138:1494–1497. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goslen JB and Bauer EA: Basal cell
carcinoma and collagenase. J Dermatol Surg Oncol. 12:812–817. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karelina TV, Goldberg GI and Eisen AZ:
Matrix metalloproteinases in blood vessel development in human
fetal skin and in cutaneous tumors. J Invest Dermatol. 105:411–417.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuonen F, Gilliet M and Perrier P:
Non-melanoma skin cancers of the fronto-temporal area
preferentially localize in the proximity of arterial blood vessels.
Dermatology. 233:199–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Folkman J and Klagsbrun M: Angiogenic
factors. Science. 235:442–447. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Velasco P and Lange-Asschenfeldt B:
Dermatological aspects of angiogenesis. Br J Dermatol. 147:841–852.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Folkman J, Parris EE and Folkman J: Tumor
angiogenesis: Therapeutic implications. N Engl J Med.
285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferrara N, Winer J, Burton T, Rowland A,
Siegel M, Phillips HS, Terrell T, Keller GA and Levinson AD:
Expression of vascular endothelial growth factor does not promote
transformation but confers a growth advantage in vivo to Chinese
hamster ovary cells. J Clin Invest. 91:160–170. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Folkman J, Watson K, Ingber D and Hanahan
D: Induction of angiogenesis during the transition from hyperplasia
to neoplasia. Nature. 339:58–61. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Srivastava A, Laidler P, Davies RP, Horgan
K and Hughes LE: The prognostic significance of tumor vascularity
in intermediate-thickness (0.76–4.0 mm thick) skin melanoma. A
quantitative histologic study. Am J Pathol. 133:419–423.
1988.PubMed/NCBI
|
|
23
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.PubMed/NCBI
|
|
24
|
Newell B, Bedlow AJ, Cliff S, Drysdale SB,
Stanton AW and Mortimer PS: Comparison of the microvasculature of
basal cell carcinoma and actinic keratosis using intravital
microscopy and immunohistochemistry. Br J Dermatol. 149:105–110.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chin CW, Foss AJ, Stevens A and Lowe J:
Differences in the vascular patterns of basal and squamous cell
skin carcinomas explain their differences in clinical behaviour. J
Pathol. 200:308–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weninger W, Rendl M, Pammer J, Grin W,
Petzelbauer P and Tschachler E: Differences in tumor microvessel
density between squamous cell carcinomas and basal cell carcinomas
may relate to their different biologic behavior. J Cutan Pathol.
24:364–369. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Loggini B, Boldrini L, Gisfredi S, Ursino
S, Camacci T, De Jeso K, Cervadoro G, Pingitore R, Barachini P,
Leocata P, et al: CD34 microvessel density and VEGF expression in
basal and squamous cell carcinoma. Pathol Res Pract. 199:705–712.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Staibano S, Boscaino A, Salvatore G,
Orabona P, Palombini L and De Rosa G: The prognostic significance
of tumor angiogenesis in nonaggressive and aggressive basal cell
carcinoma of the human skin. Hum Pathol. 27:695–700. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bosari S, Lee AKC, DeLellis RA, Wiley BD,
Heatley GJ and Silverman ML: Microvessel quantitation and prognosis
in invasive breast carcinoma. Hum Pathol. 23:755–761. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horak ER, Leek R, Klenk N, LeJeune S,
Smith K, Stuart N, Greenall M, Stepniewska K and Harris AL:
Angiogenesis, assessed by platelet/endothelial cell adhesion
molecule antibodies, as indicator of node metastases and survival
in breast cancer. Lancet. 340:1120–1124. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
33
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
34
|
Weidner N: The relationship of tumor
angiogenesis and metastasis with emphasis on invasive breast
carcinoma. In: Advances in Pathology and Laboratory Medicine.
