Checkpoint inhibitors (CPIs), adoptive cell transfer
and administration of the cytokine interleukin 2, have been
developed as effective clinical cancer immunotherapies, with no
clear identification of the immunogenic targets in human types of
cancer. Since ipilimumab, an immune CPI for CTLA4 was approved in
the United States in 2011 (1), CPIs,
as novel anticancer agents, have indicated great promise for
effective lung cancer therapy (2–7). Among
them, the programmed death receptor-1 (PD-1)/PD-1 ligand 1 (PD-L1)
pathway is a key immune checkpoint (8). Anti-PD-1 monoclonal antibodies have
been approved by the Food and Drug Administration in the USA for
treatments of a number of solid cancer types, including advanced
non-small cell lung cancer (NSCLC) (9–15). In
addition, antibodies against PD-L1 have indicated an effective
clinical response in patients with NSCLC (16).
The clinical trials and utility of CPIs have
provided key insights into the potential mechanisms of anticancer
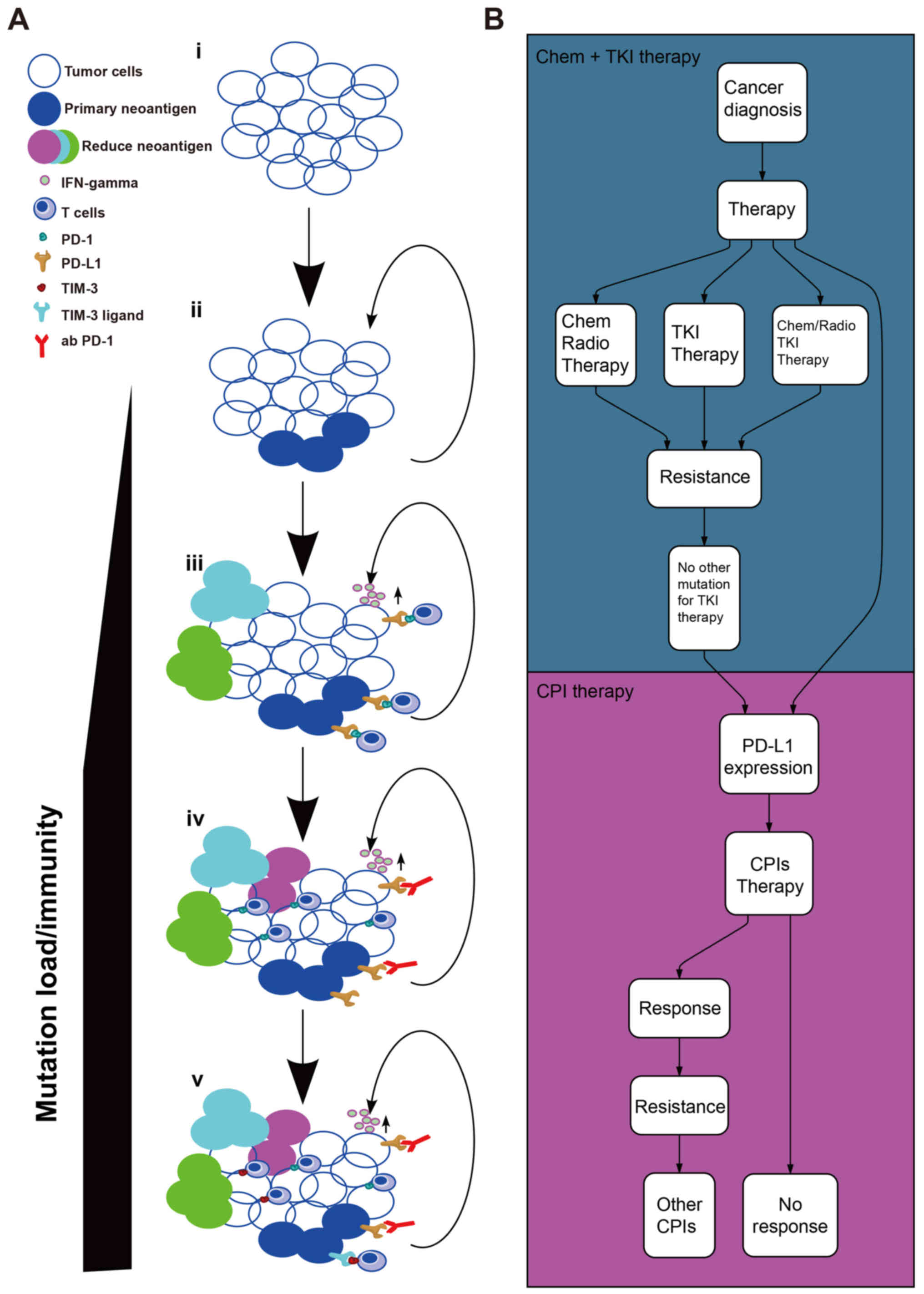
immune therapies that may underlie cancer immune escape (26). A seven-step event in an anticancer
immune response, known as the cancer-immunity cycle (27), is required to be initiated and to
sequentially lead to the effective killing of cancer cells. In the
last step, the dead cancer cells will release further
tumor-associated antigens and cycle again to increase the strength
of the immune response in subsequent cycle revolutions. However,
the cancer-immunity cycle does not function as aforementioned in
patients with cancer. The anticancer function of effector T cells
may not respond properly, owing to the factors in the tumor
microenvironment (TME) (28) as
indicated in Fig. 1A. At the early
stage, the tumor possesses a lower TMB/fewer neo-Ags (29). Subsequently, the tumor appears to
induce a greater TMB/more neo-Ags through the loss of mismatch
repair and DNA instability, enhancing the immunity of cancer, and
ultimately leading to activation of tumor neo-Ag-specific cluster
of differentiation (CD)8+ T cells and immune-mediated
tumor cell death (30–33). Heterogeneity, relevance of neo-Ag
burden and importance of clonal vs. subclonal neo-Ag in patients
with early-stage NSCLC, included in The Cancer Genome Atlas
project, have been assessed (34).
