Introduction
Ovarian cancer is one of the most common types of
gynecological malignancies worldwide with an extremely low survival
rate of 5 years (1,2). Patients diagnosed at the early stages
of ovarian cancer respond to platinum-based combinatorial
chemotherapies; however, relapse and drug resistance are prevalent,
leading to treatment failure and poor prognosis (3). At present, due to the lack of reliable
approaches for early diagnosis, ovarian cancer is generally
diagnosed at an advanced stage when surgical resection or
chemotherapy are generally ineffective (4). Therefore, molecular alterations in
tumors, particularly those involved in cell proliferation,
apoptosis and invasion signaling pathways, are being investigated
for potential developments in early diagnosis and targeted
therapy.
Potassium channels are the most widely distributed
type of ion channel and are associated with numerous biological
processes in almost all living organisms (5,6).
Potassium channels are commonly expressed in excitable cells and
are often aberrantly expressed in neoplastic cell lines and primary
types of human cancer (7,8). This contributes to the regulation of
several factors associated with neoplastic progression, including
cell proliferation, apoptosis, invasion and metastasis (9–11). The
inhibition of potassium channels has been demonstrated to exert
antineoplastic activities in various types of tumors in
vitro and in vivo (12,13).
This evidence suggests that potassium channels may be considered as
possible targets for antineoplastic therapy.
Human ether a-go-go related potassium channels
(hERGs) are voltage-dependent potassium channels that serve
important roles in the terminal repolarization on human ventricular
myocytes (14). A number of studies
over the past two decades have demonstrated that plasma membrane
hERG1 is often aberrantly expressed in various types of cancer and
serves essential roles in numerous crucial cellular events,
including electrophysiological activity and signaling conduction
(15,16). However, to the best of our knowledge,
few investigations into the function of hERG1 in human ovarian
cancer have been conducted (17,18) and
the underlying mechanisms regulating hERG1 expression in ovarian
cancer progression remain unknown.
Previous studies have revealed that numerous
traditional Chinese herbal medicines (19–21),
including berberine (BBR), a clinically important natural
isoquinoline alkaloid that was the subject of our previous study
(22), exhibit significant antitumor
activities and reverse the drug resistance of cancer cells. BBR has
been reported to exert antitumor activity against various types of
cancer, including gastric, oral, prostate and ovarian cancer
(23–25). The molecular targets of BBR include
protein kinase B, p53, mitogen-activated protein kinase, nuclear
factor-κB and signal transducer and activator of transcription 3,
which are pivotal factors in regulating cell growth, apoptosis,
invasion and angiogenesis of cancer (26–28).
However, since tumor progression is a complicated pathological
process, other molecular targets may be involved in the antitumor
effects exhibited by BBR. It is common that multi-target
combination therapy for the treatment of tumors is administered as
the main treatment approach to overcome drug resistance and improve
the prognosis of patients with cancer (29). Therefore, investigations into the
potential molecular mechanisms underlying the antitumor properties
of BBR in ovarian cancer are necessary and valuable for the
development of multi-targeted therapy.
The present study revealed that hERG1 was involved
in the regulation of proliferation, migration and invasion of
ovarian cancer cells. Treatment with BBR was observed to
efficiently inhibit these biological activities in vivo and
in vitro. The results of the present study provide novel
insights into the molecular mechanism underlying the antitumor
effects of BBR and may contribute to the development of novel
therapeutic strategies for the treatment of ovarian cancer.
Materials and methods
Tissue samples
A total of 28 ovarian carcinoma tissue samples and
matched adjacent non-tumorigenic tissues samples (2 cm from
cancerous tissues) were obtained from patients who underwent
surgical resection of the tumor at Harbin Medical University Cancer
Hospital (Harbin, China) between May 2015 and March 2016. The
average age of the patients was 48 years old. The samples were
stored in liquid nitrogen until total RNA or protein were
extracted. The present study was approved by the Ethical Committee
of Harbin Medical University Cancer Hospital and written informed
consent was obtained from all patients.
Immunohistochemical assay
All tissues were initially fixed in 10% formalin for
48 h at 4°C and embedded in paraffin. Section of 5 µm thickness
were pre-incubated with 5% goat serum (cat. no. C0265; Beyotime
Institute of Biotechnology, Shanghai, China) as a blocking buffer
for 2 h at room temperature, and then incubated with primary hERG1
antibody (1:200; cat. no. sc-377388; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) overnight at 4°C; After washing three times
with PBS at room temperature, the sections were incubated with
peroxidase-labeled anti-mouse antibody (1:50; cat. no. A0216;
Beyotime Institute of Biotechnology) for 30 min at room
temperature. The peroxidase activity was detected with
diaminobenzidine (DAB; Beyotime Institute of Biotechnology). The
stained sections were analyzed using a fluorescence Olympus BX51
microscope (magnification, ×200; Olympus Corporation, Tokyo,
Japan). The immunohistochemical staining results were scored as the
sum of intensity and percentage scores, according to the staining
intensity (0=negative; 1=weak/trace; 2=moderate; and 3=strong) and
the percentage of positive cells (0, ≤10; 1, 11–25; 2, 26–50; 3,
51–75; and 4, 76–100%). This grading produced a final score of
0–12, and samples were separated into groups based on low (0–4) or
high (6–12) scores.