Weinstein RL: Mosby-Year Book; St. Louis, MO: pp. 101–122. 1992
|
|
35
|
Winter J, Kneitz H and Bröcker EB: Blood
vessel density in basal cell carcinomas and benign trichogenic
tumors as a marker for differential diagnosis in dermatopathology.
J Skin Cancer. 2011:2413822011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bowden J, Brennan PA, Umar T and Cronin A:
Expression of vascular endothelial growth factor in basal cell
carcinoma and cutaneous squamous cell carcinoma of the head and
neck. J Cutan Pathol. 29:585–589. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aoki M, Pawankar R, Niimi Y and Kawana S:
Mast cells in basal cell carcinoma express VEGF, IL-8 and RANTES.
Int Arch Allergy Immunol. 130:216–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lupu M, Caruntu C, Ghita MA, Voiculescu V,
Voiculescu S, Rosca AE, Caruntu A, Moraru L, Popa IM, Calenic B, et
al: Gene expression and proteome analysis as sources of biomarkers
in basal cell carcinoma. Dis Markers. 2016:98312372016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bulman A, Neagu M and Constantin C:
Immunomics in skin cancer - improvement in diagnosis, prognosis and
therapy monitoring. Curr Proteomics. 10:202–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tjiu JW, Liao YH, Lin SJ, Huang YL, Tsai
WL, Chu CY, Kuo ML and Jee SH: Cyclooxygenase-2 overexpression in
human basal cell carcinoma cell line increases antiapoptosis,
angiogenesis, and tumorigenesis. J Invest Dermatol. 126:1143–1151.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nagy JA, Brown LF, Senger DR, Lanir N, Van
de Water L, Dvorak AM and Dvorak HF: Pathogenesis of tumor stroma
generation: A critical role for leaky blood vessels and fibrin
deposition. Biochim Biophys Acta. 948:305–326. 1989.PubMed/NCBI
|
|
44
|
Mikhail GR, Nims LP, Kelly AP Jr, Ditmars
DM Jr and Eyler WR: Metastatic basal cell carcinoma: Review,
pathogenesis, and report of two cases. Arch Dermatol.
113:1261–1269. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
von Domarus H and Stevens PJ: Metastatic
basal cell carcinoma. Report of five cases and review of 170 cases
in the literature. J Am Acad Dermatol. 10:1043–1060. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Altamura D, Menzies SW, Argenziano G,
Zalaudek I, Soyer HP, Sera F, Avramidis M, DeAmbrosis K, Fargnoli
MC and Peris K: Dermatoscopy of basal cell carcinoma: Morphologic
variability of global and local features and accuracy of diagnosis.
J Am Acad Dermatol. 62:67–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lallas A, Apalla Z, Argenziano G, Longo C,
Moscarella E, Specchio F, Raucci M and Zalaudek I: The
dermatoscopic universe of basal cell carcinoma. Dermatol Pract
Concept. 4:11–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Popadić M: Dermoscopic features in
different morphologic types of basal cell carcinoma. Dermatol Surg.
40:725–732. 2014.PubMed/NCBI
|
|
49
|
Seidenari S, Bellucci C, Bassoli S,
Arginelli F, Magnoni C and Ponti G: High magnification digital
dermoscopy of basal cell carcinoma: A single-centre study on 400
cases. Acta Derm Venereol. 94:677–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Popadić M: Statistical evaluation of
dermoscopic features in basal cell carcinomas. Dermatol Surg.
40:718–724. 2014.PubMed/NCBI
|
|
51
|
Puig S, Cecilia N and Malvehy J:
Dermoscopic criteria and basal cell carcinoma. G Ital Dermatol
Venereol. 147:135–140. 2012.PubMed/NCBI
|
|
52
|
Argenziano G, Zalaudek I, Corona R, Sera
F, Cicale L, Petrillo G, Ruocco E, Hofmann-Wellenhof R and Soyer
HP: Vascular structures in skin tumors: A dermoscopy study. Arch
Dermatol. 140:1485–1489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kreusch JF: Vascular patterns in skin
tumors. Clin Dermatol. 20:248–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Micantonio T, Gulia A, Altobelli E, Di
Cesare A, Fidanza R, Riitano A, Fargnoli MC and Peris K: Vascular
patterns in basal cell carcinoma. J Eur Acad Dermatol Venereol.