Generally, the human body has an immunoregulatory mechanism, known
as immune checkpoint, including CTLA4 and PD-1/PD-L1 (35). Using this pathway, the tumor evades
the lethal effects of the immune system, therefore neo-Ags,
including driver/passenger, serve an important role in this
progress. Inhibiting the immune checkpoint and killing the clonal
or subclonal neo-Ag-specific tumor cells are useful ways to unlock
the suppressed lethal response to tumors (36). Monoclonal antibodies against the
PD-1/PD-L1 pathway (Table I), have
been proved to improve outcomes in patients with NSCLC (6,11–13,37),
including patients who have relapsed following treatment with
platinum-based first-line chemotherapy or tyrosine kinase inhibitor
therapy (Fig. 1B). The clinical
trial of nivolumab in resectable NSCLC (trial no. NCT02259621),
investigates the safety, feasibility and effects of nivolumab in
this patient population (38).
Neoadjuvant nivolumab was indicated to have fewer side effects, to
not require any delay in surgery and to induce a major pathological
response in 45% of resected tumors (38). Combining immunotherapies with
chemotherapy or radiotherapy may lead to an improved response in
various patients. One reason for this observation was that
cytotoxic chemotherapies and radiation may induce a greater number
of novel subclonal mutations that are associated with the response
to immunotherapy (39). Furthermore,
the immune TME (iTME) will be evaluated or assessed to a greater
extent by immune signature/immunogenomic analysis, including
quantification of infiltrated CD8+ T cells, though
immunohistochemistry (IHC) assay, and their TCR analysis, including
next-generation sequencing (NGS) (40,41).
Currently, liquid biopsy, in particular ctDNA, can
indicate better tumor heterogeneity at a greater accuracy compared
with tumor biopsy, since it facilitates a convenient and dynamic
analysis (42,43). The question is how liquid biopsies
can be utilized for immunotherapy. As for immunotherapy, liquid
biopsies may be useful for monitoring ctDNA and the response of the
immune system in vivo, for example, the analysis of
circulating free DNA (cfDNA) released from distinct T cell clones,
on the basis of the assessment of B cell receptor and TCR immune
repertoire from blood plasma (44).
The dynamic variation in the cfDNA (45) or T cell-surface markers in the blood
(46) may provide clues to the type
of treatments that have a higher probability to be effective for
each patient. Further study of TME is required, in order to
identify suitable biomarkers for liquid biopsy, in particular the
iTME. Histologically, the primary tumors can be broadly categorized
into two classes: Inflamed or uninflamed (26,47,48). A
subset of immune-associated genes, including CD8α/β, interferon
(IFN)-γ and granzyme (GZM) A, B and H that were upregulated in the
high clonal neo-Ag group, was revealed by gene expression analysis,
indicating an inflammatory TME (49). The expression of these genes was
countered by the upregulation of immune checkpoints, including
PD-1, PD-L1 and PD-L2. The immune CPIs indicated a high efficacy
against inflamed tumors, owing to their sufficient infiltration by
cytotoxic T cells that recognize cancer-specific antigens or
neo-Ags, high density of IFN-γ-producing CD8+ T cells,
expression of PD-L1 in tumor-infiltrating immune cells, possible
genomic instability, and the presence of a pre-existing antitumor
immune response (50). However, they
have not been indicated to be effective against uninflamed tumors,
which are immunologically unknown, are poorly infiltrated by
lymphocytes, rarely express PD-L1, and are characterized by highly
proliferating tumors with low TMB and low expression of
antigen-presentation machinery markers, including major
histocompatibility complex (MHC) class I (51–53).