Reagents
BBR and DMSO were purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany). BBR was dissolved in 10% DMSO and
90% deionized water. The drug solution was diluted to
concentrations of 5, 10, 30, 50 and 100 µM in RPMI-1640 culture
medium (Beyotime Institute of Biotechnology) prior to use at room
temperature. For cellular experiments, cells were incubated with
different concentrations of BBR (5, 10, 30, 50 or 100 µM) for 24,
48 and 72 h at 37°C, respectively. Cells in the blank control group
were treated with 1% DMSO.
Cell culture and transfection
OVCAR-3, SKOV3, HO-8910, A2780 and ES-2 ovarian
carcinoma cell lines were obtained from the Cancer Institute of
Harbin Medical University. OVCAR-3 and SKOV3 cells were maintained
in RPMI-1640 medium (Beyotime Institute of Biotechnology)
supplemented with 12% (v/v) FBS (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). HO-8910, A2780 and ES-2 ovarian carcinoma cells
were maintained in DMEM (Beyotime Institute of Biotechnology)
supplemented with 12% (v/v) FBS (Thermo Fisher Scientific, Inc.).
All cell lines were cultured at 37°C with 5% CO2 in a
humidified incubator.
Short hairpin (sh)RNA-hERG1 plasmid (4 µg) and
shRNA-control (scrambled short hairpin RNA sequence) plasmids (4
µg) (Shanghai GeneChem Co., Ltd., Shanghai, China), were used to
transfect SKOV3 cells. All transfections were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Cells
were harvested at 24 h after transfection.
Cell proliferation assay
Cell proliferation was determined by Cell Counting
kit-8 (CCK8) assay. Briefly, SKOV3 cells were seeded in 96-well
plates at 1×105 cells/well and maintained for 24 h at
37°C to allow cell adhesion. Subsequently, the cells in various
groups (5, 10, 30, 50 or 100 µM berberine for 24, 48 or 72 h) were
incubated with 10 µl CCK8 for 2 h and the absorbance was measured
at 450 nm using a microplate reader (Olympus Corporation). The
proliferation of treated cells was assessed by comparison with the
blank control group. Each experiment was performed in
triplicate.
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
OVCAR-3, SKOV3, HO-8910, A2780 and ES-2 ovarian
carcinoma cells and tissue specimens were homogenized in TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) to isolate
total RNA, according to the manufacturer's protocols. The
concentration of RNA was detected with a NanoDrop spectrophotometer
(NanoDrop Technologies; Thermo Fisher Scientific, Inc.). The total
RNA of each sample (1 µg) was reverse transcribed using random
primers and a PrimeScript 1st Strand cDNA Synthesis kit (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocols. qPCR was performed using a SYBR green
fluorescent dye (PCR master mix; Applied Biosystems; Thermo Fisher
Scientific, Inc.) with the following thermocycling conditions:
Pre-denaturation at 95°C for 45 sec, followed by 40 cycles of
denaturation at 95°C for 15 sec, and annealing and extension at
55°C for 30 sec. The relative hERG1 expression levels were
calculated based on the 2−ΔΔCq method and normalized to
GAPDH level in each sample (30).
Each experiment was performed in triplicate. The primer sequences
for qPCR were as follows: hERG1 forward,
5′-CAGCGGCTGTACTCGGGCACAG-3′ and reverse,
5′-CAGAAGTGGTCGGAGAACTC-3′; and GAPDH forward,
5′-GTCAACGGATTTGGTCGTATTG-3′ and reverse,
5′-AGTGATGGCATGGACTGTGG-3′.
Western blot analysis
Briefly, total protein was extracted from tissues or
OVCAR-3, SKOV3, HO-8910, A2780 and ES-2 ovarian carcinoma cell
lines with RIPA buffer (Beyotime Institute of Biotechnology) and
the protein concentrations were determined using a bicinchoninic
acid protein assay (Pierce; Thermo Fisher Scientific, Inc.).
