Introduction
Lung cancer has been the leading cause of
cancer-associated mortality worldwide in males (24%) and females
(23%) in 2019 (1). Non-small cell
lung cancer (NSCLC) accounts for 85% of all types of lung cancer
(2). Platinum-based chemotherapy is
the standard treatment for patients with advanced epidermal growth
factor receptor (EGFR) wild-type NSCLC (3). Gemcitabine was approved as a first-line
treatment for advanced NSCLC (4–6).
However, the clinical outcome for patients with advanced stage
NSCLC remains poor, and novel effective treatment strategies are
required (7).
Immune checkpoint inhibitors have yielded promising
results in NSCLC. Programmed death ligand-1 (PD-L1) is an important
target for immunotherapy. Previous studies have revealed that PD-L1
expression may be a predictor of treatment response (8,9). High
expression of PD-L1 was associated with the presence of EGFR
mutations (10–12). Activating mutations of EGFR also
induced PD-L1 expression in NSCLC, and EGFR tyrosine kinase
inhibitors downregulated PD-L1 expression in EGFR mutation-positive
NSCLC (13–15). However, the predictive value of PD-L1
expression in patients with EGFR wild-type NSCLC remains unclear.
Furthermore, different chemotherapy regimens may affect the
clinical outcome (16,17). Therefore, the aim of the current
retrospective study was to analyze PD-L1 expression in patients
with advanced EGFR wild-type NSCLC treated with gemcitabine plus
cisplatin and to potentially determine the cut-off value of PD-L1
expression.
Materials and methods
Patients
A total of 172 eligible patients were enrolled in
the current study between August 2011 and December 2017 at The
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China). The inclusion criteria were as follows: i) Histologically
confirmed diagnosis of NSCLC based on the WHO classification
(18); ii) newly diagnosed with
cancer stage IIIB or IV; iii) ≥18 and ≤80 years of age; iv)
European Cooperative Oncology Group (ECOG) performance status (PS)
0–2; v) EGFR wild-type; vi) measurable disease according to revised
Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1
(19); vii) adequate hematological,
hepatic and organ function; and viii) adequate clinicopathological
information and follow-up data. The exclusion criteria were as
follows: i) Uncontrolled brain metastases; ii) autoimmune disease;
iii) previous malignant tumor or second primary tumor; and iv)
prior treatment with chemotherapy, radiotherapy or immunotherapy.
Clinicopathological characteristics were recorded for each patient,
including patient demographics, histology, EGFR status,
pretreatment serum C-reactive protein (CRP) level, date of
diagnosis, imaging of the involved region, cancer stage, ECOG PS,
smoking status, chemotherapy schedule, treatment response, PD-L1
expression, overall survival (OS) and progression-free survival
(PFS) times. OS was measured from the date of the initial therapy
until the last follow-up. PFS was measured from the date of the
initial treatment until the date of disease progression or death
from any cause. Patients were followed up at a median duration of 9
months (range, 2–25 months). The current study was approved by The
Ethics Committee of The First Affiliated Hospital of Zhengzhou
University, and written informed consent was obtained from all
enrolled patients. All experiments were performed in accordance
with approved guidelines and regulations (20,21).
PD-L1 expression
Pretreatment lung cancer tumor tissue was collected
for PD-L1 analyses. PD-L1 expression was retrospectively assessed
in tumor biopsies using immunohistochemical methods. Sections
(4-µm-thick) from each formalin-fixed (10% formaldehyde at 20°C for
24 h) paraffin-embedded tissue were used, followed by the modified
avidin-biotin complex method (Envision method) using an automated
immunostainer (model no. 314683; Ventana Medical Systems, Inc.,
Tucson, AZ, USA) (22). A rabbit
antihuman PD-L1 antibody (ready-to-use; cat. no. ZA-0629; OriGene
Technologies Inc., Beijing, China) was used to detect PD-L1, and
was incubated for 40 min at 37°C. The tissues were then incubated
with the horseradish peroxidase-anti-rat IgG secondary antibody
(ready-to-use; cat. no. 760-500; Roche, Basel, Switzerland) for 8
min at 37°C. The sections were counterstained with hematoxylin at
37°C for 4 min and then mounted. Images were taken using a light
microscope and analyzed using HistoQuest software (version 6.0;
TissueGnostics, Vienna, Austria) for an automated measurement.
