Introduction
Peripheral T-cell lymphoma not otherwise specified
(PTCL-NOS) is a relatively rare disease, accounting for ~6% of all
non-Hodgkin lymphomas (NHL) (1);
however, it is more common in Asian than in Western countries
(2,3). Patients with PTCL-NOS usually present
with an aggressive clinical course and poor prognosis with frequent
relapses (4). Therefore, a number of
immunophenotypical and biological factors have been studied to
determine their prognostic and predictive role in PTCL-NOS
(4).
The NME/NM23 nucleoside diphosphate kinase 1
(nm23) gene is an important metastasis suppressor gene,
whose downregulation triggers metastatic progression. A number of
previous studies have shown that the nm23 protein was expressed at
a low level in a variety of metastatic human carcinomas, including
laryngeal squamous cell carcinoma (5), breast carcinoma (6), hepatocellular carcinoma (7) and gastric carcinoma (8), suggesting that the low expression of
nm23 may increase their metastatic potential. The opposite results
have been reported in lymphomas. It has been identified that
aggressive NHL exhibited significantly higher expression levels of
nm23 compared with indolent NHL (9–11). In
addition, nm23 overexpression was associated with poor prognosis in
aggressive lymphomas, such as diffuse large B-cell lymphoma (DLBCL)
and PTCL-NOS (9–11).
Nuclear DNA topoisomerase 2-α (TOP2A) is an
essential enzyme required for DNA replication and transcription, as
it controls and alters the topological states of DNA (12). The overexpression of TOP2A in
carcinomas has been shown to be a reliable proliferation marker and
to suggest poor prognosis (12–15). The
same results were confirmed in mantle cell lymphoma (16), with shorter survival in patients with
higher TOP2A.
Interferon regulatory factor 4/multiple myeloma
oncogene-1 (MUM-1) is a member of the interferon regulatory factor
family of transcriptional factors (17). It is usually expressed in aggressive
B-cell lymphomas (17). A recent
study suggested that the MUM-1 expression may be associated with
poor survival outcomes in patients with PTCL (18).
Vascular endothelial growth factor (VEGF) is an
important signaling protein belonging to the platelet-derived
growth factor family produced by cells that is involved in
vasculogenesis and angiogenesis (19). VEGF may also be beneficial in the
development of hematolymphoid cells (20). A number of studies have shown that
VEGF overexpression was associated with a worse prognosis and tumor
progression in classical Hodgkin and NHL (21,22). It
has been reported that elevated VEGF expression levels may be an
indicator of biologically aggressive DLBCL (20).
Although the roles of nm23, TOP2A, MUM-1 and VEGF
have been investigated in certain types of lymphomas, thus far, to
the best of our knowledge, there have been only a limited number of
studies on PTCL-NOS. The aim of the present study was to determine
the expression of nm23, TOP2A, MUM-1 and VEGF in PTCL-NOS, and
evaluate the clinical and prognostic significance.
Materials and methods
Case selection
All cases of T-cell lymphoma diagnosed between
November 1999 and October 2011 at the National Cancer
Center/National Clinical Research Center for Cancer/Cancer
Hospital, Chinese Academy of Medical Sciences and Peking Union
Medical College (Beijing, China) were reviewed. All cases were
reclassified according to the 2017 World Health Organization
classification of tumors of hematopoietic and lymphoid tissues by
two experienced hematopathologists using a multi-head microscope
(4). When there were different
opinions regarding a case, there was discussion until an agreement
was reached. A total of 124 cases [88 men and 36 women with a
median age of 49 years (range, 4–88 years)] of de novo
PTCL-NOS were reassessed following hematoxylin and eosin and
immunohistochemical staining, and included in the study. The
corresponding bone marrow aspiration smear and bone marrow biopsy
slice, as well as cerebrospinal fluid smear were also reviewed by
two experienced pathologists using a multi-head microscope, to
identify whether tumor cells were present.
Clinical information, including age at diagnosis,
gender, primary site, number and sites of involvement, Ann Arbor
stage (23), International
Prognostic Index (IPI) score (24),
treatment regimens, response to therapy and overall survival (OS),
was collected from the patients' medical records. The last
follow-up date was November 16, 2017. This was a retrospective
study therefore it was exempt from consent, which was approved by
the Ethics Committee of the Cancer Hospital, Chinese Academy of
Medical Sciences.
Immunohistochemical analysis
All the samples were fixed in 10% formalin at room
temperature for 6 to 48 h. Immunohistochemical analysis was
performed on 4 µm paraffin-embedded tissue sections using an
autostainer, a Ventana Benchmark XT (Ventana Medical Systems, Inc),
according to the manufacturer's protocols. The panel of monoclonal
antibodies included nm23 (clone 37.6; cat. no. sc-56928; Genetex
Santa Cruz Biotechnology, Inc.), TOP2A (clone 3F6; cat. no.