Subsequently, proteins (50 µg/lane) were separated by SDS-PAGE (8%
gel) and transferred onto a nitrocellulose membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). To prevent non-specific
binding, the membrane was blocked with 5% skimmed milk in PBS with
Tween-20 (0.25%) for 2 h at room temperature. Subsequently, the
membrane was incubated with hERG1 antibody (1:1,000; cat. no.
sc-377388; Santa Cruz Biotechnology, Inc.) or β-actin antibody
(1:1,000; cat. no. ab8227; Abcam, Cambridge, UK) in PBS at 4°C
overnight. Subsequently, the membrane was rinsed with PBST three
times, which was followed by incubation with corresponding
IRDye® 800CW fluorescent secondary antibodies (1:8,000;
cat. nos. anti-rabbit 926–32211 and anti-mouse 926–32210; LI-COR
Biosciences, Lincoln, NE, USA) for 1 h at room temperature.
Finally, the protein bands were visualized using an Odyssey
infrared imaging system (LI-COR Biosciences) at 800 nm.
Transwell assays
SKOV3 cells in serum-free RPMI-1640 medium (200 µl
containing 5×105 cells for BBR group, 200 µl containing
2.5×105 cells for other groups) were added to the upper
Transwell chambers (pore size, 8 µm; Corning Inc., Corning, NY,
USA). The chambers were coated with Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA) for the invasion assays but not the
migration assays. The bottom chamber was filled with 700 µl
RPMI-1640 medium containing 15% FBS. The cells were cultured in an
incubator at 37°C with 5% CO2 for 24 h, then the cells
were fixed in 4% paraformaldehyde for 15 min. Subsequently, the
cells were washed with PBS and stained with 0.1% crystal violet for
10 min at room temperature. For analysis, five fields were randomly
selected and the number of stained cells was counted under a light
microscope at magnification of ×200 (Nikon Corporation, Tokyo,
Japan). Data are expressed as the mean number of cells per
insert.
In vivo tumor xenograft study
Five-week-old BALB/c-nu/nu nude mice were purchased
from the Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China). The experimental protocols were approved by the
Ethics Committee of Harbin Medical University (Harbin, China). A
total of 72 mice (36 females, 36 males) weighing 20–25 g each, were
maintained under a 12-h light/dark cycle at 20.0–26.1°C and 35–70%
humidity. Food and water were freely accessible to mice. SKOV3
cells (2×106 per mouse) were subcutaneously injected
into the flank of each mouse. Following the outgrowth of palpable
tumors, the mice were randomly divided into four groups (n=3 for
each group) and fed by oral gavage with saline or BBR (20 mg/kg),
twice per week. BBR was dissolved in carboxymethylcellulose sodium
for use via oral gavage. Tumors volumes were calculated using the
following formula: Volume=L × (W)2/2, where L is the
longest diameter and W is the shorter diameter. At the end of the
present study, mice were euthanized and tumor tissues were
harvested for further research.
Statistical analysis
Data are presented as the mean ± standard error of
the mean of triplicate experiments and were analyzed with SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). The
χ2 test was used to analyze the association of hERG1
expression with clinicopathologic features of ovarian cancer.
Unpaired Student's t-test and one-way analysis of variance with a
Student-Newman-Keuls post hoc test were used to determine the
significance of differences between two groups and three or more
groups, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
hERG1 is upregulated in human ovarian
cancer
In order to determine the role of hERG1 in ovarian
cancer development, the mRNA and protein expression levels of hERG1
were detected by RT-qPCR and western blotting. A total of 28 tumor
tissues and matched non-tumor tissues were obtained from patients
with ovarian cancer with a median age of 48 years in the present
study. The association of hERG1 expression with clinicopathologic
features of ovarian cancer is presented in Table I. These results demonstrate that
hERG1 expression level is significantly associated with ovarian
cancer distant metastasis and lymph node metastasis. The
immunohistochemical assay results revealed that the protein
expression levels of hERG1 were markedly higher in ovarian cancer
tissues compared with non-tumor tissues (Fig. 1A). As presented in Fig. 1B and C, the mRNA and protein
expression levels of hERG1 were significantly upregulated in tumor
tissues compared with non-tumor tissues, as expected. Furthermore,
the expression level of hERG1 in OVCAR-3, SKOV3, HO-8910, A2780 and
ES-2 ovarian cancer cell lines was investigated. The results
(Fig. 1D and E) revealed that hERG1
was generally expressed in these ovarian cancer cell lines and
highly expressed in SKOV3, in particular. Therefore, in the present
study, SKOV3 cells were used for further analysis.
 | Table I.Associations between hERG1 expression
and clinicopathological features of patients with ovarian
cancer. |
Table I.