PD-L1 expression was defined by tumor cell membrane expression
levels, and classified according to prespecified levels (≥1, ≥5,
≥10 and ≥50%) (23–25).
Treatment and response
Eligible patients received platinum-based doublet
chemotherapy (gemcitabine 1,000 mg/m2 on days 1 and 8;
cisplatin 25 mg/m2 on days 1, 2 and 3, repeated every 3
weeks; both from Hanson Pharma, Lianyungang, China). This treatment
is the standard of care for managing patients with advanced NSCLC
in China (7). Assessment of
treatment response was based on the RECIST version 1.1 guidelines
(19).
Statistical analysis
The association between PD-L1 expression and
clinicopathological characteristics was analyzed using the
χ2 test. Survival curves and rates were estimated using
the Kaplan-Meier method and groups were compared using the log-rank
test. Multivariate analysis using Cox proportional hazard
regression model was performed to evaluate the prognostic and
predictive role of PD-L1 expression. The hazard ratio (HR) and 95%
confidence interval (CI) were estimated using a stratified Cox
proportional hazards model. Statistical analyses were performed
using GraphPad Prism software (version 6; GraphPad Software Inc.,
La Jolla CA, USA) and SPSS software (version 21.0; IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The clinicopathological characteristics of the
enrolled patients are presented in Table
I. Among 172 patients, positive PD-L1 expression was observed
in 48.3% (84/172), 40.7% (70/172), 21.5% (37/172) and 8.1% (14/172)
of patients when using cut-off values of 1, 5, 10 and 50%,
respectively.
 | Table I.Association of PD-L1 expression and
clinicopathological characteristics (n=number of patients). |
Table I.
Association of PD-L1 expression and
clinicopathological characteristics (n=number of patients).
|
|
| PD-L1 (1% cut-off)
(n) | PD-L1 (5% cut-off)
(n) | PD-L1 (10% cut-off)
(n) | PD-L1 (50% cut-off)
(n) |
|---|
|
|
|
|
|
|