MAB9689; Abnova), MUM-1 (clone MUM1p; cat. no. IS644; Agilent
Technologies, Inc.) and VEGF (clone A20; cat. no. sc-152; Santa
Cruz Biotechnology, Inc.). All of these four antibodies were
working solution purchased from ZSGB-Bio, Beijing. The temperature
for the primary antibody was 37°C and the duration of incubation of
VEGF, NM23, and TOP2A was 32 min, and for MUM-1 it was 1 h. The
ultraView Universal horseradish peroxidase (HRP) Mutimer (cat. no.
05269806001; Roche Diagnostics) was used as the secondary antibody
and the temperature and duration of incubation was 37°C and 8 min,
respectively. The blocking reagent was included in the dispenser of
HRP Mutimer (<50 µg/ml), in the ultraView Universal DAB
Detection kit. The staining reagent used was the ultraView
Universal DAB Chromogen (0.2% DAB), DAB H2O2
(0.04% H2O2), DAB Copper (CuSO4 5
g/l), and temperature was 37°C, the duration of staining was 8 min.
Positive controls were used, and PBS was used as the negative
control, replacing the primary antibody. nm23 and VEGF exhibited
cytoplasmic staining, and TOP2A and MUM-1 nuclear staining. The
cut-offs for positive nm23, TOP2A, MUM-1 and VEGF expression were
≥30% of tumor cells, as previously reported (9,25). All
samples were observed and evaluated by two experienced
hematopathologists using a multi-head microscope.
Statistical analysis
OS was calculated from the date of diagnosis to the
date of death of the patient from any cause or last follow-up. The
one-year OS rate was calculated using the life-table method
(26,27). Patient survival was analyzed using
the Kaplan-Meier method and compared using the log-rank test.
Multivariate analysis was performed using Cox regression analysis.
Clinicopathological characteristics of different groups were
compared using the Fisher's exact or χ2 tests.
Statistical analysis was performed using SPSS 18.0 software (SPSS,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical features
The study group included 88 men and 36 women with a
median age of 49 years (range, 4–88 years). A total of 96 (77.4%)
patients had extranodal disease at diagnosis and 13 (13.5%)
presented with ≥2 extranodal sites of involvement. The extranodal
sites involved included bone marrow, central nervous system (CNS),
lungs, breasts, liver, spleen, skin, soft tissue, bones, small
intestine and nasopharynx. Bone marrow involvement was observed in
10/97 (10.3%) patients assessed. A total of 98 patients underwent
cerebrospinal fluid analysis or imaging examination, and tumor
cells in cerebrospinal fluid smear were detected in 2 (2.0%)
patients. The serum lactate dehydrogenase (LDH) level was elevated
in 31/79 (39.2%) patients assessed. A total of 58/104 (55.8%)
patients presented with a high Ann Arbor stage (III/IV) disease,
and 16/66 (24.2%) had high-intermediate to high IPI score (Table I).
 | Table I.The association between the
expression of m23, TOP2A, MUM-1 and VEGF and clinical features in
