Introduction
Malignant tumors are a serious disease and since
1981 have the highest risk of death in Japan. In particular, colon
cancer is the second most common type of cancer death (1). The association between fruit and
vegetable intake and colorectal cancer risk has been investigated
by many epidemiologic studies (2–4). Fruit
and vegetables contain cancer-preventive agents, and dietary
chemoprevention strategies have gained significant interest
(5–7).
Sulforaphane is an isothiocyanate that is abundant
in cruciferous vegetables, including broccoli sprouts, cabbage, and
cauliflower (8,9). Several reports showed that
sulforaphane, a well-known phytochemical, induces cell cycle arrest
and apoptosis in cancer cells (10–14).
Sulforaphane is a promising compound for cancer prevention and
therapy (15–18). Sulforaphane downregulates the
expression of Bcl-2, inhibitor of apoptosis (cIAP)-1/2, and XIAP in
prostate cancer (14) and the
expression of Bcl-2 family proteins in human hepatoma cells
(19).
There are several reports that suggest that
Lactobacilli have antiumor activity. Takeda et al
(20) found that drinking fermented
products containing Lactobacilli have immunological effects
on the immune system of healthy individuals, and
Lactobacilli have anticancer effects in vitro and
in vivo (21–23). Ishikawa et al (24) found in randomized clinical studies
that a Lactobacillus strain prevents colorectal tumors.
Lactobacillus strains induce tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL), tumor necrosis factor (TNF) α,
interleukin (IL)-1β, and IL-10 from normal human peripheral blood
mononuclear cells (PBMCs) (25–27).
Izumo et al (28,29) and
Hayama et al (30) reported
that the Lactobacillus (L.) pentosus strain
S-PT84, isolated from Kyoto pickles, has inhibitory activity
against influenza virus, Salmonella Typhimurium, and
Candida albicans infection in mice, and S-PT84 enhances
IFN-α production from plasmacytoid dendritic cells by virus
stimulation (31).
Previously, we proposed ‘combination-oriented
molecular-targeting prevention’ of cancer (32). A combination of two agents can
synergistically enhance their preventive effects, even if the
effect of each single agent is weak. A combination of sulforaphane
and curcumin has a synergistic anti-inflammatory effect (33), and a combination of sulforaphane and
epigallocatechin-gallate has a synergistic growth-inhibitory effect
in human colon carcinoma cells (34). Additionally, sulforaphane enhances
TRAIL-induced apoptosis in human cancer cells (35,36).
TRAIL is a member of the TNF family of cytokines, which is crucial
for cancer prevention.
Therefore, we hypothesized that the combination of
sulforaphane and a TRAIL inducer, such as Lactobacillus, may
have a beneficial effect for cancer prevention. In this study, we
evaluated the effect of a combination of sulforaphane and
co-culture with L. pentosus S-PT84-treated PBMCs in human
colon cancer cells.
Materials and methods
Reagents and antibodies
Sulforaphane was purchased from LKT Laboratories
(St. Paul, MN, USA) and dissolved in dimethyl sulfoxide (DMSO).
Lactobacillus pentosus strain S-PT84 was kindly gifted from
the Suntory Global Innovation Center (Kyoto, Japan) (37). Heat-killed L. pentosus S-PT84
was freeze-dried and used for the following experiments: Propidium
iodide was purchased from Sigma (St. Louis, MO, USA). 5, 5′, 6,
6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolylcarbocyanine
iodide (JC-1) was purchased from FUJIFILM Wako pure chemical
corporation (Osaka, Japan). Annexin V-FITC apoptosis detection kit
was purchased from Nacalai Tesque (Kyoto, Japan). Antibodies
against cIAP-1, cIAP-2, Bcl-xL, acetylated-Histone H4, and Histone
H4 were obtained from Cell Signaling Technology (Danvers, MA, USA).