|
|---|
| Variables | No. of patients
(%) | Positive (84) | Negative (88) | P-value | Positive (70) | Negative (102) | P-value | Positive (37) | Negative (135) | P-value | Positive (14) | Negative (158) | P-value |
|---|
| Gender |
|
|
| 0.304 |
|
| 0.645 |
|
| 0.947 |
|
| 0.776 |
|
Female | 55 (32.0) | 30 | 25 |
| 21 | 34 |
| 12 | 43 |
| 4 | 51 |
|
|
Male | 117 (68.0) | 54 | 63 |
| 49 | 68 |
| 25 | 92 |
| 10 | 107 |
|
| Age (years) |
|
|
| 0.281 |
|
| 0.035 |
|
| 0.071 |
|
| 0.124 |
|
<60 | 83 (48.3) | 37 | 46 |
| 27 | 56 |
| 13 | 70 |
| 4 | 79 |
|
|
≥60 | 89 (51.7) | 47 | 42 |
| 43 | 46 |
| 24 | 65 |
| 10 | 79 |
|
| Histology |
|
|
| 0.634 |
|
| 0.905 |
|
| 0.757 |
|
| 0.568 |
|
Adenocarcinoma | 122 (70.9) | 61 | 61 |
| 50 | 72 |
| 27 | 95 |
| 9 | 113 |
|
|
Non-adenocarcinoma | 50 (29.1) | 23 | 27 |
| 20 | 30 |
| 10 | 40 |
| 5 | 45 |
|
| Stage |
|
|
| 0.001 |
|
| 0.001 |
|
| 0.001 |
|
| 0.018 |
|
IIIB | 89 (51.7) | 32 | 57 |
| 22 | 67 |
| 12 | 77 |
| 3 | 86 |
|
| IV | 83 (48.3) | 52 | 31 |
| 48 | 35 |
| 25 | 58 |
| 11 | 72 |
|
| ECOG PS |
|
|
| 0.706 |
|
| 0.291 |
|
| 0.368 |
|
| 0.160 |
|
0–1 | 104 (60.5) | 52 | 52 |
| 39 | 65 |
| 20 | 84 |
| 6 | 198 |
|
| 2 | 68 (39.5) | 32 | 36 |
| 31 | 37 |
| 17 | 51 |
| 8 | 60 |
|
| Smoking status |
|
|
| 0.551 |
|
| 0.908 |
|
| 0.812 |
|
| 0.856 |
| Former
or current smoker | 82 (47.7) | 42 | 40 |
| 33 | 49 |
| 17 | 65 |
| 7 | 75 |
|
|
Non-smoker | 90 (52.3) | 42 | 48 |
| 37 | 53 |
| 20 | 70 |
| 7 | 83 |
|
| CRP |
|
|
| 0.001 |
|
| 0.001 |
|
| 0.001 |
|
| 0.008 |
|
Elevated | 66 (38.4) | 43 | 23 |
| 37 | 29 |
| 23 | 43 |
| 10 | 56 |
|
|
Normal | 106 (61.7) | 41 | 65 |
| 33 | 73 |
| 14 | 92 |
| 4 | 102 |
|
Association of PD-L1 expression with
clinicopathological characteristics
The χ2 test revealed that elevated
pretreatment serum CRP (≥10 mg/l) was significantly associated with
positive PD-L1 expression for 1, 5, 10 and 50% cut-off values