124 cases of peripheral T-cell lymphoma not otherwise
specified. |
Table I.
The association between the
expression of m23, TOP2A, MUM-1 and VEGF and clinical features in
124 cases of peripheral T-cell lymphoma not otherwise
specified.
|
| nm23 | TOP2A | MUM-1 | VEGF | Co-expression
nm23/TOP2A/VEGF |
|---|
|
|
|
|
|
|
|
|---|
| Feature | % patients
(P/E) | P-value | % patients
(P/E) | P-value | % patients
(P/E) | P-value | % patients
(P/E) | P-value | % patients
(P/E) | P-value |
|---|
| Age, years |
| 0.515 |
| 0.129 |
| 0.079 |
| 0.197 |
| 0.189 |
|
≤60 | 55.3 (52/94) |
| 54.3 (51/94) |
| 20.9 (19/91) |
| 48.9 (46/94) |
| 37.6 (35/93) |
|
|
>60 | 64.3 (18/28) |
| 71.4 (20/28) |
| 39.3 (11/28) |
| 64.3 (18/28) |
| 53.6 (15/28) |
|
| Sex |
| 0.225 |
| 0.841 |
| 0.106 |
| 0.554 |
| 0.160 |
|
Male | 61.6 (53/86) |
| 59.3 (51/86) |
| 29.8 (25/84) |
| 54.7 (47/86) |
| 45.9 (39/85) |
|
|
Female | 48.6 (17/35) |
| 57.1 (20/35) |
| 14.7 (5/34) |
| 48.6 (17/35) |
| 31.4 (11/35) |
|
| Serum LDH |
| 0.166 |
| 0.361 |
| 0.071 |
| 0.355 |
| 0.351 |
|
Normal | 59.6 (28/47) |
| 59.6 (28/47) |
| 39.1 (18/46) |
| 55.3 (26/47) |
| 43.5 (20/46) |
|
|
>Normal | 41.9 (13/31) |
| 48.4 (15/31) |
| 17.2 (5/29) |
| 41.9 (13/31) |
| 32.3 (10/31) |
|
| Ann Arbor
stage |
| 0.690 |
| 0.162 |
| 0.247 |
| 0.431 |
| 0.157 |
|
I–II | 51.1 (23/45) |
| 46.7 (21/45) |
| 18.6 (8/43) |
| 46.7 (21/45) |
| 31.1 (14/45) |
|
|
III–IV | 56.9 (33/58) |
| 62.1 (36/58) |
| 29.8 (17/57) |
| 55.2 (32/58) |
| 45.6 (26/57) |
|
| Bone marrow
involvement |
| 0.727 |
| 0.727 |
| 0.435 |
| 0.492 |
| 1.000 |
|
Absent | 55.8 (48/86) |
| 55.8 (48/86) |
| 28.9 (24/83) |
| 51.2 (44/86) |
| 40.0 (34/85) |
|
|
Present | 66.7 (6/9) |
| 66.7 (6/9) |
| 11.1 (1/9) |
| 66.7 (6/9) |
| 44.4 (4/9) |
|
| CNS
involvement |
| 1.000 |
| 1.000 |
| 1.000 |
| 1.000 |
| 1.000 |
|
Absent | 55.8 (53/95) |
| 55.8 (53/95) |
| 27.2 (25/92) |
| 51.6 (49/95) |
| 39.4 (37/94) |
|
|
Present | 50.0 (1/2) |
| 50.0 (1/2) |
| 0 (0/2) |
| 50.0 (1/2) |
| 50.0 (1/2) |
|
| Extranodal
sites |
| 1.000 |
| 0.226 |
| 0.060 |
| 0.370 |
| 0.529 |
| ≤2 | 47.7 (31/65) |
| 52.3 (34/65) |
| 23.8 (15/63) |
| 46.2 (30/65) |
| 34.4 (22/64) |
|
|
>2 | 46.2 (6/13) |
| 30.8 (4/13) |
| 0 (0/13) |
| 30.8 (4/13) |
| 23.1 (3/13) |
|
| IPI |
| 0.775 |
| 0.394 |
| 1.000 |
| 1.000 |
| 0.549 |
|
L/L-I | 48.0 (24/50) |
| 48 (24/50) |
| 31.9 (15/47) |
| 46.0 (23/50) |
| 32.7 (16/49) |
|
|
H-I/H | 56.3 (9/16) |
| 62.5 (10/16) |
| 31.3 (5/16) |
| 50.0 (8/16) |
| 43.8 (7/16) |
|
Expression of nm23, TOP2A, MUM-1 and VEGF in
PTCL-NOS. nm23, TOP2A and VEGF was performed on 122 samples. The
present study indicated that 70/122 (57.4%) cases were positive for
nm23, 71/122 (58.2%) cases for TOP2A, 30/119 (25.2%) for MUM-1, and
64/122 (52.5%) for VEGF. Of note, 50/122 cases concurrently
expressed nm23, TOP2A and VEGF (Fig.
1).