25:358–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Trigoni A, Lazaridou E, Apalla Z, Vakirlis
E, Chrysomallis F, Varytimiadis D and Ioannides D: Dermoscopic
features in the diagnosis of different types of basal cell
carcinoma: A prospective analysis. Hippokratia. 16:29–34.
2012.PubMed/NCBI
|
|
56
|
Pan Y, Chamberlain AJ, Bailey M, Chong AH,
Haskett M and Kelly JW: Dermatoscopy aids in the diagnosis of the
solitary red scaly patch or plaque-features distinguishing
superficial basal cell carcinoma, intraepidermal carcinoma, and
psoriasis. J Am Acad Dermatol. 59:268–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Staindl O and Lametschwandtner A: Die
Angioarchitektur solidzystischer Basaliome (The angioarchitecture
of solid-cystical basaliomas). HNO. 29:112–117. 1981.PubMed/NCBI
|
|
58
|
Giacomel J and Zalaudek I: Dermoscopy of
superficial basal cell carcinoma. Dermatol Surg. 31:1710–1713.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liebman TN, Jaimes-Lopez N, Balagula Y,
Rabinovitz HS, Wang SQ, Dusza SW and Marghoob AA: Dermoscopic
features of basal cell carcinomas: Differences in appearance under
non-polarized and polarized light. Dermatol Surg. 38:392–399. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kreusch J and Koch F:
Auflichtmikroskopische Charakterisierung von Gefässmustern in
Hauttumoren. Hautarzt. 47:264–272. 1996.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Scope A, Benvenuto-Andrade C, Agero AL and
Marghoob AA: Nonmelanocytic lesions defying the two-step dermoscopy
algorithm. Dermatol Surg. 32:1398–1406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zalaudek I, Argenziano G, Leinweber B,
Citarella L, Hofmann-Wellenhof R, Malvehy J, Puig S, Pizzichetta
MA, Thomas L, Soyer HP, et al: Dermoscopy of Bowens disease. Br J
Dermatol. 150:1112–1116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Menzies SW, Westerhoff K, Rabinovitz H,
Kopf AW, McCarthy WH and Katz B: Surface microscopy of pigmented
basal cell carcinoma. Arch Dermatol. 136:1012–1016. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Stolz W, Braun-Falco O, Bilek P,
Landthaler M, Burgdorf WH and Cognetta AB: Dermatoscopic diagnostic
criteria. Color Atlas of Dermatoscopy. Stolz W, Braun-Falco O,
Bilek P, Landthaler M, Burgdorf WHC and Cognetta AB: 2nd. Blackwell
Science; Berlin: pp. p312002
|
|
65
|
Scalvenzi M, Lembo S, Francia MG and
Balato A: Dermoscopic patterns of superficial basal cell carcinoma.
Int J Dermatol. 47:1015–1018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Püspök-Schwarz M, Steiner A, Binder M,
Partsch B, Wolff K and Pehamberger H: Statistical evaluation of
epiluminescence microscopy criteria in the differential diagnosis
of malignant melanoma and pigmented basal cell carcinoma. Melanoma
Res. 7:307–311. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Demirtaşoglu M, İlknur T, Lebe B, Kuşku E,
Akarsu S and Özkan S: Evaluation of dermoscopic and histopathologic
features and their correlations in pigmented basal cell carcinomas.
J Eur Acad Dermatol Venereol. 20:916–920. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zalaudek I, Ferrara G, Broganelli P,
Moscarella E, Mordente I, Giacomel J and Argenziano G: Dermoscopy
patterns of fibroepithelioma of pinkus. Arch Dermatol.