(P=0.001, 0.001, 0.001 and 0.008, respectively). Similarly, stage
IV cancer was significantly associated with positive PD-L1
expression for 1, 5, 10 and 50% cut-off values (P=0.001, 0.001,
0.001 and 0.018, respectively; Table
I). These data suggested that PD-L1 expression was associated
with pretreatment serum CRP level and cancer stage.
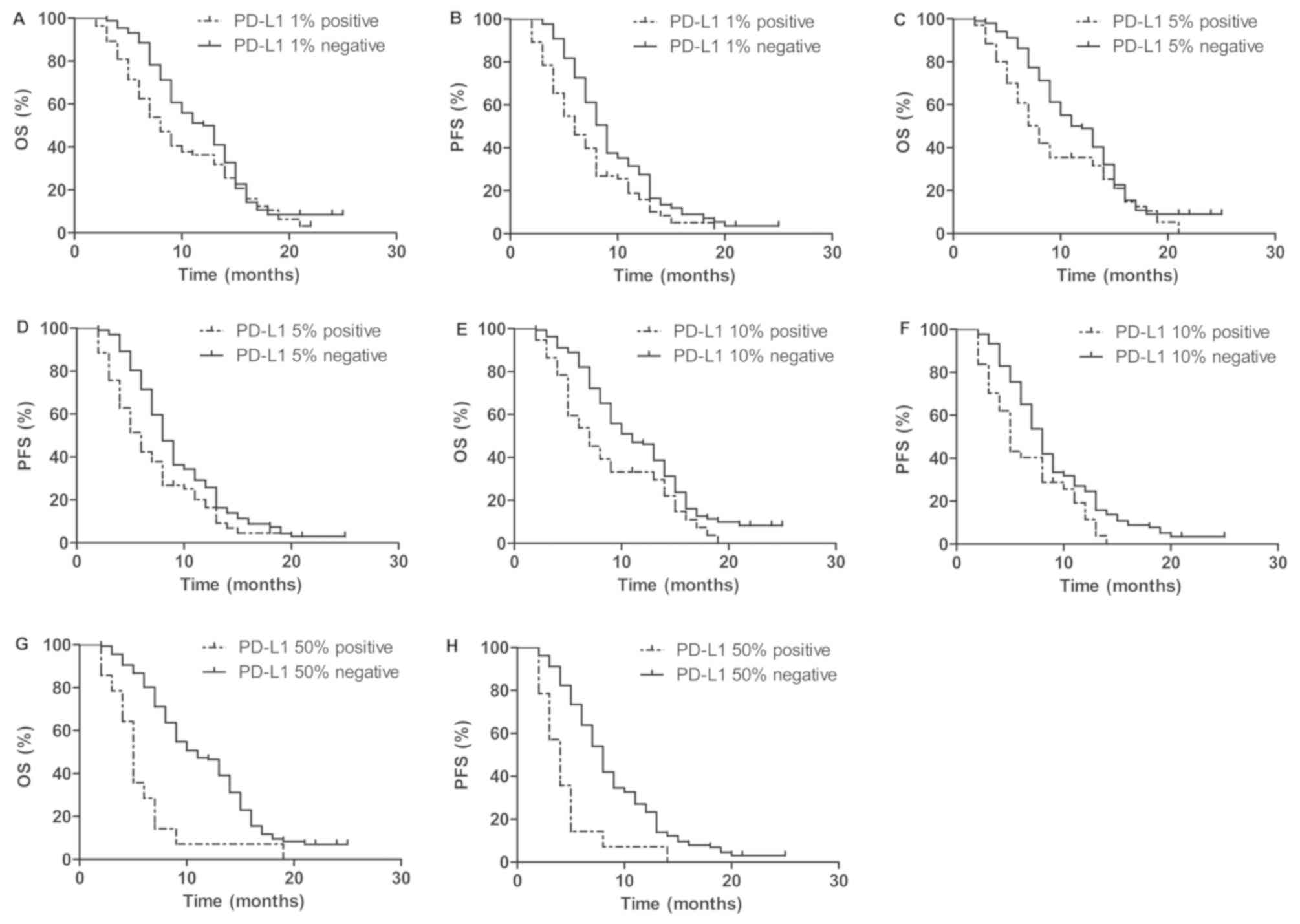
Survival time
To illustrate the prognostic value of PD-L1, the was
association between PD-L1 expression and OS and PFS times was
determined. At the median follow-up duration of 9 months, the
one-year OS and PFS times of all patients were 43.3 and 22.0%
respectively. Positive PD-L1 expression group was significantly
associated with shorter OS and PFS times compared with negative
PD-L1 expression group (Fig. 1). The
one-year OS time for positive PD-L1 expression group were shorter
than negative PD-L1 expression group (36.4 vs. 50% at 1% cut-off;
35.4 vs. 49.0% at 5% cut-off; 33.2 vs. 46.5% at 10% cut-off; and
7.1 vs. 46.2% at 50% cut-off, respectively). The one-year PFS time
for positive PD-L1 expression group were also shorter than negative
PD-L1 expression group (16.4 vs. 27.6% at 1% cut-off; 16.0 vs.
25.8% at 5% cut-off; 11.5 vs. 24.6% at 10% cut-off; and 7.0 vs.
23.3% at 50% cut-off, respectively; Fig.
2). Representative immunohistochemical staining images of tumor
biopsies with PD-L1 were shown in Figure
3. Univariate survival analysis revealed that cancer stage IV,
positive PD-L1 (1% cut-off) expression, ECOG PS 2 and age ≥60 were
significantly associated with shorter OS and PFS times
(P<0.0001, 0.0481, 0.0050 and <0.0001 for OS time;
P<0.0001, 0.0035, 0.0278 and 0.0010 for PFS time, respectively;
Table II). These results indicated
that positive PD-L1 (1% cut-off) expression predicted a shorter
survival time.
 | Table II.Univariate and multivariate analysis
of the association between clinicopathological characteristics and
survival. |
Table II.