According to a useful pragmatic framework reported by Teng et
al (54) and Smyth et al
(55), TME can be stratified into
four types: Type I [tumor-infiltrating lymphocyte (TIL)+, PD-L1+],
Type II (TILs-, PD-L1-), Type III (TIL-, PD-L1+) and Type IV (TIL+,
PD-L1-) (Fig. 2A). Researchers have
attempted to use this classification for lung cancer immunotherapy,
in order to provide an explanation for its contribution of its poor
prognosis (56–58). Biomarkers associated with
distinguishing the four types of iTME will be beneficial to
clinical cancer management of individualized and precise cancer
treatment.
According to the use of CTLA4, PD-L1 and PD-1,
co-inhibitory receptor targets, including lymphocyte activating 3
(59), T cell immunoglobulin-3
(TIM-3) (60) and T cell
immunoglobulin and ITIM domain (61), which are safer and less toxic
(62), are being investigated in
clinical trials. Adaptive resistance to anti-PD-1 therapy is
associated with the upregulation of TIM-3 expression in lung cancer
(63). Patients with cancer may
receive more optimal effects when receiving the anti-TIM-3 agent.
The expression level of PD-L2, GZMA and human leukocyte antigens A
has indicated that these factors are novel potential biomarkers for
predicting the effective response for CPIs in pre-anti-PD-1
antibody-treatment (nivolumab) melanoma tissues (50). Gros et al (64) reported that mutation-specific T cells
may be isolated from blood in 75% of patients with melanoma. This
study was focused on melanoma; however, it is becoming clear that
immunotherapies can be used to treat numerous types of cancer,
including lung cancer. These mutation-specific T cells have made it
possible to determine the neo-Ag status of tumors from blood, and
they may serve as a liquid biopsy technique for cancer
immunotherapy or a novel immunotherapy (41). In Fig.
2B, the biomarkers associated with iTME are depicted, which may
be used in liquid biopsy for lung cancer immunotherapy. The
detection of PD-L1+ circulating tumor cells (CTCs) in
patients with NSCLC treated with the PD-1 inhibitor nivolumab
indicated that CTCs was a good liquid biopsy material associated
with immunotherapy (65). At the
American Association for Cancer Research Annual Meeting 2018, data
from the CheckMate-227 trial indicated that the first-line
treatment of nivolumab and ipilimumab in combination has improved
progression-free survival (7.2 months) compared with chemotherapy
(5.5 months) for patients with advanced NSCLC with high TMB
(66). The importance of TMB
detection makes blood-based TMB (bTMB) a considerable clinical
biomarker (67). Using ctDNA, bTMB
analysis may be performed more easily and at a higher rate, as
indicated by the clinical outcomes stratified by bTMB in the POPLAR
(clincaltrials.gov. no. NCT01903993) and
OAK (clincaltrials.gov. no. NCT02008227)
clinical trials (68), which
assessed the efficacy of anti-PD-L1 CPI (atezolizumab) for
recurrent advanced NSCLC. In this meeting, another trial
(clinicaltrials.gov no. KEYNOTE-189)
reported an improvement in overall survival by 8.8 months in the
pembrolizumab-combination group and 4.9 months in the
placebo-combination group across all PD-L1 categories that were
evaluated, indicating the key role of PD-L1 detection in CPI
therapy. In another trial (clincaltrials.gov. no. NCT02259621), TMB was used as
an indicator of the pathological response to anti-PD-1 CPI therapy
(38). At between 2 and 4 weeks
after neoadjuvant nivolumab treatment, rapid expansion of
mutation-associated neo-Ag-specific T-cell clones, from a primary
tumor, along with a positive pathological assessment, was detected
in peripheral blood in 8/9 patients assessed. A number of these
clones were not detected prior to the administration of anti-PD-1
CPI (nivolumab).
Immunotherapies are developed to help strengthen the
immune attack against tumor cells. One approach is CPIs, as
aforementioned, and the other is TCR-engineered adoptive therapy
(64,69). The increased sensitivity of the
sequencing method allows for the detection of early-stage lung
cancer by means of cfDNA analysis, as this technique will provide
additional information about patients with cancer after a
radiological screening method. Rizvi et al (70) indicated that a smoking signature and
neo-Ags in the tumor were factors, which were associated with the
response to anti-PD-1 CPI. It has been reported that tumor
regression was associated with a neo-Ag-specific response by
CD8+ T cells (71). The
accumulated evidence indicates that the genomic characteristics of
a tumor may potentially assist in selecting and customizing
immunotherapy. Consistent with these data, researchers have also
been able to identify tumor-infiltrating CD8+ T cells
reactive to clonal neo-Ags in patients with NSCLC with homogenous
and heterogeneous early-stage tumors (72). Adoptive T cell therapy was developed
further in a number of ways on the basis of current knowledge.