Associations between hERG1 expression
and clinicopathological features of patients with ovarian
cancer.
|
| hERG1 expression,
n |
|
|---|
|
|
|
|
|---|
| Variables | Low | High | P-value |
|---|
| Age, years |
|
| 0.6193 |
|
≤50 | 7 | 8 |
|
|
>50 | 8 | 5 |
|
| Tumor diameter,
cm |
|
| 0.8232 |
|
<8 | 3 | 8 |
|
| ≥8 | 4 | 13 |
|
| Ascite |
|
| 0.7051 |
|
Negative | 6 | 7 |
|
|
Positive | 9 | 6 |
|
| Lymph node
metastasis |
|
| 0.0044a |
|
Negative | 9 | 5 |
|
|
Positive | 1 | 13 |
|
| Distant
metastasis |
|
| 0.0204b |
|
Negative | 7 | 3 |
|
|
Positive | 4 | 14 |
|
|
Differentiation |
|
| 0.7483 |
|
Good | 3 | 4 |
|
|
Moderate | 5 | 3 |
|
|
Poor | 7 | 6 |
|
| Tumor location |
|
| 0.694 |
|
Bilateral | 8 | 10 |
|
|
Unilateral | 6 | 4 |
|
BBR inhibits cell proliferation and
hERG1 in SKOV3 cells
Subsequently, the effects of BBR on SKOV3 cells were
investigated using a CCK8 assay. SKOV3 cells were treated for 24,
48 and 72 h with BBR at concentrations ranging between 5 and 100
µM, and the results were compared with the control group treated
with DMSO. The CCK8 assay results demonstrated that BBR inhibited
the proliferation of SKOV3 cells in a time- and dose-dependent
manner (Fig. 2A). The proliferation
of cells treated for 48 h decreased more significantly compared
with cells treated for 24 h (P<0.05); however, no difference was
observed between cells in the 48 and 72 h groups. Simultaneously,
BBR significantly reduced the mRNA and protein expression levels of
hERG1 in a dose-dependent manner (Fig.
2B-D), which suggests that hERG1 may serve a role in the
proliferation of SKOV3 cells. Expression levels of hERG1 decreased
significantly in response to 10 µM BBR and the IC50 of
BBR was 9.8 µM at 48 h. Therefore, in subsequent experiments BBR
was administered to SKOV3 cells for 48 h at a concentration of 10
µM.
Knockdown of hERG1 in SKOV3 cells
To investigate the effects of hERG1 on tumor
biological activities, expression levels of hERG1 in SKOV3 cells
were downregulated using a shRNA-hERG1 plasmid. As presented in
Fig. 3, the mRNA and protein
expression levels of hERG1 in SKOV3 cells transfected with the
shRNA-hERG1 plasmid were significantly decreased compared with in
the control groups.
hERG1 is involved in the
pathophysiological process of SKOV3 cells
The effects of hERG1 on the proliferation of SKOV3
cells were detected in vitro using a CCK8 assay (Fig. 4A). The results demonstrated that
knockdown of hERG1 in SKOV3 cells significantly reduced the cell
proliferation activity compared with the control cells. BBR (10 µM)
was considered as a positive control group. As expected, knockdown
of hERG1 exhibited similar cytotoxic effects as BBR treatment.
These results suggest that hERG1 may serve a role in cell
proliferation.
Furthermore, to determine whether hERG1 is involved
in the process of cell migration and invasion, which represent the
tumor migration and invasive abilities, Transwell assays were
performed following the transfection of SKOV3 cells with sh-hERG1
or scramble control. As presented in Fig. 4B-E, knockdown of hERG1 significantly
reduced the migration and invasion abilities of SKOV3 cells. BBR
(10 µM) was used as a positive control. The results of the present
study indicate that hERG1 may be involved in the proliferation,
migration and invasion processes of SKOV3 cells, and that BBR may
be a potential therapeutic drug in the treatment of ovarian
cancer.