Univariate and multivariate analysis
of the association between clinicopathological characteristics and
survival.
|
|
| OS | PFS |
|---|
|
|
|
|
|
|---|
|
|
|
| Multivariate
analysis |
| Multivariate
analysis |
|---|
|
|
|
|
|
|
|
|---|
| Variable | Category | Univariate analysis
P-value | HR (95% CI) | P-value | Univariate analysis
P-value | HR (95% CI) | P-value |
|---|
| Gender | Male | 0.094 |
|
| 0.681 |
|
|
| Age | ≥60 | <0.0001 | 1.537
(1.067–2.213) | 0.021 | 0.0010 | 1.298
(0.915–1.840) | 0.144 |
| Histology |
Non-adenocarcinoma | 0.5878 |
|
| 0.5422 |
|
|
| Stage | IV | <0.0001 | 1.700
(1.187–2.434) | 0.004 | <0.0001 | 1.860
(1.299–2.665) | 0.001 |
| ECOG PS | 2 | 0.0050 | 1.346
(0.937–1.935) | 0.108 | 0.0278 | 1.390
(0.971–1.988) | 0.072 |
| Smoking status | Non-smoker | 0.8710 |
|
| 0.7849 |
|
|
| CRP | Elevated | 0.6622 |
|
| 0.1117 |
|
|
| PD-L1 (1%
cut-off) | Positive | 0.0481 | 1.125
(0.783–1.617) | 0.524 | 0.0035 | 1.266
(0.884–1.813) | 0.199 |
Prognostic significance of PD-L1
expression cut-off values
To investigate the cut-off value of PD-L1
expression, the association between survival and PD-L1 expression
at 1, 5, 10 and 50% levels was investigated. Univariate survival
analysis revealed that positive PD-L1 expression for 1, 5, 10 and
50% cut-off values was significantly associated with shorter OS and
PFS times (OS time: P=0.0481, 0.0212, 0.0068 and <0.0001,
respectively; PFS time: P=0.0035, 0.0044, 0.0051 and <0.0001,
respectively; Fig. 1 and Table III). All parameters that were
statistically significant according to the univariate analysis were
included in the multivariate analysis, which demonstrated that
positive PD-L1 (50% cut-off) expression was significantly
associated with shorter survival time (OS time, P=0.001; HR=2.768,
95% CI, 1.551–4.940; PFS, P=0.002; HR=2.537, 95% CI, 1.423–4.524;
Table III). These results
suggested that positive PD-L1 (50% cut-off) expression was an
independent predictor of poor prognosis for patients with advanced
NSCLC treated with gemcitabine plus cisplatin.
 | Table III.Survival and PD-L1 expression
level. |
Table III.
Survival and PD-L1 expression
level.
|
| OS | PFS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| PD-L1 cut-off | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| ≥1% | 0.0481 | 1.434 | 1.003–2.051 | 0.524 | 1.125 | 0.783–1.617 | 0.0035 | 1.691 | 1.188–2.406 | 0.199 | 1.266 | 0.884–1.813 |
| ≥5% | 0.0212 | 1.558 | 1.069–2.272 | 0.807 | 1.049 | 0.716–1.537 | 0.0044 | 1.721 | 1.185–2.499 | 0.469 | 1.147 | 0.791–1.665 |
| ≥10% | 0.0068 | 1.952 | 1.202–3.171 | 0.084 | 1.421 | 0.954–2.117 | 0.0051 | 2.001 | 1.232–3.249 | 0.073 | 1.439 | 0.937–2.141 |
| ≥50% | <0.0001 | 7.768 | 3.031–19.91 | 0.001 | 2.768 | 1.551–4.940 | <0.0001 | 8.123 | 3.137–21.04 | 0.002 | 2.537 | 1.423–4.524 |
Discussion
Increased PD-L1 expression was observed in NSCLC and
neuroendocrine tumors of the lung (23,26),
suggesting that patients with NSCLC may benefit from PD-L1
inhibitors. The results obtained in the current study revealed that
high PD-L1 expression was observed in patients with advanced NSCLC,
compared with normal lung tissue.