First, the CD8+/PD-1+ T cell subset, which
was isolated and expanded from peripheral blood, was reinfused into
the patient with cancer (41). Using
high-throughput screening platforms, including NGS and
high-performance liquid chromatography-mass spectrometry (HPLC-MS),
neo-Ag-specific T cells in the PD-1+ T cells may be
identified, and may be used with their respective TCRs in
immunotherapy (73). Another method
of identification is the combination of HPLC-MS and sequencing,
where novel neo-Ags can be identified (74). The focus of the study of Khodadoust
et al (75) was on direct
proteomic analysis of cancer MHC ligands and epitopes, using
HPLC-MS rather than simply performing whole exome sequencing (WES)
of DNA to identify tumor-associated non-synonymous somatic
mutations. Neo-Ags are personalized antigens, except for certain
common oncogene-specific antigens, including the KRAS
proto-oncogene. A summary of TCR-engineered adoptive therapy
targeting neo-Ags for lung cancer is presented in Fig. 3. The deep sequencing on tumor tissue,
CTCs or ctDNA is used to determine the potential neo-Ags and TCR,
in order to identify the sequences of the most dominant clonotypes
within the PD-1+ T cell subset. CTCs, a liquid biopsy
material, can be enriched from the blood using a number of methods,
including microfluidic isolation based on the epithelial cell
adhesion molecule expression (76).
This may be another way of obtaining neo-Ags from CTCs, based on
NGS, since they provide more information about the primary or
metastatic tumor sites.
Immunotherapy serves a key role in lung cancer
therapy. CPIs have already been used for lung cancer therapy in
various locations, including United States (77) and China (78). However, novel therapies targeting
CPIs, including anti-CTLA4, anti-PD-1 and anti-PD-L1, are changing
the prognosis of patients with advanced lung cancer. Randomized
trials have reported improvements in OS compared with standard
treatments, including chemotherapy and radiotherapy (6,11,13,77–81).
Since there are biomarkers suitable for use in immunotherapy, a
great deal of attention has been drawn to the assessment of PD-1 or
PD-L1 expression in TME, challenged by the difficulty of accessing
tissue samples, heterogeneity and the lack of gold-standard
antibodies for IHC staining (82).
WES for determination of TMB in liquid biopsy from patients with
advanced NSCLC suggests that liquid biopsy-derived TMB may be used
as a useful biomarker for predicting the CPI response, particularly
in cases where tumor biopsy is not accessible or has been resampled
(83). Theoretically, there are
numerous potential biomarkers for immunotherapy in liquid biopsy;
however, to the best of our knowledge, none has been identified to
be reliable enough, particularly with respect to evaluating their
efficiency or even their selection following drug resistance. It is
important to identify liquid biopsy biomarkers for prognostic and
response prediction associated with CPIs to guide future clinical
decisions.
Successes with CPIs in the second-line treatment of
NSCLCs have inevitably led to trials in the first-line setting
(11). However, not all patients
have reported an effective response. In clinical trials, patients
who have presented with immunogenic tumors, including high TMB or
neo-Ags, and pre-existing intratumoral immune infiltrate and immune
escape ligands (i.e., PD-1/PD-L1) being targeted, seem to benefit
the most from CPI therapy. As the first approved IHC assay for
anti-PD-1 (pembrolizumab) in NSCLC, the PD-L1 (22C3) diagnostic
(Dako PD-L1 IHC 22C3 pharmDx) (84,85) is
still a key biomarker for the selection of patients with cancer
(86). Pembrolizumab had been used
as the first-line treatment (87),
instead of cytotoxic chemotherapy, in patients with lung cancer
whose proportion score for PD-L1 was ≥50% in TME (88). However, activating the immune system
also presents with its own risks, since the immune CPIs give rise
to grade 3/4 immune-associated adverse events (irAEs) with
ipilimumab (15-25%), permbrolizumab (13%) and nivolumab (14%)
(89,90). It is therefore necessary to elucidate
the immune status in individual patients with cancer to identify a
predictive method for these irAE risks. Biomarkers associated with
these CPIs that predict efficacy, prognosis or risk of irAE risk
may assist in the identification of patients who may benefit from
these therapies. Biomarkers associated with clinical response
prediction and the acquired resistance monitoring of lung cancer
immunotherapy may be assessed in a dynamic manner using liquid
biopsy based on blood samples, which would be beneficial to
patients. However, extensive further investigation is required for
the practical application of this treatment, largely due to the
limitations of its sensitivity.
Although neo-Ag vaccines or TCR-engineered T cells
targeting neo-Ags can be used for the majority of patients with
cancer, the truly but rare tumor-specific T cells among the
selected subset, may limit the therapeutic utility of T cell
products (91). Therefore, more
comprehensive technologies, including NGS, TCR sequencing and
HPLC-MS are required. Furthermore, current methods for predicting
tumor neo-Ags remain at an early stage and are limited by class I
rather than class II MHC antigens (92). Additional efforts are required in the
development of MHC class I- and class II-restricted neo-Ags as
these will provide additional information about the immune
surveillance in tumor development. The neo-Ag identification can be
classified into direct and reverse identification using different
techniques (93). The direct
identification requires validation by exome and transcriptome
sequencing data, whereas the MS-based reverse identification allows
the identification of CD8+ and CD4+ T-cell
neo-epitopes (29). However, to the
best of our knowledge, the capacity of neo-Ag identification by
direct identification has yet to be improved. It may eventually
serve as a key tool in antigen discovery.