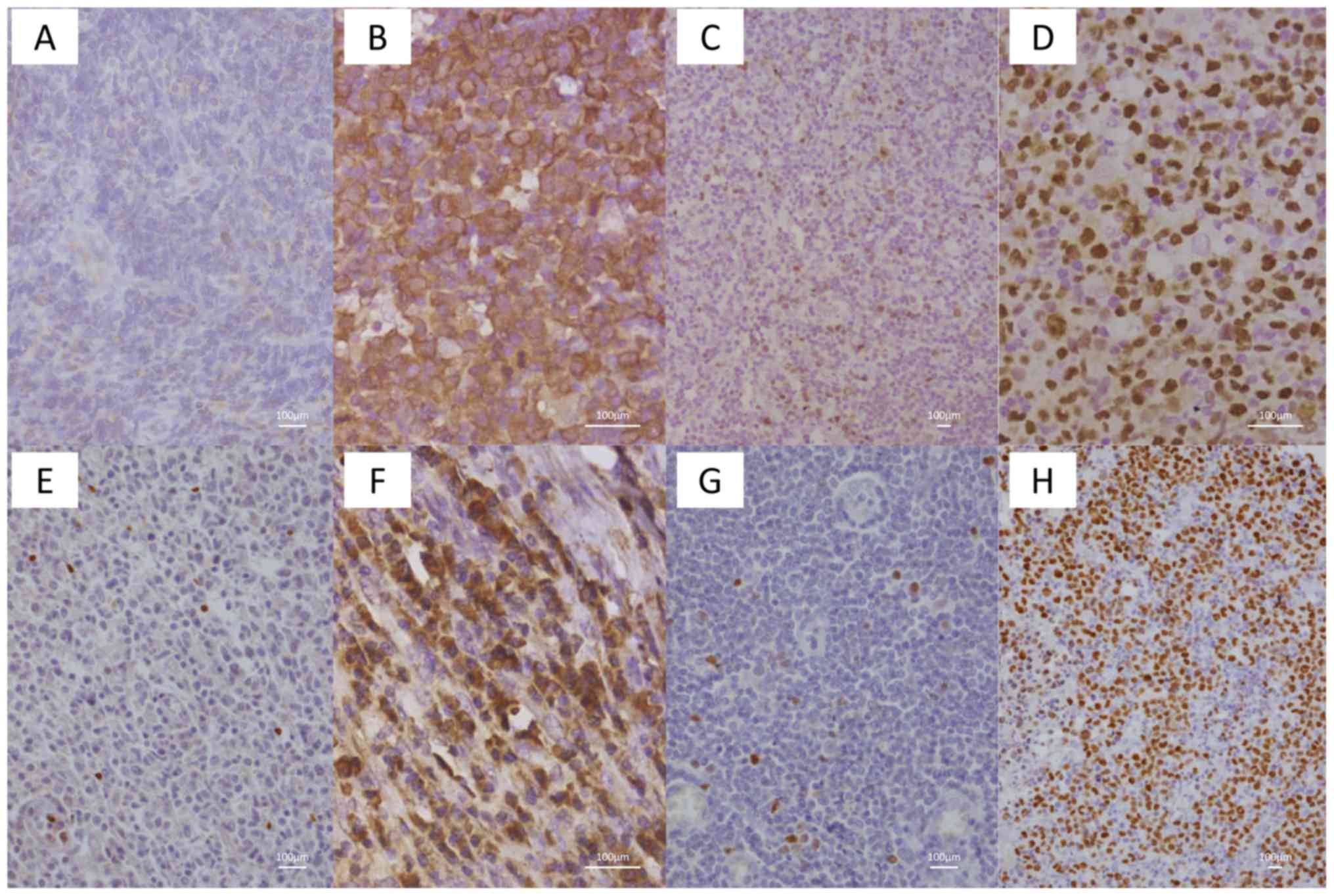 | Figure 1.Immunohistochemical staining of
peripheral T-cell lymphoma not otherwise specified for nm23, TOP2A,
VEGF and MUM-1. (A) nm23-positive cells in the negative case, 2±2%
(magnification, ×200). (B) nm23-positive cells in the positive
case, 90±5% (magnification, ×400). (C) TOP2A-positive cells in the
negative case, 10±3% (magnification, ×100). (D) TOP2A-positive
cells in the positive case, 80±5% (magnification, ×200). (E)
VEGF-positive cells in the negative case, 3±2% (magnification,
×200). (F) VEGF-positive cells in the positive case, 50±5%
(magnification, ×400). (G) MUM-1-positive cells in the negative
case, 5±2% (magnification, ×200). (H) MUM-1-positive cells in the
positive case, 70±5% (magnification, ×100). nm23, NME/NM23
nucleoside diphosphate kinase 1; TOP2A, topoisomerase 2-α; VEGF,
vascular endothelial growth factor; MUM-1, multiple myeloma
oncogene-1. |
Association between nm23, TOP2A, MUM-1
or VEGF expression and clinicopathological features of patients
with PTCL-NOS
TOP2A positivity in patients that were Ann Arbor
stage I–II was 46.7%, which was less than in Ann Arbor stage III–IV
(62.1%). However, association analysis by χ2 test did
not show any significance (P=0.162). In addition, the concurrent
expression of nm23, TOP2A and VEGF in Ann Arbor stages I–II and
III–IV were 31.1 and 45.6%, respectively (P=0.157), with no
significant difference observed. No statistical association was
identified between the nm23, TOP2A, MUM-1, VEGF or concurrent
nm23/TOP2A/VEGF expression and any other clinicopathological
feature, including age, sex, IPI score and serum LDH level
(Table I).
Prognostic analysis
Although 124 patients were enrolled in this study,
37 were lost in follow-up. With a median follow-up of 13.5 months
(range, 0.0–199.0 months), 13/87 (14.9%) patients died, most of
which (11/13, 84.6%) died within 12 months from diagnosis. The
one-year OS rate was 82% calculated by using the life-table
method.
The present results showed that high Ann Arbor stage
(III/IV) and high-intermediate/high IPI score were associated with
a worse OS (P=0.034 and P=0.032; Fig. 2A
and B). All other factors were assessed by univariate analysis
for their impact on OS. It was found that the nm23, TOP2A and VEGF
expression had a negative prognostic effect in patients with
PTCL-NOS (Fig. 2C-E). The OS of
nm23-, TOP2A- or VEGF-positive patients was significantly shorter
than that of nm23-(P=0.012), TOP2A-(P=0.002) or VEGF-negative
(P=0.008) patients. However, MUM-1 expression was not associated
with the prognosis of the patients with PTCL-NOS (P=0.918) (data
not shown). In patients with PTCL-NOS characterized by concurrent
nm23/TOP2A/VEGF expression, the OS was significantly worse than
that in other patients in this cohort (P=0.002; Fig. 2F). Other variables, including age,
sex and serum LDH level were not associated with the OS of patients
with PTCL-NOS (data not shown).