Knockdown of hERG1 inhibits ovarian
tumor growth in vivo
To assess whether hERG1 is involved in ovarian
cancer growth in vivo, nude mice were inoculated with SKOV3
cells, which were previously transfected with sh-hERG1 or
shRNA-control plasmids. Following the outgrowth of palpable tumors,
nude mice were randomly divided into four groups (n=3 for each
group) and fed via oral gavage with saline or BBR (20 mg/kg) twice
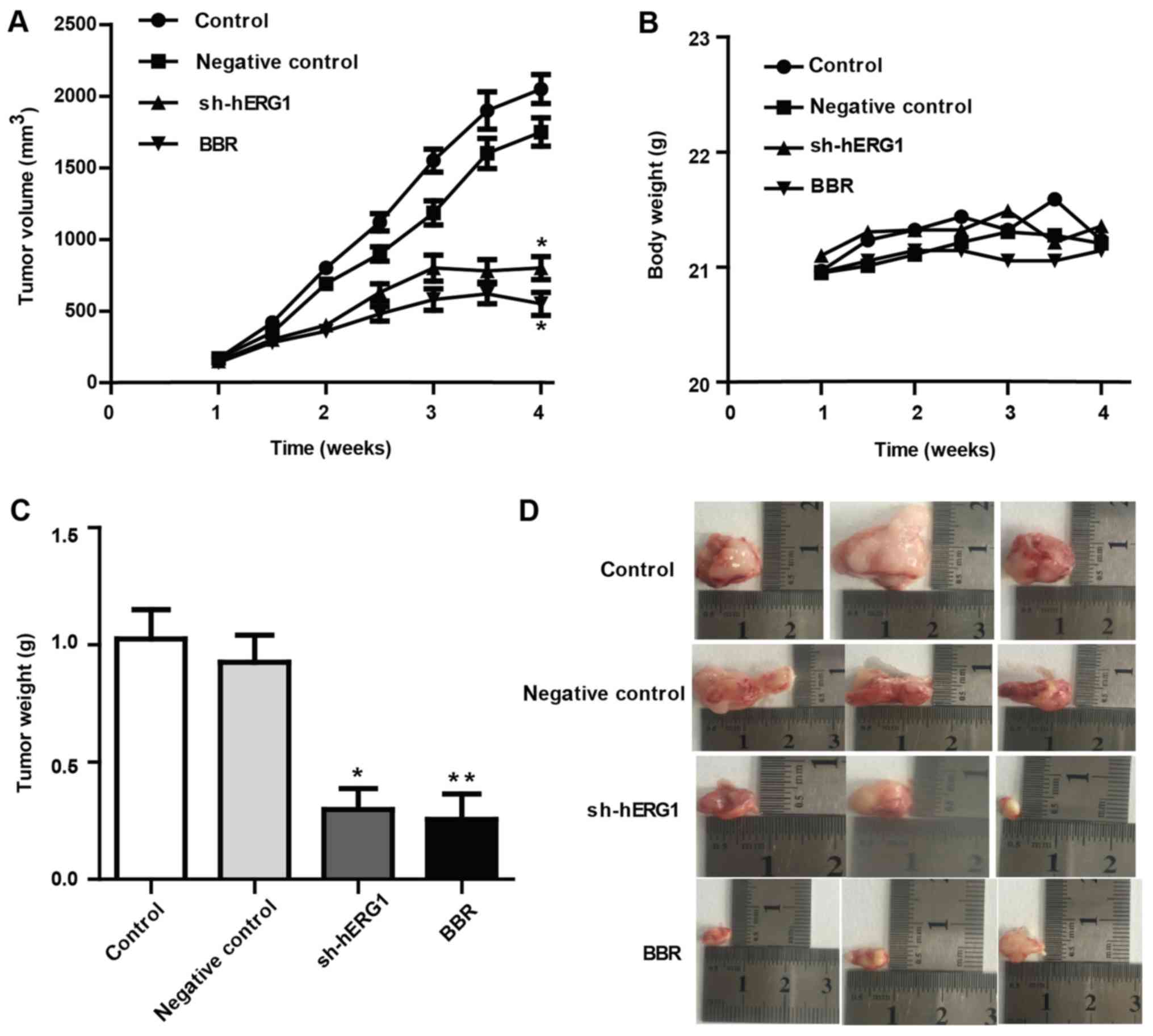
per week. As presented in Fig. 5,
the size and weight of ovarian cancer mass significantly decreased
following the administration of BBR by oral gavage compared with
the control group. Similarly, knockdown of hERG1 also significantly
decreased tumor growth of xenografts compared with the control
group. During the experiments, no notable weight loss was observed
in mice of the different groups. These findings indicate that hERG1
may be involved in the pathogenesis of ovarian cancer and may be
considered as a potential prognostic indicator in the treatment of
ovarian cancer.
Discussion
Great efforts have been made in the treatment of
ovarian cancer clinically; however, the prognosis of ovarian cancer
remains poor due to drug resistance and tumor recurrence. In
addition, tumor markers with appropriate specificity and
sensitivity for the diagnosis and treatment of ovarian cancer
remain to be identified (31,32).
Therefore, it is urgent to determine novel molecular markers to
improve early diagnosis and effective therapy of ovarian
cancer.
Over the past decades, BBR has drawn extensive
attention due to its antitumorigenic effects in various types of
human cancer cells and animal models (33,34).
Treatment with BBR has been reported to act on numerous targets and
signaling pathways underlying pathophysiological process, including
proliferation, apoptosis, angiogenesis, migration and invasion in a
variety of cancer cells (35–37). In
our previous study, BBR was observed to exert a strong inhibitory
effect on hERG potassium channels (22,38,39),
which are often aberrantly expressed in carcinoma. Therefore, the
present study proposed that BBR may inhibit ovarian cancer by
targeting hERG1.