There is no universal method for PD-L1
immunostaining and antibodies used in different studies vary
(21). The definition of a positive
PD-L1 test result differs depending on which biomarker assay is
used. Four immunohistochemical assays are approved by the US Food
and Drug Administration as diagnostic tests in advanced NSCLC
including PD-L1 IHC 22C3 pharmDx (Dako Omnis), PD-L1 IHC 28-8
pharmDx, VENTANA PD-L1 (SP142) assay and VENTANA PD-L1 (SP263)
assay (23). The comparative
accuracy and utility between the immunohistochemical assay used in
the current study and the aforementioned assays have been verified
(27).
Previously published studies reported conflicting
results on the association between PD-L1 expression and age,
gender, histology, ECOG PS, smoking status and cancer stage
(28). The present study revealed a
significant association between PD-L1 expression and cancer stage
and pretreatment serum CRP level. Expression of the PD-L1 gene may
be controlled by inflammatory signaling (29). Expression of the PD-L1 was regulated
by interferon-γ through the Janus kinase/signal transducer of
activation pathway in NSCLC (30). A
recently published study demonstrated that the serum CRP level was
associated with PD-L1 expression in patients with NSCLC (29). The inflammatory markers, CRP and
neutrophil-lymphocyte ratio, were predictive for the efficacy of
nivolumab in patients with NSCLC (29). The results obtained in the current
study indicated that pretreatment elevated serum CRP level was
associated with positive PD-L1 expression. However, future studies
are required to verify this association.
PD-L1 is a co-regulatory molecule that can be
expressed on tumor cells and its expression suppress T-cell
activity (31). Compared with PD-L2,
PD-L1 is the dominant inhibitory ligand of PD-1 on T cells
(25). Immune checkpoints inhibitors
which target the PD-L1/PD-1 pathway (including pembrolizumab and
nivolumab which target PD-1, and atezolizumab and duralumab which
target PD-L1) are a promising treatment method for patients with
advanced NSCLC (8,24,32–36).
Thus, the identification of potential biomarkers may guide the
choice of inhibitor used.
Currently known biomarkers include the ALK receptor
tyrosine kinase fusion oncogene, ROS proto-oncogene 1, receptor
tyrosine kinase gene rearrangements, sensitizing EGFR gene
mutations and B-Raf proto-oncogene, serine/threonine kinase V600E
point mutations (37,38). Activation of the immune checkpoint
pathways is one of the main mechanisms underlying tumor development
(39). PD-L1 expression on tumor
cells negatively regulates the immune response and may lead to
cancer progression (31). Although
PD-L1 expression may not be an optimal biomarker (40,41),
PD-L1 expression is currently used to assess whether patients with
NSCLC are candidates for treatment with pembrolizumab (42,43).
Identification of PD-L1 expression using immunohistochemical
methods may aid in treatment selection (21). Previously published studies have
reported conflicting results on whether positive PD-L1 expression
may be a predictor of treatment response (44,45). The
definition of positive PD-L1 expression is variable in different
studies and it may impact the results (21). Future studies are required to define
the prognostic role and cut-off value of PD-L1. A previous study
reported that a PD-L1 expression level of ≥50% was a positive test
result for first-line pembrolizumab therapy (8). In the present study, PD-L1 expression
predicted poor clinical outcome at the prespecified PD-L1
expression levels of 1, 5, 10 and 50% and results obtained
suggested that the optimal cut-off value may be 50%. The current
study was limited by the small sample size and retrospective
analysis. Therefore, a future prospective study with a larger
sample is required to validate the results obtained.
In conclusion, the present study demonstrated that
positive PD-L1 expression was associated with poor outcomes in
patients with advanced NSCLC treated with gemcitabine plus
cisplatin.
Acknowledgements
The authors would like to thank Miss Dandan Zhang of
The First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) for providing technical assistance for the present
study.
Funding
This present study was supported by the National
Natural Science Foundation of China (grant no. 81570203).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YJQ analyzed and interpreted the data of the study,
and wrote the manuscript. MZ, JJ and YRQ participated in the design
of this research. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldstraw P, Ball D, Jett JR, Le Chevalier
T, Lim E, Nicholson AG and Shepherd FA: Non-small-cell lung cancer.