 | Figure 2.OS of patients with peripheral T-cell
lymphoma not otherwise specified. Patients were subdivided
according to (A) Ann Arbor Stage, (B) IPI, (C) nm23, (D) TOP2A, (E)
VEGF and (F) concurrent nm23, TOP2A and VEGF expression. OS,
overall survival; IPI, International Prognostic Index; nm23,
NME/NM23 nucleoside diphosphate kinase 1; TOP2A, topoisomerase 2-α;
VEGF, vascular endothelial growth factor. |
Based on the univariate analysis, nm23, TOP2A and
VEGF expression, and IPI score were assessed by multivariate Cox
regression analysis. The results showed that none of the variables
were independent prognostic factors (nm23, P=0.751; TOP2A, P=0.459;
VEGF, P=0.245). The concurrent expression of nm23, TOP2A and VEGF,
and IPI were also analyzed. It was found that the concurrent
expression of nm23, TOP2A and VEGF tended to predict a worse
prognosis, however the P-value was borderline [hazard ratio (HR),
1.495; 95% confidence interval (CI), 0.993–2.250; P=0.054]
(Table II).
 | Table II.Multivariate analysis of
clinicopathological features predictive of overall survival rate in
all patients with peripheral T-cell lymphoma not otherwise
specified. |
Table II.
Multivariate analysis of
clinicopathological features predictive of overall survival rate in
all patients with peripheral T-cell lymphoma not otherwise
specified.
| Features | Hazard ratio | 95% confidence
interval | P-value |
|---|
| IPI (H-I/H vs.
L/L-I) | 3.035 | 0.742–12.414 | 0.122 |
| Co-expression of
nm23/TOP2A/VEGF | 1.495 | 0.993–2.250 | 0.054 |
Discussion
PTCL-NOS, accounting for 60–70% of T-cell lymphomas,
does not conform to known entities of mature T-cell lymphoma in the
current classification (4,28). The majority of patients suffering
from it are adults, and the male to female ratio is usually 2:1
(4). Similar to previous studies
from Western and Asian countries (29–32), the
median age at diagnosis of this study's cohort was 49 years, and
there was a sex bias with a male to female ratio of 2.4:1.
PTCL-NOS is one of the most aggressive subtypes of
NHLs, and it is associated with poor prognosis (4). Patients with PTCL-NOS more often
exhibit a high Ann Arbor stage, high IPI score and more extranodal
sites of involvement at diagnosis (4,30). As
illustrated in the present study, 55.8% of patients had high Ann
Arbor stage (III/IV) disease. Furthermore, most patients (77.4%)
presented with extranodal sites of involvement, and 13.5% with ≥2
extranodal sites of involvement. It has been reported that the
3-year OS rate of PTCL-NOS was ~40% and the 5-year OS rate was ~30%
(33,34). Recently, a small cohort study on a
Japanese population with PTCL-NOS reported a 2-year OS rate of 61%
(29). In this study, there were 37
patients lost to follow-up. Since a number of the present cases had
a short follow-up time (median follow-up, 13.5 months), only the
1-year OS rate was assessed using the life-table method, which was
found to be 82%. In addition, 13/87 (14.9%) patients in this cohort
succumbed to the disease, most of them (11/13, 84.6%) within 12
months from diagnosis. Therefore, the present results also provided
evidence that patients with PTCL-NOS have an aggressive clinical
progress, generally consistent with previous reports (35,36). It
has been reported that the median time of failure-free survival was
only 3.1 months (35), and nearly
half of the patients experienced relapse or progression within 6
months after primary therapy (36).
Although many studies evaluating the contribution of
clinical and biological factors in influencing the prognosis of
PTCL-NOS have been performed, IPI score remains the most commonly
used and effective predictive prognostic factor (37,38). In
the past few years; however, several studies have demonstrated that
IPI score did not effectively predict the prognosis of PTCL-NOS
(28,39). Furthermore, it is considered that the
IPI score does not provide specific biological insight or potential
targets for drug therapy (28).
Therefore, various prognostic models of PTCL-NOS have been
investigated in previous studies, each of which incorporated
different clinical parameters (31,34).
Similar to IPI score, these prognostic models also did not include
tumor-specific biological factors, and can therefore not be used
for targeted therapies (31,34).
nm23, TOP2A, MUM-1 and VEGF are very common markers
used to estimate the progression, infiltration and metastasis
ability of many malignant tumors, particularly carcinomas (14,40–44).
Although their prognostic role in various carcinomas has been
widely investigated over time, such as breast (45), pancreatic (12) and renal cell carcinoma (42), to the best of our knowledge, a
limited number of have focused on their value in lymphomas.
In the present study, the expression of nm23, TOP2A,
MUM-1 and VEGF was examined in 122 cases of PTCL-NOS. The
association between their expression and clinicopathological
features, as well as their prognostic value, was analyzed. It was
found that 57.4% cases were positive for nm23, which was slightly
higher than in other studies (9,46).
Similar to the nm23 expression, >50% of cases were positive for
TOP2A (58.2%) and VEGF (52.5%). Of note, 50/122 (41.0%) cases
concurrently expressed nm23, TOP2A and VEGF. This study therefore
speculated that there may be an association among them. Further
studies are required to illustrate the mechanism of their
interactions. No significant difference was observed between the
expression of nm23, TOP2A, MUM-1 or VEGF, and clinicopathological
characteristics.