142:1318–1322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Crowson AN: Basal cell carcinoma: Biology,
morphology and clinical implications. Mod Pathol. 19 (Suppl
2):S127–S147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Verduzco-Martínez AP, Quiñones-Venegas R,
Guevara-Gutiérrez E and Tlacuilo-Parra A: Correlation of
dermoscopic findings with histopathologic variants of basal cell
carcinoma. Int J Dermatol. 52:718–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pyne J, Sapkota D and Wong JC: Aggressive
basal cell carcinoma: Dermatoscopy vascular features as clues to
the diagnosis. Dermatol Pract Concept. 2:0203a022012.doi:
10.5826/dpc.0203a02. View Article : Google Scholar
|
|
72
|
Popadić M: Dermoscopy of aggressive basal
cell carcinomas. Indian J Dermatol Venereol Leprol. 81:608–610.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cheng B, Erdos D, Stanley RJ, Stoecker WV,
Calcara DA and Gómez DD: Automatic detection of basal cell
carcinoma using telangiectasia analysis in dermoscopy skin lesion
images. Skin Res Technol. 17:278–287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hames SC, Sinnya S, Tan JM, Morze C,
Sahebian A, Soyer HP and Prow TW: Automated detection of actinic
keratoses in clinical photographs. PLoS One. 10:e01124472015.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Choi JW, Kim BR, Lee HS and Youn SW:
Characteristics of subjective recognition and computer-aided image
analysis of facial erythematous skin diseases: A cornerstone of
automated diagnosis. Br J Dermatol. 171:252–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ghiţă MA, Căruntu C, Rosca AE, Căruntu A,
Moraru L, Constantin C, Neagu M and Boda D: Real-time investigation
of skin blood flow changes induced by topical capsaicin. Acta
Dermatovenerol Croat. 25:223–227. 2017.PubMed/NCBI
|
|
77
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55 (Suppl 3):1191–1196.
2014.PubMed/NCBI
|
|
78
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI
|
|
79
|
Lupu M, Caruntu A, Caruntu C, Boda D,
Moraru L, Voiculescu V and Bastian A: Non-invasive imaging of
actinic cheilitis and squamous cell carcinoma of the lip. Mol Clin
Oncol. 8:640–646. 2018.PubMed/NCBI
|
|
80
|
Lupu M, Caruntu C, Solomon I, Popa A,
Lisievici C, Draghici C, Papagheorghe L, Voiculescu VM and
Giurcaneanu C: The use of in vivo reflectance confocal microscopy
and dermoscopy in the preoperative determination of basal cell
carcinoma histopathological subtypes. DermatoVenerol. 62:7–13.
2017.
|
|
81
|
Ghita MA, Caruntu C, Rosca AE, Kaleshi H,
Caruntu A, Moraru L, Docea AO, Zurac S, Boda D, Neagu M, et al:
Reflectance confocal microscopy and dermoscopy for in vivo,
non-invasive skin imaging of superficial basal cell carcinoma.
Oncol Lett. 11:3019–3024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Căruntu C, Boda D, Guţu DE and Căruntu A:
In vivo reflectance confocal microscopy of basal cell carcinoma
with cystic degeneration. Rom J Morphol Embryol. 55:1437–1441.
2014.PubMed/NCBI
|
|
83
|
Diaconeasa A, Boda D, Neagu M, Constantin
C, Căruntu C, Vlădău L and Guţu D: The role of confocal microscopy
in the dermato-oncology practice. J Med Life. 4:63–74.