Lancet. 378:1727–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hellmann MD, Li BT, Chaft JE and Kris MG:
Chemotherapy remains an essential element of personalized care for
persons with lung cancers. Ann Oncol. 27:1829–1835. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohe Y, Ohashi Y, Kubota K, Tamura T,
Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y and Fukuoka
M: Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Danson S, Middleton MR, O'Byrne KJ,
Clemons M, Ranson M, Hassan J, Anderson H, Burt PA, Fairve-Finn C,
Stout R, et al: Phase III trial of gemcitabine and carboplatin
versus mitomycin, ifosfamide, and cisplatin or mitomycin,
vinblastine, and cisplatin in patients with advanced nonsmall cell
lung carcinoma. Cancer. 98:542–553. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herbst RS, Baas P, Kim DW, Felip E,
Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui S, Su X, Dong L, Qian J, Ye L, Zhang
T, Fu H, Han H, Huang J, Yao Y, et al: Programmed cell death ligand
1 protein levels predicted survival of non-small cell lung cancer.
J Cancer. 8:4075–4082. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azuma K, Ota K, Kawahara A, Hattori S,
Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, et
al: Association of PD-L1 overexpression with activating EGFR
mutations in surgically resected nonsmall-cell lung cancer. Ann
Oncol. 25:1935–1940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin C, Chen X, Li M, Liu J, Qi X, Yang W,
Zhang H, Cai Z, Dai Y and Ouyang X: Programmed death-ligand 1
expression predicts tyrosine kinase inhibitor response and better
prognosis in a cohort of patients with epidermal growth factor
receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer.
16:e25–e35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang N, Zeng Y, Du W, Zhu J, Shen D, Liu
Z and Huang JA: The EGFR pathway is involved in the regulation of
PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in
EGFR-mutated non-small cell lung cancer. Int J Oncol. 49:1360–1368.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin K, Cheng J, Yang T, Li Y and Zhu B:
EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through
inhibiting NF-kappaB. Biochem Biophys Res Commun. 463:95–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akbay EA, Koyama S, Carretero J, Altabef
A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp
EM, Pugh TJ, et al: Activation of the PD-1 pathway contributes to
immune escape in EGFR-driven lung tumors. Cancer Discov.
3:1355–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen N, Fang W, Zhan J, Hong S, Tang Y,
Kang S, Zhang Y, He X, Zhou T, Qin T, et al: Upregulation of PD-L1
by EGFR activation mediates the immune escape in EGFR-Driven NSCLC:
Implication for optional immune targeted therapy for NSCLC patients
with EGFR mutation. J Thorac Oncol. 10:910–923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fossella F, Pereira JR, von Pawel J,
Pluzanska A, Gorbounova V, Kaukel E, Mattson KV, Ramlau R, Szczesna
A, Fidias P, et al: Randomized, multinational, phase III study of
docetaxel plus platinum combinations versus vinorelbine plus
cisplatin for advanced non-small-cell lung cancer: The TAX 326
study group. J Clin Oncol. 21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun C, Mezzadra R and Schumacher TN:
Regulation and function of the PD-L1 checkpoint. Immunity.
48:434–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thunnissen E, de Langen AJ and Smit EF:
PD-L1 IHC in NSCLC with a global and methodological perspective.
Lung Cancer. 113:102–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramos-Vara JA and Miller MA: Comparison of
two polymer-based immunohistochemical detection systems: ENVISION+
and ImmPRESS. J Microsc. 224:135–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brody R, Zhang Y, Ballas M, Siddiqui MK,
Gupta P, Barker C, Midha A and Walker J: PD-L1 expression in
advanced NSCLC: Insights into risk stratification and treatment
selection from a systematic literature review. Lung Cancer.