Consistent with several previous studies (9,46,47), the
univariate analysis results showed that the expression of nm23,
TOP2A and VEGF was associated with a poor prognosis in patients
with PTCL-NOS. Similarly, concurrent nm23/TOP2A/VEGF expression
predicted a worse prognosis for patients with PTCL-NOS. However,
multivariate Cox regression analysis revealed that only concurrent
nm23/TOP2A/VEGF expression was usually associated with an inferior
outcome in patients with PTCL-NOS, however no significance was
observed. Due to the short follow-up time in this cohort, further
studies are required.
In conclusion, the present results indicated that
the expression of nm23, TOP2A and VEGF could serve as a promising
prognostic factor for PTCL-NOS, and could be included in an
effective prognostic model for PTCL-NOS. In addition, the
development of novel treatments targeting nm23, TOP2A and VEGF is
required for patients diagnosed with PTCL-NOS.
Acknowledgements
Not applicable.
Funding
This research was supported by the Capital Clinical
Characteristic Application Research (grant no. Z141107002514046)
from Beijing Municipal Science & Technology Commission.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH contributed to experiment guidance, data analysis
and manuscript writing. ZC and LZ contributed to clinical follow-up
and acquisition of clinical data. LG contributed to tissue
collection and performing immunohistochemistry staining. XL
contributed to immunohistochemistry staining. NL contributed to the
study design. XF contributed to coordination, discussion and
manuscript editing, and provided data interpretation. All authors
commented on the manuscript and have read and approved the final
version of this manuscript.
Ethics approval and consent to
participate
This study was a retrospective study, and the ethics
approval of this study was obtained from the Independent Ethics
Committee of Cancer Hospital, Chinese Academy of Medical Sciences,
National GCP Center for Anticancer Drugs (approval no.
NCC2014ST-08).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PTCL-NOS
|
peripheral T-cell lymphoma, not
otherwise specified
|
|
TOP2A
|
nuclear DNA topoisomerase 2-α
|
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
MUM-1
|
multiple myeloma oncogene-1
|
|
OS
|
overall survival
|
|
HR
|
hazard ratio
|
|
IPI
|
International Prognostic Index
|
References
|
1
|
You JM: Peripheral T-cell Lymphoma, Not
Otherwise Specified. Medeiros LJ, Miranda RN, Wang SA, Vega F,
Muzzafar T, Yin CC, Bueso-Ramos CE and Lin P: Diagnostic pathology
lymph nodes and spleen with extranodal lymphomas. Lippincott
Williams & Wilkins; Baltimore, MD: pp. 1006–1027. 2011
|
|
2
|
Dotlic S, Perry AM, Petrusevska G, Fetica
B, Diebold J, MacLennan KA, Müller-Hermelink HK, Nathwani BN,
Boilesen E, Bast M, et al: Classification of non-Hodgkin lymphoma
in South-eastern Europe: Review of 632 cases from the international
non-Hodgkin lymphoma classification project. Br J Haematol.
171:366–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park S and Ko YH: Peripheral T cell
lymphoma in Asia. Int J Hematol. 99:227–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pileri SA, Weisenburger DD, Sng I,
Nakamura S, Muller-Hermelink HK, Chan WC and Jaffe ES: Peripheral
T-Cell Lymphoma, NOS. Swerdlow EH, Harris NL, Jaffe ES, Pileri SA,
Stein H and Thiele J: WHO classification of tumours of
haematopoietic and lymphoid tissues. IARC; Lyon: pp. 403–407.
2017
|
|
5
|
Zheng Z, Tian R and Wang P: Roles of KAI1
and nm23 in lymphangiogenesis and lymph metastasis of laryngeal
squamous cell carcinoma. World J Surg Oncol. 15:2112017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Durán E, Cárdenas JM, Reina MÁ and Arriazu
R: Loss of Nm23 is associated with a more favorable tumor
microenvironment in patients with breast cancer. Histol
Histopathol. 30:345–352. 2015.PubMed/NCBI
|
|
7
|
Khera L, Paul C and Kaul R: Hepatitis C
Virus E1 protein promotes cell migration and invasion by modulating
cellular metastasis suppressor Nm23-H1. Virology. 506:110–120.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Radović S, Dorić M, Hukić A, Babić M,
Kuskunović S and Spahović N: Immunohistochemical expression and
significance of NM23 suppressor protein in primary gastric
adenocarcinoma. Bosn J Basic Med Sci. 13:72–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niitsu N, Nakamine H and Okamoto M:
Expression of nm23-H1 is associated with poor prognosis in
peripheral T-cell lymphoma, not otherwise specified. Clin Cancer
Res. 17:2893–2899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niitsu N, Okabe-Kado J, Kasukabe T,
Yamamoto-Yamaguchi Y, Umeda M and Honma Y: Prognostic implications
of the differentiation inhibitory factor nm23-H1 protein in the
plasma of aggressive non-Hodgkin's lymphoma. Blood. 94:3541–3550.