2011.PubMed/NCBI
|
|
84
|
Malvehy J, Hanke-Martinez M, Costa J,
Salerni G, Carrera C and Puig S: Semiology and pattern analysis in
nonmelanocytic lesions. Reflectance Confocal Microscopy for Skin
Diseases. Springer; Berlin, Heidelberg: pp. 239–252. 2011
|
|
85
|
Scope A, Benvenuto-Andrade C, Agero A-LC,
Malvehy J, Puig S, Rajadhyaksha M, Busam KJ, Marra DE, Torres A,
Propperova I, et al: In vivo reflectance confocal microscopy
imaging of melanocytic skin lesions: Consensus terminology glossary
and illustrative images. J Am Acad Dermatol. 57:644–658. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gerger A, Koller S, Weger W, Richtig E,
Kerl H, Samonigg H, Krippl P and Smolle J: Sensitivity and
specificity of confocal laser-scanning microscopy for in vivo
diagnosis of malignant skin tumors. Cancer. 107:193–200. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ulrich M, Maltusch A, Rius-Diaz F,
Röwert-Huber J, González S, Sterry W, Stockfleth E and Astner S:
Clinical applicability of in vivo reflectance confocal microscopy
for the diagnosis of actinic keratoses. Dermatol Surg. 34:610–619.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
González S and Tannous Z: Real-time, in
vivo confocal reflectance microscopy of basal cell carcinoma. J Am
Acad Dermatol. 47:869–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Grazzini M, Stanganelli I, Rossari S, Gori
A, Oranges T, Longo AS, Lotti T, Bencini PL and De Giorgi V:
Dermoscopy, confocal laser microscopy, and hi-tech evaluation of
vascular skin lesions: Diagnostic and therapeutic perspectives.
Dermatol Ther (Heidelb). 25:297–303. 2012. View Article : Google Scholar
|
|
90
|
Ulrich M, Lange-Asschenfeldt S and
González S: In vivo reflectance confocal microscopy for early
diagnosis of nonmelanoma skin cancer. Actas Dermosifiliogr.
103:784–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hui D and Ai-E X: The vascular features of
psoriatic skin: Imaging using in vivo confocal laser scanning
microscopy. Skin Res Technol. 19:e545–e548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ulrich M, Kanitakis J, González S,
Lange-Asschenfeldt S, Stockfleth E and Roewert-Huber J: Evaluation
of Bowen disease by in vivo reflectance confocal microscopy. Br J
Dermatol. 166:451–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Braga JC, Scope A, Klaz I, Mecca P,
González S, Rabinovitz H and Marghoob AA: The significance of
reflectance confocal microscopy in the assessment of solitary pink
skin lesions. J Am Acad Dermatol. 61:230–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sauermann K, Gambichler T, Wilmert M,
Rotterdam S, Stucker M, Altmeyer P and Hoffmann K: Investigation of
basal cell carcionoma by confocal laser scanning microscopy in
vivo. Skin Res Technol. 8:141–147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Incel P, Gurel MS and Erdemir AV: Vascular
patterns of nonpigmented tumoral skin lesions: Confocal
perspectives. Skin Res Technol. 21:333–339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ahlgrimm-Siess V, Cao T, Oliviero M,
Hofmann-Wellenhof R, Rabinovitz HS and Scope A: The vasculature of
nonmelanocytic skin tumors in reflectance confocal microscopy:
Vascular features of basal cell carcinoma. Arch Dermatol.
146:353–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Agero A, Cuevas J, Jaen P, Marghoob A,
Gill M and Gonzalez S: Basal cell carcinoma. In: Reflectance
Confocal Microscopy of Cutaneous Tumors. González S, Gill M and
Halpern AC: CRC. 60–75. 2008.
|
|
98
|
Grunt TW, Lametschwandtner A and Staindl
O: The vascular pattern of basal cell tumors: Light microscopy and
scanning electron microscopic study on vascular corrosion casts.
Microvasc Res. 29:371–386. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Eichert S, Möhrle M, Breuninger H, Röcken
M, Garbe C and Bauer J: Diagnosis of cutaneous tumors with in vivo
confocal laser scanning microscopy. J Dtsch Dermatol Ges.
8:400–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
McDonald DM and Foss AJ: Endothelial cells
of tumor vessels: Abnormal but not absent. Cancer Metastasis Rev.