Not applicable.
The present study was supported by the National
Natural Sciences Foundation Key Program (grant no. 81630071), the
Aiyou Foundation (grant no. KY201701) and the Ministry of Education
Innovation Team development project (grant no. IRT-17R10).
Not applicable.
LLC and JW wrote the review, and have read and
approved the final version of this manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Cameron F, Whiteside G and Perry C:
Ipilimumab: First global approval. Drugs. 71:1093–1104. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou
J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, et al:
Bevacizumab plus ipilimumab in patients with metastatic melanoma.
Cancer Immunol Res. 2:632–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L and Han X: Anti-PD-1/PD-L1 therapy
of human cancer: Past, present, and future. J Clin Invest.
125:3384–3391. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brahmer J, Reckamp KL, Baas P, Crino L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizvi NA, Mazieres J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herbst RS, Baas P, Kim DW, Felip E,
Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borghaei H and Brahmer J: Nivolumab in
nonsquamous non-small-cell lung cancer. N Engl J Med. 374:493–494.
2016.PubMed/NCBI
|
|
15
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spigel DR, Schrock AB, Fabrizio D,
Frampton GM, Sun J and He J: Total mutation burden (TMB) in lung
cancer (LC) and relationship with response to PD-1/PD-L1 targeted
therapies. Am Soc Clin Oncol. 2016. View Article : Google Scholar
|
|
18
|
Anagnostou V, Smith KN, Forde PM, Niknafs
N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N,
et al: Evolution of neoantigen landscape during immune checkpoint
blockade in non-small cell lung cancer. Cancer Discov. 7:264–276.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vitale SG, Lagana AS, Capriglione S,
Angioli R, La Rosa VL, Lopez S, Valenti G, Sapia F, Sarpietro G,
Butticè S, et al: Target therapies for uterine carcinosarcomas:
Current evidence and future perspectives. Int J Mol Sci.
18:E11002017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai H, Mao L, Wang HS, Zhao J, Yang L, An
TT, Wang X, Duan CJ, Wu NM, Guo ZQ, et al: Epidermal growth factor
receptor mutations in plasma DNA samples predict tumor response in
Chinese patients with stages IIIB to IV non-small-cell lung cancer.
J Clin Oncol. 27:2653–2659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai H, Wang Z, Chen K, Zhao J, Lee JJ,
Wang S, Zhou Q, Zhuo M, Mao L, An T, et al: Influence of
chemotherapy on EGFR mutation status among patients with
non-small-cell lung cancer. J Clin Oncol. 30:3077–3083. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aparicio S and Caldas C: The implications
of clonal genome evolution for cancer medicine. N Engl J Med.
368:842–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aguiar PN Jr, De Mello RA, Barreto CMN,
Perry LA, Penny-Dimri J, Tadokoro H and Lopes GL Jr: Immune
checkpoint inhibitors for advanced non-small cell lung cancer:
Emerging sequencing for new treatment targets. ESMO Open.
2:e0002002017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alexander W: European society for medical
oncology 2016 congress. P T. 41:796–800. 2016.PubMed/NCBI
|
|
25
|
Cyriac G and Gandhi L: Emerging biomarkers
for immune checkpoint inhibition in lung cancer. Semin Cancer Biol.
52:269–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hegde PS, Karanikas V and Evers S: The
where, the when, and the how of immune monitoring for cancer
immunotherapies in the era of checkpoint inhibition. Clin Cancer
Res. 22:1865–1874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bobisse S, Foukas PG, Coukos G and Harari
A: Neoantigen-based cancer immunotherapy. Ann Transl Med.
4:2622016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miller A, Asmann Y, Cattaneo L, Braggio E,
Keats J, Auclair D, Lonial S; MMRF CoMMpass Network, ; Russell SJ
and Stewart AK: High somatic mutation and neoantigen burden are
correlated with decreased progression-free survival in multiple
myeloma. Blood Cancer J. 7:e6122017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Turajlic S, Litchfield K, Xu H, Rosenthal
R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M,
et al: Insertion-and-deletion-derived tumour-specific neoantigens
and the immunogenic phenotype: A pan-cancer analysis. Lancet Oncol.