1999.PubMed/NCBI
|
|
11
|
Niitsu N, Nakamine H, Okamoto M, Akamatsu
H, Higashihara M, Honma Y, Okabe-Kado J and Hirano M: Clinical
significance of intracytoplasmic nm23-H1 expression in diffuse
large B-cell lymphoma. Clin Cancer Res. 10:2482–2490. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Z, Liu S, Zhang M, Zhou R, Liu J,
Chang Y and Zhao Q: overexpression of topoisomerase 2-alpha confers
a poor prognosis in pancreatic adenocarcinoma identified by
co-expression analysis. Dig Dis Sci. 62:2790–2800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen D, Maruschke M, Hakenberg O,
Zimmermann W, Stief CG and Buchner A: TOP2A, HELLS, ATAD2, and TET3
are novel prognostic markers in renal cell carcinoma. Urology.
102:265.e1–e7. 2017. View Article : Google Scholar
|
|
14
|
Ito F, Furukawa N and Nakai T: Evaluation
of TOP2A as a predictive marker for endometrial cancer with
Taxane-containing adjuvant chemotherapy. Int J Gynecol Cancer.
26:325–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El Rebey HS, Aiad HA, Abulkheir IL, Asaad
NY, El-Wahed MM, Abulkasem FM and Mahmoud SF: The predictive and
prognostic role of topoisomerase IIα and tissue inhibitor of
metalloproteinases 1 expression in locally advanced breast
carcinoma of egyptian patients treated with anthracycline-based
neoadjuvant chemotherapy. Appl Immunohistochem Mol Morphol.
24:167–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brizova H, Kalinova M, Krskova L, Mrhalova
M and Kodet R: A novel quantitative PCR of proliferation markers
(Ki-67, topoisomerase IIalpha and TPX2): An immunohistochemical
correlation, testing, and optimizing for mantle cell lymphoma.
Virchows Arch. 456:671–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heo MH, Park HY, Ko YH, Kim WS and Kim SJ:
IRF4/MUM1 expression is associated with poor survival outcomes in
patients with peripheral T-cell lymphoma. J Cancer. 8:1018–1024.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Nakayama M, Pitulescu ME, Schmidt
TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U,
et al: Ephrin-B2 controls VEGF-induced angiogenesis and
lymphangiogenesis. Nature. 465:483–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Medinger M, Fischer N and Tzankov A:
Vascular endothelial growth factor-related pathways in
hemato-lymphoid malignancies. J Oncol 2010. 7297252010.
|
|
21
|
Koh YW, Park C, Yoon DH, Suh C and Huh J:
Prognostic significance of COX-2 expression and correlation with
Bcl-2 and VEGF expression, microvessel density and clinical
variables in classical Hodgkin lymphoma. Am J Surg Pathol.
37:1242–1251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Li W, He X, Zhang G, Yue L and
Chai Y: VEGF overexpression is a valuable prognostic factor for
non-Hodgkin's lymphoma evidence from a systemic meta-analysis. Dis
Markers 2015. 7867902015.
|
|
23
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on Hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
24
|
International Non-Hodgkin's Lymphoma
Prognostic Factors Project: A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee WJ, Kim YJ, Lee YJ, Won CH, Chang SE,
Choi JH and Lee MW: Vascular endothelial growth factor protein
expression is associated with a poor prognosis in patients with
cutaneous extranodal natural killer/T-cell lymphoma. Br J Dermatol.
178:e11–e12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen JG, Chen HZ, Zhu J, Yang YL, Zhang
YH, Huang PX, Chen YS, Zhu CY, Yang LP, Shen K, et al: Cancer
survival in patients from a hospital-based cancer registry, China.
J Cancer. 9:851–860. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krzanowski M, Drelicharz L, Belowski A,
Partyka L, Sumek-Brandys B, Ramakrishnan PK and Nizankowski R:
Costs of Real-life endovascular treatment of critical limb
ischemia: Report from Poland-A European union country with a
low-budget health care system. Ann Vasc Surg. 31:111–123. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piccaluga PP, Agostinelli C, Gazzola A,
Mannu C, Bacci F, Sabattini E and Pileri SA: Prognostic markers in
peripheral T-cell lymphoma. Curr Hematol Malig Rep. 5:222–228.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suzuki T, Kawamoto K, Tamura S, Uemura S,
Kaihatsu A, Nemoto H, Kobayashi H, Ushiki T, Fuse K, Shibazaki Y,
et al: Peripheral T-cell lymphoma, not otherwise specified: A
retrospective single-center analysis. Rinsho Ketsueki. 58:905–911.