Antibody against TNFR1 was purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Antibodies against Bcl-2 and Bim were
purchased from Abcam (Cambridge, MA, USA). Antibody against Bax was
purchased from BD Biosciences (San Jose, CA, USA). Antibody against
GAPDH was purchased from HyTest (Turku, Finland). Antibodies
against XIAP, human recombinant TNFα/Fc chimera, human recombinant
DR5/Fc chimera, human recombinant Fas/Fc chimera, and zVAD-fmk were
obtained from R&D Systems (Minneapolis, MN, USA). Granzyme B
inhibitor I was obtained from Calbiochem (San Diego, CA, USA).
HRP-conjugated anti-mouse IgG and HRP-conjugated anti-rabbit IgG
were purchased from GE Healthcare (Piscataway, NJ, USA).
Cell culture
Human colon cancer HCT116 and SW480 cells and normal
human colon epithelial CCD 841 CoN cells were obtained from the
American Type Culture Collection (Rockville, MD, USA). HCT116 and
SW480 cells were maintained in Dulbecco's modified Eagle's medium
supplemented with 10% (v/v) fetal bovine serum, 4 mmol/l glutamine,
50 U/ml of penicillin G, and 100 µg/ml of streptomycin. CCD 841 CoN
cells were maintained in Eagle's minimum essential medium
supplemented with 10% (v/v) fetal bovine serum, 4 mmol/l glutamine,
50 U/ml of penicillin G, and 100 µg/ml of streptomycin. Normal
human PBMCs were isolated as previously described (25). All cells were incubated at 37°C in a
humidified atmosphere containing 5% CO2.
For co-culture, HCT116 or SW480 cells were seeded
onto 12-well plates (BD Falcon, no. 353503). The next day, PBMCs
were seeded on cell culture inserts (0.4 µm pores; BD Falcon no.
353494) in a 12-well plate.
Cell proliferation assay
The number of viable cells was determined using the
Cell Counting Kit-8 according to the manufacturer's instructions
(Dojindo Molecular Technology, Kumamoto, Japan).
Detection of sub-G1 population
Cells were stained with 10 µg/ml of propidium
iodide. Flow cytometry was performed with FACSCalibur (BD
Biosciences, Franklin Lakes, NJ, USA) and CellQuest software (BD
Biosciences).
Detection of apoptosis (JC-1)
Cells were stained with 2 µmol/l JC-1 and incubated
for 15 min. Cells were washed twice and resuspended with PBS. Flow
cytometry was performed with FACSCalibur and CellQuest
software.
Detection of apoptosis (Annexin
V)
Annexin V-FITC apoptosis detection kit was used
according to the manufacturer's protocol. Briefly, cells were
resuspended with Annexin V binding buffer and incubated with
Annexin V and propidium iodide for 15 min. Flow cytometry was
performed with FACSCalibur and CellQuest software.
Enzyme-linked immunosorbent assay
(ELISA)
Cells were centrifuged and the supernatant was
collected. A human TNFα ELISA kit (Abcam, Cambridge, UK) was used
according to the manufacturer's instructions.
Western blotting
Cells were lysed in buffer containing 50 mmol/l
Tris-HCl (pH 7.5), 1% SDS, 2 µg/ml leupeptin, 2 µg/ml aprotinin,
and 1 mmol/l dithiothreitol (DTT). The lysate was sonicated and
centrifuged at 14,000 g for 20 min at 4°C, and the supernatant was
collected. Equal amounts of lysate were analyzed by SDS-PAGE and
transferred to PVDF membranes (Millipore, Bedford, MA, USA). The
blots were blocked in blocking buffer for 1 h at room temperature
and incubated with the appropriate primary antibody in blocking
buffer for 1 h at room temperature. The blots were then washed and
incubated with the appropriate horseradish peroxidase-conjugated
secondary antibody for 1 h, and signals were detected using a
Chemilumi-one chemiluminescent kit (Nacalai Tesque, Kyoto, Japan)
or Immobilon Western Chemiluminescent HRP Substrate (Millipore).
The band intensities were quantified by ImageJ software (NIH).