In the present study, it was demonstrated that the
potassium ion channel protein, hERG1, serves a pivotal role in the
pathophysiological process of SKOV3 ovarian cancer cells and may be
inhibited by BBR in vitro and in vivo. The results of
the present study suggest that BBR exerts a strong cytotoxic effect
against ovarian cancer cells. This was demonstrated by the time-
and dose-dependent inhibition of the proliferation of human ovarian
cancer SKOV3 cells. A similar cytotoxic effect was achieved by
knockdown of hERG1, which strongly suggests that hERG1 is involved
in the ovarian cancer cell proliferation process. Additionally, the
migration and invasion abilities of ovarian cancer SKOV3 cells were
investigated. The results of the present study revealed that
knockdown of hERG1 markedly reduced the migration and invasion
abilities of ovarian cancer SKOV3 cells, which further suggests
that hERG1 is an important regulator in the ovarian cancer
phenotype. The results of the in vivo experimental results
similarly supported the hypothesis of the present study, in which
hERG1 was involved in the process of tumor growth and BBR could be
a potential drug for the treatment of ovarian cancer.
During past decades, a number of studies have
demonstrated the association between the activity of ion channels
and the progression of different types of cancer, and considerable
achievements have been made in understanding the role of ion
channels in cancer (7,40,41).
hERG1 has been reported to exhibit oncogenic properties, is often
aberrantly expressed in cancer and has essential roles in numerous
crucial cellular events (15,16). To
the best of our knowledge, the present study revealed for the first
time, that hERG1, as an important part of the potassium channel,
may be the pivotal molecule to sense stimuli, transmit signals and
eventually cause a series of signal pathway alterations within the
cell.
In conclusion, hERG1 was demonstrated to be
associated with the pathophysiological process of ovarian cancer
SKOV3 cells, and the extract of a natural Chinese herbal medicine,
BBR, may be of potential use as an anticancer agent for patients
with ovarian cancer. The anticancer effects of BBR may be mediated
by targeting the potassium hERG1 channel and eventually inducing a
series of pathophysiological alterations in ovarian cancer cells.
However, further studies are required to evaluate how BBR regulates
the expression of hERG1 and the mechanism underlying the
hERG1-mediated phenotypic alterations in ovarian cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the Haiyan Foundation
from Harbin Medical University Cancer Hospital (grant no.
JJQN2017-12) and the Harbin Medical University Scientific Research
Innovation Fund (grant no. 2017LCZX97).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ was responsible for the design of the study and
writing of the article. KZ was a major contributor in data analysis
and was responsible for some experimental operations. XF performed
the western blotting experiments. JZ performed the cell culture,
transfection and proliferation assays. XL performed RT-qPCR,
Transwell assays and the in vivo tumor xenograft study. DY
was responsible for the immunohistochemical assays, and the
revision of the article. MD was responsible for the design of the
study and revision of the article. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study has been approved by the Ethical
Committee of the Harbin Medical University Cancer Hospital (Harbin,
China) and written informed consent was obtained from all recruited
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wright JD, Chen L, Hou JY, Burke WM,
Tergas AI, Ananth CV, Neugut AI and Hershman DL: Association of
hospital volume and quality of care with survival for ovarian
cancer. Obstet Gynecol. 130:545–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee YK, Chung HH, Kim JW, Song YS and Park
NH: Expression of phosphorylated Akt and hTERT is associated with
prognosis of epithelial ovarian carcinoma. Int J Clin Exp Pathol.
8:14971–14976. 2015.PubMed/NCBI
|
|
3
|
Stefanou DT, Bamias A, Episkopou H,
Kyrtopoulos SA, Likka M, Kalampokas T, Photiou S, Gavalas N,
Sfikakis PP, Dimopoulos MA and Souliotis VL: Aberrant DNA damage
response pathways may predict the outcome of platinum chemotherapy
in ovarian cancer. PLoS One. 10:e01176542015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bandera EV, Lee VS, Qin B,
Rodriguez-Rodriguez L, Powell CB and Kushi LH: Impact of body mass
index on ovarian cancer survival varies by stage. Br J Cancer.
117:282–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Littleton JT and Ganetzky B: Ion channels
and synaptic organization: Analysis of the Drosophila
genome. Neuron. 26:35–43. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Curran ME: Potassium ion channels and
human disease: Phenotypes to drug targets? Curr Opin Biotechnol.