112:200–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen X and Zhao B: Efficacy of PD-1 or
PD-L1 inhibitors and PD-L1 expression status in cancer:
Meta-analysis. BMJ. 362:k35292018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takada K, Okamoto T, Toyokawa G, Kozuma Y,
Matsubara T, Haratake N, Akamine T, Takamori S, Katsura M, Shoji F,
et al: The expression of PD-L1 protein as a prognostic factor in
lung squamous cell carcinoma. Lung Cancer. 104:7–15. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kerr KM, Tsao MS, Nicholson AG, Yatabe Y,
Wistuba II, Hirsch FR and IASLC Pathology Committee: Programmed
death-ligand 1 immunohistochemistry in lung cancer: In what state
is this art? J Thorac Oncol. 10:985–989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang YL, Yang CY, Huang YL, Wu CT and
Yang PC: High PD-L1 expression is associated with stage IV disease
and poorer overall survival in 186 cases of small cell lung
cancers. Oncotarget. 8:18021–18030. 2017.PubMed/NCBI
|
|
29
|
Akamine T, Takada K, Toyokawa G, Kinoshita
F, Matsubara T, Kozuma Y, Haratake N, Takamori S, Hirai F, Tagawa
T, et al: Association of preoperative serum CRP with PD-L1
expression in 508 patients with non-small cell lung cancer: A
comprehensive analysis of systemic inflammatory markers. Surg
Oncol. 27:88–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ikeda S, Okamoto T, Okano S, Umemoto Y,
Tagawa T, Morodomi Y, Kohno M, Shimamatsu S, Kitahara H, Suzuki Y,
et al: PD-L1 is upregulated by simultaneous amplification of the
PD-L1 and JAK2 genes in non-small cell lung cancer. J Thorac Oncol.
11:62–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horn L, Spigel DR, Vokes EE, Holgado E,
Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L,
et al: Nivolumab versus docetaxel in previously treated patients
with advanced non-small-cell lung cancer: Two-year outcomes from
two randomized, open-label, phase III trials (checkmate 017 and
checkmate 057). J Clin Oncol. 35:3924–3933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brahmer J, Reckamp KL, Baas P, Crino L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: Durvalumab after chemoradiotherapy in stage III non-small-cell
lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sequist LV, Martins RG, Spigel D, Grunberg
SM, Spira A, Janne PA, Joshi VA, McCollum D, Evans TL, Muzikansky
A, et al: First-line gefitinib in patients with advanced
non-small-cell lung cancer harboring somatic EGFR mutations. J Clin
Oncol. 26:2442–2449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miller VA, Riely GJ, Zakowski MF, Li AR,
Patel JD, Heelan RT, Kris MG, Sandler AB, Carbone DP, Tsao A, et
al: Molecular characteristics of bronchioloalveolar carcinoma and
adenocarcinoma, bronchioloalveolar carcinoma subtype, predict
response to erlotinib. J Clin Oncol. 26:1472–1478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Petrelli F, Maltese M, Tomasello G, Conti
B, Borgonovo K, Cabiddu M, Ghilardi M, Ghidini M, Passalacqua R,
Barni S and Brighenti M: Clinical and molecular predictors of PD-L1
expression in non-small-cell lung cancer. Systematic Clin Lung
Cancer. 19:315–322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martinez P, Peters S, Stammers T and Soria
JC: Immunotherapy for the first-line treatment of patients with
metastatic non-small cell lung cancer. Clin Cancer Res. 2019.
View Article : Google Scholar
|
|
42
|
Kerr KM and Nicolson MC: Non-small cell
lung cancer, PD-L1, and the pathologist. Arch Pathol Lab Med.
140:249–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kerr KM and Hirsch FR: Programmed death
ligand-1 immunohistochemistry: Friend or foe? Arch Pathol Lab Med.
140:326–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou Y, Chen C, Zhang X, Fu S, Xue C, Ma
Y, Fang W, Yang Y, Hou X, Huang Y, et al: Immune-checkpoint
inhibitor plus chemotherapy versus conventional chemotherapy for
first-line treatment in advanced non-small cell lung carcinoma: A
systematic review and meta-analysis. J Immunother Cancer.
6:1552018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bethmann D, Feng Z and Fox BA:
Immunoprofiling as a predictor of patient's response to cancer
therapy-promises and challenges. Curr Opin Immunol. 45:60–72. 2017.
View Article : Google Scholar : PubMed/NCBI
|