18:1009–1021. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tran E, Robbins PF and Rosenberg SA:
‘Final common pathway’ of human cancer immunotherapy: Targeting
random somatic mutations. Nat Immunol. 18:255–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tran E, Turcotte S, Gros A, Robbins PF, Lu
YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS,
et al: Cancer immunotherapy based on mutation-specific CD4+ T cells
in a patient with epithelial cancer. Science. 344:641–645. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McGranahan N, Furness AJ, Rosenthal R,
Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak
NJ, Hiley CT, et al: Clonal neoantigens elicit T cell
immunoreactivity and sensitivity to immune checkpoint blockade.
Science. 351:1463–1469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jenkins RW, Barbie DA and Flaherty KT:
Mechanisms of resistance to immune checkpoint inhibitors. Br J
Cancer. 118:9–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McGranahan N and Swanton C: Clonal
heterogeneity and tumor evolution: Past, present, and the future.
Cell. 168:613–628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reck M, Bondarenko I, Luft A, Serwatowski
P, Barlesi F, Chacko R, Sebastian M, Lu H, Cuillerot JM and Lynch
TJ: Ipilimumab in combination with paclitaxel and carboplatin as
first-line therapy in extensive-disease-small-cell lung cancer:
Results from a randomized, double-blind, multicenter phase 2 trial.
Ann Oncol. 24:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Forde PM, Chaft JE and Pardoll DM:
Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med.
379:e142018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samstein RM and Riaz N: The DNA damage
response in immunotherapy and radiation. Adv Radiat Oncol.
3:527–533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakamura Y: Immunopharmacogenomics.
Springer; Japan: pp. 4832015
|
|
41
|
Schumacher TN and Scheper W: A liquid
biopsy for cancer immunotherapy. Nat Med. 22:340–341. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heitzer E, Ulz P and Geigl JB: Circulating
tumor DNA as a liquid biopsy for cancer. Clin Chem. 61:112–123.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng F, Su L and Qian C: Circulating
tumor DNA: A promising biomarker in the liquid biopsy of cancer.
Oncotarget. 7:48832–48841. 2016.PubMed/NCBI
|
|
44
|
Akyuz N, Brandt A, Stein A, Schliffke S,
Mahrle T, Quidde J, Goekkurt E, Loges S, Haalck T, Ford CT, et al:
T-cell diversification reflects antigen selection in the blood of
patients on immune checkpoint inhibition and may be exploited as
liquid biopsy biomarker. Int J Cancer. 140:2535–2544. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gedvilaitė V, Schveigert D and Cicėnas S:
Cell-free DNA in non-small cell lung cancer. Acta Med Litu.
24:138–144. 2017.PubMed/NCBI
|
|
46
|
Mingari MC and Moretta L: Surface markers
of human T lymphocytes. Ric Clin Lab. 12:439–448. 1982.PubMed/NCBI
|
|
47
|
Gajewski TF: The next hurdle in cancer
immunotherapy: Overcoming the Non-T-Cell-inflamed tumor
microenvironment. Semin Oncol. 42:663–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Inoue H, Park JH, Kiyotani K, Zewde M,
Miyashita A, Jinnin M, Kiniwa Y, Okuyama R, Tanaka R, Fujisawa Y,
et al: Intratumoral expression levels of PD-L1, GZMA, and HLA-A
along with oligoclonal T cell expansion associate with response to
nivolumab in metastatic melanoma. Oncoimmunology. 5:e12045072016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duan J, Wang Y and Jiao S: Checkpoint
blockade-based immunotherapy in the context of tumor
microenvironment: Opportunities and challenges. Cancer Med.
7:4517–4529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kandoth C, McLellan MD, Vandin F, Ye K,
Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al:
Mutational landscape and significance across 12 major cancer types.
Nature. 502:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kinoshita Y, Ishiguro T, Sano Y, Azuma Y,
Tsunenari T, Ono N, Kayukawa Y, Ueda O, Wada NA, Hino H, et al:
Anti-GPC3 TRAB, a first-in-class T cell-redirecting bispecific
antibody targeting glypican-3 with potent in vitro and in vivo
antitumor efficacy against solid tumors. Cancer Research.
76:14822016. View Article : Google Scholar
|
|
54
|
Teng MW, Ngiow SF, Ribas A and Smyth MJ:
Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer
Res. 75:2139–2145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Smyth MJ, Ngiow SF, Ribas A and Teng MW:
Combination cancer immunotherapies tailored to the tumour
microenvironment. Nat Rev Clin Oncol. 13:143–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kawai O, Ishii G, Kubota K, Murata Y,
Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, et al:
Predominant infiltration of macrophages and CD8(+) T Cells in
cancer nests is a significant predictor of survival in stage IV
nonsmall cell lung cancer. Cancer. 113:1387–1395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mu CY, Huang JA, Chen Y, Chen C and Zhang
XG: High expression of PD-L1 in lung cancer may contribute to poor
prognosis and tumor cells immune escape through suppressing tumor
infiltrating dendritic cells maturation. Med Oncol. 28:682–688.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
He Y, Yu H, Rozeboom L, Rivard CJ, Ellison
K, Dziadziuszko R, Suda K, Ren S, Wu C, Hou L, et al: LAG-3 protein
expression in non-small cell lung cancer and its relationship with
PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol.