9:565–572. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji CD, Wang YX, Xiang DF, Liu Q, Zhou ZH,
Qian F, Yang L, Ren Y, Cui W, Xu SL, et al: Kir2.1 interaction with
Stk38 promotes invasion and metastasis of human gastric cancer by
enhancing MEKK2-MEK1/2-ERK1/2 signaling. Cancer Res. 78:3041–3053.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng YY, Wright CM, Kirschner MB,
Williams M, Sarun KH, Sytnyk V, Leshchynska I, Edelman JJ, Vallely
MP, McCaughan BC, et al: KCa1.1, a calcium-activated potassium
channel subunit alpha 1, is targeted by miR-17-5p and modulates
cell migration in malignant pleural mesothelioma. Mol Cancer.
15:442016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lastraioli E, Lottini T, Bencini L,
Bernini M and Arcangeli A: hERG1 potassium channels: Novel
biomarkers in human solid cancers. Biomed Res Int. 2015:8964322015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patanè S: HERG-targeted therapy in both
cancer and cardiovascular system with cardiovascular drugs. Int J
Cardiol. 176:1082–1085. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leanza L, Biasutto L, Managò A, Gulbins E,
Zoratti M and Szabò I: Intracellular ion channels and cancer. Front
Physiol. 4:2272013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
García-Quiroz J, García-Becerra R,
Santos-Martínez N, Barrera D, Ordaz-Rosado D, Avila E, Halhali A,
Villanueva O, Ibarra-Sánchez MJ, Esparza-López J, et al: In vivo
dual targeting of the oncogenic Ether-à-go-go-1 potassium channel
by calcitriol and astemizole results in enhanced antineoplastic
effects in breast tumors. BMC Cancer. 14:7452014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
D'Amico M, Gasparoli L and Arcangeli A:
Potassium channels: Novel emerging biomarkers and targets for
therapy in cancer. Recent Pat Anticancer Drug Discov. 8:53–65.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balijepalli SY, Lim E, Concannon SP, Chew
CL, Holzem KE, Tester DJ, Ackerman MJ, Delisle BP, Balijepalli RC
and January CT: Mechanism of loss of Kv11.1 K+ current
in mutant T421M-Kv11.1-expressing rat ventricular myocytes:
Interaction of trafficking and gating. Circulation. 126:2809–2818.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fortunato A: The role of hERG1 ion
channels in epithelial-mesenchymal transition and the capacity of
riluzole to reduce cisplatin resistance in colorectal cancer cells.
Cell Oncol (Dordr). 40:367–378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Becchetti A, Crescioli S, Zanieri F,
Petroni G, Mercatelli R, Coppola S, Gasparoli L, D'Amico M,
Pillozzi S, Crociani O, et al: The conformational state of hERG1
channels determines integrin association, downstream signaling, and
cancer progression. Sci Signal. 10(pii): eaaf32362017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sundby E, Han J, Kaspersen SJ and Hoff BH:
In vitro baselining of new pyrrolopyrimidine EGFR-TK inhibitors
with Erlotinib. Eur J Pharm Sci. 80:56–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fraley ME, Garbaccio RM, Arrington KL,
Hoffman WF, Tasber ES, Coleman PJ, Buser CA, Walsh ES, Hamilton K,
Fernandes C, et al: Kinesin spindle protein (KSP) inhibitors. Part
2: The design, synthesis, and characterization of
2,4-diaryl-2,5-dihydropyrrole inhibitors of the mitotic kinesin
KSP. Bioorg Med Chem Lett. 16:1775–1779. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J, Wang G, Wu J, Zuo Y, Zhang J and
Chen J: Arsenic trioxide inhibits Skp2 expression to increase
chemosensitivity to gemcitabine in pancreatic cancer cells. Am J
Transl Res. 11:991–997. 2019.PubMed/NCBI
|
|
20
|
Qu X, Wang F, Zhang Y, Du Z, Ren J, Liu Z
and Zhang L: Biocompatible heterogeneous MOF-Cu catalyst used for
in vivo drug synthesis at targeted subcellular organelles. Angew
Chem Int Ed Engl. 19–Mar;2019.(Epub ahead of print). doi
org/10.1002/anie.201901760.
|
|
21
|
Park HH, Choi SW, Lee GJ, Kim YD, Noh HJ,
Oh SJ, Yoo I, Ha YJ, Koo GB, Hong SS, et al: A formulated red
ginseng extract inhibits autophagic flux and sensitizes to
doxorubicin-induced cell death. J Ginseng Res. 43:86–94. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhi D, Feng PF, Sun JL, Guo F, Zhang R,
Zhao X and Li BX: The enhancement of cardiac toxicity by
concomitant administration of Berberine and macrolides. Eur J Pharm
Sci. 76:149–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ortiz LM, Lombardi P, Tillhon M and
Scovassi AI: Berberine, an epiphany against cancer. Molecules.