2017.(In Japanese). PubMed/NCBI
|
|
30
|
Lee Y, Uhm JE, Lee HY, Park MJ, Kim H, Oh
SJ, Jang JH, Kim K, Jung CW, Ahn YC, et al: Clinical features and
prognostic factors of patients with ‘peripheral T cell lymphoma,
unspecified’. Ann Hematol. 88:111–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gallamini A, Stelitano C, Calvi R, Bellei
M, Mattei D, Vitolo U, Morabito F, Martelli M, Brusamolino E,
Iannitto E, et al: Peripheral T-cell lymphoma unspecified (PTCL-U):
A new prognostic model from a retrospective multicentric clinical
study. Blood. 103:2474–2479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang QP, Zhang WY, Yu JB, Zhao S, Xu H,
Wang WY, Bi CF, Zuo Z, Wang XQ, Huang J, et al: Subtype
distribution of lymphomas in Southwest China: Analysis of 6,382
cases using WHO classification in a single institution. Diagn
Pathol. 6:772011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vose J, Armitage J and Weisenburger D;
International T-Cell Lymphoma Project, : International peripheral
T-cell and natural killer/T-cell lymphoma study: Pathology findings
and clinical outcomes. J Clin Oncol. 26:4124–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Federico M, Bellei M, Marcheselli L,
Schwartz M, Manni M, Tarantino V, Pileri S, Ko YH, Cabrera ME,
Horwitz S, et al: Peripheral T cell lymphoma, not otherwise
specified (PTCL-NOS). A new prognostic model developed by the
International T cell Project Network. Br J Haematol. 181:760–769.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chihara D, Fanale MA, Miranda RN, Noorani
M, Westin JR, Nastoupil LJ, Hagemeister FB, Fayad LE, Romaguera JE,
Samaniego F, et al: The survival outcome of patients with
relapsed/refractory peripheral T-cell lymphoma-not otherwise
specified and angioimmunoblastic T-cell lymphoma. Br J Haematol.
176:750–758. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mak V, Hamm J, Chhanabhai M, Shenkier T,
Klasa R, Sehn LH, Villa D, Gascoyne RD, Connors JM and Savage KJ:
Survival of patients with peripheral T-cell lymphoma after first
relapse or progression: Spectrum of disease and rare long-term
survivors. J Clin Oncol. 31:1970–1976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weisenburger DD, Savage KJ, Harris NL,
Gascoyne RD, Jaffe ES, MacLennan KA, Rüdiger T, Pileri S, Nakamura
S, Nathwani B, et al: Peripheral T-cell lymphoma, not otherwise
specified: A report of 340 cases from the International Peripheral
T-cell Lymphoma Project. Blood. 117:3402–3408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu P, Yu D, Wang L, Shen Y, Shen Z and
Zhao W: Analysis of prognostic factors and comparison of prognostic
scores in peripheral T cell lymphoma, not otherwise specified: A
single-institution study of 105 Chinese patients. Ann Hematol.
94:239–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Savage KJ, Harris NL, Vose JM, Ullrich F,
Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne
RD, et al: ALK-anaplastic large-cell lymphoma is clinically and
immunophenotypically different from both ALK+ ALCL and peripheral
T-cell lymphoma, not otherwise specified: Report from the
International Peripheral T-Cell Lymphoma Project. Blood.
111:5496–5504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marioni G, Cappellesso R, Ottaviano G,
Fasanaro E, Marchese-Ragona R, Favaretto N, Giacomelli L, Guzzardo
V, Martini A, Fassina A and Blandamura S: Nuclear nonmetastatic
protein 23-H1 expression and epithelial-mesenchymal transition in
laryngeal carcinoma: A pilot investigation. Head Neck.
40:2020–2028. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fu JW and Chu XQ: Correlation between
non-metastatic protein 23 expression and clinicopathological
features of colorectal cancer in Asians. Genet Mol Res.
14:15597–15608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuan L, Chen L, Qian K, Qian G, Wu CL,
Wang X and Xiao Y: Co-expression network analysis identified six
hub genes in association with progression and prognosis in human
clear cell renal cell carcinoma (ccRCC). Genom Data. 14:132–140.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Piao J, Sun J, Yang Y, Jin T, Chen L and
Lin Z: Target gene screening and evaluation of prognostic values in
non-small cell lung cancers by bioinformatics analysis. Gene.
647:306–311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li K, Sun H, Lu Z, Xin J, Zhang L, Guo Y
and Guo Q: Value of [18F]FDG PET radiomic features and
VEGF expression in predicting pelvic lymphatic metastasis and their
potential relationship in early-stage cervical squamous cell
carcinoma. Eur J Radiol. 106:160–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han W, Zhang C, Cao FY, Cao F, Jiang L and
Ding HZ: Prognostic and clinicopathological value of NM23
expression in patients with breast cancer: A systematic review and
meta-analysis. Curr Probl Cancer. 41:80–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Niitsu N, Nakamine H, Okamoto M, Akamatsu
H, Honma Y, Higashihara M, Okabe-Kado J and Hirano M; Adult
Lymphoma Treatment Study Group ALTSG, : Expression of nm23-H1 is
associated with poor prognosis in peripheral T-cell lymphoma. Br J
Haematol. 123:621–630. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cuadros M, Dave SS, Jaffe ES, Honrado E,
Milne R, Alves J, Rodríguez J, Zajac M, Benitez J, Staudt LM and
Martinez-Delgado B: Identification of a proliferation signature
related to survival in nodal peripheral T-cell lymphomas. J Clin
Oncol. 25:3321–3329. 2007. View Article : Google Scholar : PubMed/NCBI
|
