12:814–823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Y, Cai P, Liang T, Wang L and Hu L:
TIM-3 is a potential prognostic marker for patients with solid
tumors: A systematic review and meta-analysis. Oncotarget.
8:31705–31713. 2017.PubMed/NCBI
|
|
61
|
Liu XG, Hou M and Liu Y: TIGIT, a novel
therapeutic target for tumor immunotherapy. Immunol Invest.
46:172–182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Anderson AC, Joller N and Kuchroo VK:
Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized
functions in immune regulation. Immunity. 44:989–1004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Koyama S, Akbay EA, Li YY, Herter-Sprie
GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ,
Asahina H, et al: Adaptive resistance to therapeutic PD-1 blockade
is associated with upregulation of alternative immune checkpoints.
Nat Commun. 7:105012016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gros A, Parkhurst MR, Tran E, Pasetto A,
Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts
IM, et al: Prospective identification of neoantigen-specific
lymphocytes in the peripheral blood of melanoma patients. Nat Med.
22:433–438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nicolazzo C, Raimondi C, Mancini M,
Caponnetto S, Gradilone A, Gandini O, Mastromartino M, Del Bene G,
Prete A, Longo F, et al: Monitoring PD-L1 positive circulating
tumor cells in non-small cell lung cancer patients treated with the
PD-1 inhibitor Nivolumab. Sci Rep. 6:317262016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gandara DR, Paul SM, Kowanetz M,
Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G,
Malboeuf C, et al: Blood-based tumor mutational burden as a
predictor of clinical benefit in non-small-cell lung cancer
patients treated with atezolizumab. Nat Med. 24:1441–1448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gandara DR, Kowanetz M, Mok TSK, Rittmeyer
A, Fehrenbacher L, Fabrizio D, Otto G, Malboeuf C, Lieber D, Paul
SM, et al: 1295OBlood-based biomarkers for cancer immunotherapy:
Tumor mutational burden in blood (bTMB) is associated with improved
atezolizumab (atezo) efficacy in 2L+ NSCLC (POPLAR and OAK). Ann
Oncol. 28 (Suppl 5):2017. View Article : Google Scholar
|
|
69
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Durgeau A, Virk Y, Corgnac S and
Mami-Chouaib F: Recent advances in targeting CD8 T-Cell immunity
for more effective cancer immunotherapy. Front Immunol. 9:142018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pasetto A, Gros A, Robbins PF, Deniger DC,
Prickett TD, Matus-Nicodemos R, Douek DC, Howie B, Robins H,
Parkhurst MR, et al: Tumor-and neoantigen-reactive T-cell receptors
can be identified based on their frequency in fresh tumor. Cancer
Immunol Res. 4:734–743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bassani-Sternberg M and Coukos G: Mass
spectrometry-based antigen discovery for cancer immunotherapy. Curr
Opin Immunol. 41:9–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Caron E, Kowalewski DJ, Chiek Koh C, Sturm
T, Schuster H and Aebersold R: Analysis of major histocompatibility
complex (MHC) immunopeptidomes using mass spectrometry. Mol Cell
Proteomics. 14:3105–3117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Khodadoust MS, Olsson N, Wagar LE, Haabeth
OA, Chen B, Swaminathan K, Rawson K, Liu CL, Steiner D, Lund P, et
al: Antigen presentation profiling reveals recognition of lymphoma
immunoglobulin neoantigens. Nature. 543:723–727. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cai LL, Ye HM, Zheng LM, Ruan RS and Tzeng
CM: Circulating tumor cells (CTCs) as a liquid biopsy material and
drug target. Curr Drug Targets. 15:965–972. 2014.PubMed/NCBI
|
|
77
|
Rizvi NA, Hellmann MD, Brahmer JR,
Juergens RA, Borghaei H, Gettinger S, Chow LQ, Gerber DE, Laurie
SA, Goldman JW, et al: Nivolumab in combination with platinum-based
doublet chemotherapy for first-line treatment of advanced
non-small-cell lung cancer. J Clin Oncol. 34:2969–2979. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu SY and Wu YL: Ongoing clinical trials
of PD-1 and PD-L1 inhibitors for lung cancer in China. J Hematol
Oncol. 10:1362017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ribas A, Puzanov I, Dummer R, Schadendorf
D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD,
et al: Pembrolizumab versus investigator-choice chemotherapy for
ipilimumab-refractory melanoma (KEYNOTE-002): A randomised,
controlled, phase 2 trial. Lancet Oncol. 16:908–918. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen Q, Li T and Yue W: Drug response to
PD-1/PD-L1 blockade: Based on biomarkers. Onco Targets Ther.