19:109–120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sari Aslani F and Aledavood A:
Angiogenesis assessment in basal cell carcinoma. Med J Islam Repub
Iran. 15:73–77. 2001.
|
|
102
|
Miettinen M, Lindenmayer AE and Chaubal A:
Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and
Y-antigens - evaluation of their specificity and sensitivity in the
diagnosis of vascular tumors and comparison with von Willebrand
factor. Mod Pathol. 7:82–90. 1994.PubMed/NCBI
|
|
103
|
Yerebakan O, Ciftçioglu MA, Akkaya BK and
Yilmaz E: Prognostic value of Ki-67, CD31 and epidermal growth
factor receptor expression in basal cell carcinoma. J Dermatol.
30:33–41. 2003.PubMed/NCBI
|
|
104
|
Rasi A, Safaii Naraghi Z, Tavangar SM,
Taghizadeh AR and Davoodi F: Angiogenesis evaluation in cutaneous
basal cell carcinoma and squamous cell carcinoma. Majallah-i Ulum-i
Pizishki-i Razi. 12:63–70. 2006.(In Persian).
|
|
105
|
Vuletic MS, Jancic SA, Ilic MB, Azanjac G,
Joksimovic IS, Milenkovic SM, Janicijevic-Petrovic MA and Stankovic
VD: Expression of vascular endothelial growth factor and
microvascular density assessment in different histotypes of basal
cell carcinoma. J BUON. 19:780–786. 2014.PubMed/NCBI
|
|
106
|
Oh CK, Kwon YW, Kim YS, Jang HS and Kwon
KS: Expression of basic fibroblast growth factor, vascular
endothelial growth factor, and thrombospondin-1 related to
microvessel density in nonaggressive and aggressive basal cell
carcinomas. J Dermatol. 30:306–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Cernea CR, Ferraz AR, de Castro IV, Sotto
MN, Logullo AF, Bacchi CE and Potenza AS: Angiogenesis and skin
carcinomas with skull base invasion: A case-control study. Head
Neck. 26:396–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Dunstan S, Powe DG, Wilkinson M, Pearson J
and Hewitt RE: The tumour stroma of oral squamous cell carcinomas
show increased vascularity compared with adjacent host tissue. Br J
Cancer. 75:559–565. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Fox SB, Gatter KC, Bicknell R, Going JJ,
Stanton P, Cooke TG and Harris AL: Relationship of endothelial cell
proliferation to tumor vascularity in human breast cancer. Cancer
Res. 53:4161–4163. 1993.PubMed/NCBI
|
|
110
|
Holash J, Maisonpierre PC, Compton D,
Boland P, Alexander CR, Zagzag D, Yancopoulos GD and Wiegand SJ:
Vessel cooption, regression, and growth in tumors mediated by
angiopoietins and VEGF. Science. 284:1994–1998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Srivastava A, Laidler P, Hughes LE,
Woodcock J and Shedden EJ: Neovascularization in human cutaneous
melanoma: A quantitative morphological and Doppler ultrasound
study. Eur J Cancer Clin Oncol. 22:1205–1209. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Holmgren L, OReilly MS and Folkman J:
Dormancy of micrometastases: Balanced proliferation and apoptosis
in the presence of angiogenesis suppression. Nat Med. 1:149–153.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Christenson LJ, Borrowman TA, Vachon CM,
Tollefson MM, Otley CC, Weaver AL and Roenigk RK: Incidence of
basal cell and squamous cell carcinomas in a population younger
than 40 years. JAMA. 294:681–690. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Polacheck WJ, German AE, Mammoto A, Ingber
DE and Kamm RD: Mechanotransduction of fluid stresses governs 3D
cell migration. Proc Natl Acad Sci USA. 111:2447–2452. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Shields JD, Fleury ME, Yong C, Tomei AA,
Randolph GJ and Swartz MA: Autologous chemotaxis as a mechanism of
tumor cell homing to lymphatics via interstitial flow and autocrine
CCR7 signaling. Cancer Cell. 11:526–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ng CP, Helm C-LE and Swartz MA:
Interstitial flow differentially stimulates blood and lymphatic
endothelial cell morphogenesis in vitro. Microvasc Res. 68:258–264.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sabine A, Agalarov Y, Maby-El Hajjami H,
Jaquet M, Hägerling R, Pollmann C, Bebber D, Pfenniger A, Miura N,
Dormond O, et al: Mechanotransduction, PROX1, and FOXC2 cooperate
to control connexin37 and calcineurin during lymphatic-valve
formation. Dev Cell. 22:430–445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Menzies SW: Dermoscopy of pigmented basal
cell carcinoma. Clin Dermatol. 20:268–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Neale RE, Davis M, Pandeya N, Whiteman DC
and Green AC: Basal cell carcinoma on the trunk is associated with
excessive sun exposure. J Am Acad Dermatol. 56:380–386. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Felder S, Rabinovitz H, Oliviero M and
Kopf A: Dermoscopic differentiation of a superficial basal cell
carcinoma and squamous cell carcinoma in situ. Dermatol Surg.