19:12349–12367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ayati SH, Fazeli B, Momtazi-Borojeni AA,
Cicero AFG, Pirro M and Sahebkar A: Regulatory effects of berberine
on microRNome in cancer and other conditions. Crit Rev Oncol
Hematol. 116:147–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Yang S, Cai X, Dong J, Chen Z,
Wang R, Zhang S, Cao H, Lu D, Jin T, et al: Berberine inhibits EGFR
signaling and enhances the antitumor effects of EGFR inhibitors in
gastric cancer. Oncotarget. 7:76076–76086. 2016.PubMed/NCBI
|
|
26
|
Wang H, Li K, Ma L, Wu S, Hu J, Yan H,
Jiang J and Li Y: Berberine inhibits enterovirus 71 replication by
downregulating the MEK/ERK signaling pathway and autophagy. Virol
J. 14:22017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Puthdee N, Seubwai W, Vaeteewoottacharn K,
Boonmars T, Cha'on U, Phoomak C and Wongkham S: Berberine induces
cell cycle arrest in cholangiocarcinoma cell lines via inhibition
of NF-κB and STAT3 pathways. Biol Pharm Bull. 40:751–757. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsu WH, Hsieh YS, Kuo HC, Teng CY, Huang
HI, Wang CJ, Yang SF, Liou YS and Kuo WH: Berberine induces
apoptosis in SW620 human colonic carcinoma cells through generation
of reactive oxygen species and activation of JNK/p38 MAPK and FasL.
Arch Toxicol. 81:719–728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xing Y, Ding T, Wang Z, Wang L, Guan H,
Tang J, Mo D and Zhang J: Temporally-controlled
photothermal/photodynamic and combined therapy for overcoming
multidrug resistance of cancer by polydopamine nanoclustered
micelles. ACS Appl Mater Interfaces. 2–Apr;2019.(Epub ahead of
print). doi: 10.1021/acsami.9b00472. View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Capriglione S, Luvero D, Plotti F,
Terranova C, Montera R, Scaletta G, Schirò T, Rossini G, Benedetti
Panici P and Angioli R: Ovarian cancer recurrence and early
detection: May HE4 play a key role in this open challenge? A
systematic review of literature. Med Oncol. 34:1642017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yi H, Zheng X, Song J, Shen R, Su Y and
Lin D: Exosomes mediated pentose phosphate pathway in ovarian
cancer metastasis: A proteomics analysis. Int J Clin Exp Pathol.
8:15719–15728. 2015.PubMed/NCBI
|
|
33
|
Zheng F, Wu J, Tang Q, Xiao Q, Wu W and
Hann SS: The enhancement of combination of berberine and metformin
in inhibition of DNMT1 gene expression through interplay of SP1 and
PDPK1. J Cell Mol Med. 22:600–612. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Wang N, Li H, Liu M, Cao F, Yu X,
Zhang J, Tan Y, Xiang L and Feng Y: Up-regulation of PAI-1 and
down-regulation of uPA are involved in suppression of invasiveness
and motility of hepatocellular carcinoma cells by a natural
compound berberine. Int J Mol Sci. 17:5772016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li D, Zhang Y, Liu K, Zhao Y, Xu B, Xu L,
Tan L, Tian Y, Li C, Zhang W, et al: Berberine inhibits
colitis-associated tumorigenesis via suppressing inflammatory
responses and the consequent EGFR signaling-involved tumor cell
growth. Lab Invest. 97:1343–1353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chu SC, Yu CC, Hsu LS, Chen KS, Su MY and
Chen PN: Berberine reverses epithelial-to-mesenchymal transition
and inhibits metastasis and tumor-induced angiogenesis in human
cervical cancer cells. Mol Pharmacol. 86:609–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H,
Fan Z, Cai J and Li Q: Berberine inhibits invasion and metastasis
of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3
signaling pathway. PLoS One. 10:e01234782015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan M, Zhang K, Shi Y, Feng L, Lv L and Li
B: Mechanism and pharmacological rescue of berberine-induced hERG
channel deficiency. Drug Des Devel Ther. 9:5737–5747.
2015.PubMed/NCBI
|
|
39
|
Zhang K, Zhi D, Huang T, Gong Y, Yan M,
Liu C, Wei T, Dong Z, Li B and Yang B: Berberine induces hERG
channel deficiency through trafficking inhibition. Cell Physiol
Biochem. 34:691–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xia J, Wang H, Li S, Wu Q, Sun L, Huang H
and Zeng M: Ion channels or aquaporins as novel molecular targets
in gastric cancer. Mol Cancer. 16:542017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arcangeli A and Becchetti A: Novel
perspectives in cancer therapy: Targeting ion channels. Drug Resist
Updat 21–22. 11–19. 2015. View Article : Google Scholar
|