11:4673–4683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Koeppel F, Blanchard S, Jovelet C, Genin
B, Marcaillou C, Martin E, Rouleau E, Solary E, Soria JC, André F
and Lacroix L: Whole exome sequencing for determination of tumor
mutation load in liquid biopsy from advanced cancer patients. PLoS
One. 12:e01881742017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ilie M, Khambata-Ford S, Copie-Bergman C,
Huang L, Juco J, Hofman V and Hofman P: Use of the 22C3 anti-PD-L1
antibody to determine PD-L1 expression in multiple automated
immunohistochemistry platforms. PLoS One. 12:e01830232017.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Marchetti A, Barberis M, Franco R, De Luca
G, Pace MV, Staibano S, Volante M, Buttitta F, Guerini-Rocco E,
Righi L, et al: Multicenter comparison of 22c3 pharmDx (Agilent)
and SP263 (Ventana) assays to test PD-L1 expression for NSCLC
patients to be treated with immune checkpoint inhibitors. J
Thoracic Oncol. 12:1654–1663. 2017. View Article : Google Scholar
|
|
86
|
Kowanetz M, Koeppen H, Zou W, Mariathasan
S, Hellmann M, Kockx M, Chappey C, Kadel E, Smith D, Miley N, et
al: Abstract A017: PD-L1 as a predictive biomarker for atezolizumab
(MPDL3280A; anti-PDL1) in non-small cell lung cancer (NSCLC). AACR.
2016.
|
|
87
|
Hu X and Hay JW: First-line pembrolizumab
in PD-L1 positive non-small-cell lung cancer: A cost-effectiveness
analysis from the UK health care perspective. Lung Cancer.
123:166–171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dang TO, Ogunniyi A, Barbee MS and Drilon
A: Pembrolizumab for the treatment of PD-L1 positive advanced or
metastatic non-small cell lung cancer. Expert Rev Anticancer Ther.
16:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ibrahim RA, Berman DM, Depril V, Humphrey
RW, Chen T and Messina M: Ipilimumab safety profile: Summary of
findings from completed trials in advanced melanoma. J Clin Oncol.
29 (15-Suppl):85832011. View Article : Google Scholar
|
|
90
|
Teply BA and Lipson EJ: Identification and
management of toxicities from immune checkpoint-blocking drugs.
Oncology (Williston Park). 3 (28 Suppl):30–38. 2014.
|
|
91
|
Efremova M, Finotello F, Rieder D and
Trajanoski Z: Neoantigens generated by individual mutations and
their role in cancer immunity and immunotherapy. Front Immunol.
8:16792017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wilson EA and Anderson KS: Lost in the
crowd: Identifying targetable MHC class I neoepitopes for cancer
immunotherapy. Expert Rev Proteomics. 15:1065–1077. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bassani-Sternberg M, Bräunlein E, Klar R,
Engleitner T, Sinitcyn P, Audehm S, Straub M, Weber J,
Slotta-Huspenina J, Specht K, et al: Direct identification of
clinically relevant neoepitopes presented on native human melanoma
tissue by mass spectrometry. Nat Commun. 7:134042016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Klebanoff CA, Rosenberg SA and Restifo NP:
Prospects for gene-engineered T cell immunotherapy for solid
cancers. Nat Med. 22:26–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Politi K and Herbst RS: Lung cancer in the
era of precision medicine. Clin Cancer Res. 21:2213–2220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ramalingam S, Hui R, Gandhi L, Carcereny
E, Felip E, Ahn MJ, Eder JP, Balmanoukian AS, Leighl N, Aggarwal C,
et al: P2. 39: Long-Term OS for patients with advanced NSCLC
enrolled in the KEYNOTE-001 study of pembrolizumab. J Thoracic
Oncol. 11:S241–S242. 2016. View Article : Google Scholar
|
|
97
|
Gettinger S, Rizvi NA, Chow LQ, Borghaei
H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman
JW, et al: Nivolumab monotherapy for first-line treatment of
advanced non-small-cell lung cancer. J Clin Oncol. 34:2980–2987.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Forde PM, Smith KN, Chaft JE, Hellmann M,
Merghoub T, Wolchok JD, Yang SC, Battafarano RJ, Gabrielson E,
Georgiades CS, et al: NSCLC, early stageNeoadjuvant anti-PD1,
nivolumab, in early stage resectable non-small-cell lung cancer.
Ann Oncol. 27 (Suppl 6):LBA41–PR. 2016. View Article : Google Scholar
|
|
99
|
Antonia S, Rizvi N, Brahmer JR, Ou SHL,
Khleif SN, Hwu WJ, Gutierrez M, Schoffski P, Hamid O, Weiss J, et
al: Abstract A047: Safety and clinical activity of durvalumab
(MEDI4736), an anti-programmed cell death ligand-1 (PD-L1)
antibody, in patients with non-small cell lung cancer (NSCLC).
AACR. 2016.
|