32:423–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Căruntu C and Boda D: Evaluation through
in vivo reflectance confocal microscopy of the cutaneous neurogenic
inflammatory reaction induced by capsaicin in human subjects. J
Biomed Opt. 17:0850032012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Des Guetz G, Uzzan B, Nicolas P, Cucherat
M, Morere JF, Benamouzig R, Breau JL and Perret GY: Microvessel
density and VEGF expression are prognostic factors in colorectal
cancer. Meta-analysis of the literature. Br J Cancer. 94:1823–1832.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Uzzan B, Nicolas P, Cucherat M and Perret
GY: Microvessel density as a prognostic factor in women with breast
cancer: A systematic review of the literature and meta-analysis.
Cancer Res. 64:2941–2955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Aurello P, Bellagamba R, Rossi Del Monte
S, DAngelo F, Nigri G, Cicchini C, Ravaioli M and Ramacciato G:
Apoptosis and microvessel density in gastric cancer: Correlation
with tumor stage and prognosis. Am Surg. 75:1183–1188.
2009.PubMed/NCBI
|
|
127
|
Stefanou D, Batistatou A, Arkoumani E,
Ntzani E and Agnantis NJ: Expression of vascular endothelial growth
factor (VEGF) and association with microvessel density in
small-cell and non-small-cell lung carcinomas. Histol Histopathol.
19:37–42. 2004.PubMed/NCBI
|
|
128
|
Barrascout E, Medioni J, Scotte F, Ayllon
J, Mejean A, Cuenod CA, Tartour E, Elaidi R and Oudard S:
Angiogenesis inhibition: Review of the activity of sorafenib,
sunitinib and bevacizumab. Bull Cancer. 97:29–43. 2010.PubMed/NCBI
|
|
129
|
Franco OE, Shaw AK, Strand DW and Hayward
SW: Cancer associated fibroblasts in cancer pathogenesis. Semin
Cell Dev Biol. 21:33–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hundeiker M and Brehm K: Capillary
architecture of basal cell carcinoma. Hautarzt. 23:169–171.
1972.(In German). PubMed/NCBI
|
|
131
|
Barnhill RL, Fandrey K, Levy MA, Mihm MC
Jr and Hyman B: Angiogenesis and tumor progression of melanoma.
Quantification of vascularity in melanocytic nevi and cutaneous
malignant melanoma. Lab Invest. 67:331–337. 1992.PubMed/NCBI
|
|
132
|
Smolle J, Soyer HP, Hofmann-Wellenhof R,
Smolle-Juettner FM and Kerl H: Vascular architecture of melanocytic
skin tumors. A quantitative immunohistochemical study using
automated image analysis. Pathol Res Pract. 185:740–745. 1989.
View Article : Google Scholar : PubMed/NCBI
|