Statistical analysis
Data are the means ± standard deviation of three
experiments. For simple group means analysis, data were analyzed
using a Student's t-test and differences were considered
significant at P<0.05. For between-group comparisons, one-way
ANOVA and Tukey's post hoc test were performed using Microsoft
Excel 2007 (Microsoft Corporation). P<0.05 was considered to
indicate a statistically significant difference.
Results
Sulforaphane enhances apoptosis
induced by co-culture with Lactobacillus-treated PBMCs in human
colon cancer cells
Sulforaphane, a well-known phytochemical, induces
apoptosis in cancer cells (10–14). We
investigated the effect of sulforaphane on cell growth in human
colon cancer HCT116 and SW480 cells and found that sulforaphane
suppressed the growth of both cells in a dose-dependent manner
(Fig. 1A).
Previously, we showed that Lactobacillus
strains facilitated natural killer activity against human cancer
cells in vitro (25). Here,
we evaluated the effect of L. pentosus S-PT84 on cellular
immunity by inducing apoptosis in human colon cancer HCT116 and
SW480 cells in co-culture with PBMCs treated by L. pentosus
S-PT84. First, isolated PBMCs from a healthy volunteer were
pre-treated by L. pentosus S-PT84 for 24 h. Second, PBMCs
were co-cultured with human colon cancer cells. After 48 h, the
sub-G1 population was examined by flow cytometry. Although
non-treated PBMCs showed basal levels of cytotoxicity,
Lactobacillus-treated PBMCs induced apoptosis against HCT116
and SW480 cells (Fig. 1). Similarly,
the sub-G1 population was significantly increased by sulforaphane
in both human colon cancer cell lines tested (Fig. 1B).
We evaluated the effect of the combination of
sulforaphane and co-culture with Lactobacillus-treated PBMCs
in human colon cancer cells. As shown in Fig. 1B, the addition of sulforaphane
increased the sub-G1 population from 33 to 57% in HCT116 cells and
from 19 to 49% in SW480 cells under co-culture with
Lactobacillus-treated PBMCs. Next, Annexin V/propidium
iodide staining was used to analyze apoptosis by treatment in human
colon cancer HCT116 and SW480 cells or in normal human colon CCD
841 CoN cells. Sulforaphane markedly enhanced apoptosis induced by
co-culture with Lactobacillus-treated PBMCs in human colon
cancer cells more than in normal human colon cells (Fig. 2A). In addition, we used PBMCs from
another volunteer to assess the combination of sulforaphane and
L. pentosus S-PT84-treated PBMCs (Fig. S1). As a result, the combination also
induced apoptosis in HCT116 cells markedly. Furthermore, we
assessed the mitochondrial transmembrane potential in human colon
cancer HCT116 and SW480 cells by JC-1 staining (Fig. 2B). To investigate the mitochondria
membrane potential (ΔΨm) involved in apoptotic induction, we
analyzed apoptosis using a mitochondria-specific dye JC-1. As shown
in Fig. 2B, the combination of
sulforaphane and co-culture with Lactobacillus-treated PBMCs
markedly decreased ΔΨm in human colon cancer HCT116 and
SW480 cells, which indicates an increase in apoptosis.
 | Figure 2.SFN enhances apoptosis induced by
Lactobacillus in human colon cancer cells. (A) Annexin V and
propidium iodide staining. PBMCs were pre-incubated with 10 µg/ml
L. pentosus S-PT84 for 24 h. HCT116, SW480, or CCD 841 CoN
cells were co-incubated with PBMCs (E/T ratio=100:1), incubated
with SFN for 48 h, and then harvested. After staining with Annexin
V and propidium iodide, the cells were subjected to apoptosis
analysis using FACSCalibur. The bottom right quadrant indicates
early apoptotic cells, whereas the top right quadrant indicates
late apoptotic cells. (B) JC-1 staining to measure mitochondrial
depolarization. HCT116 or SW480 cells were co-incubated with PBMCs
(E/T ratio=100:1), incubated with SFN for 48 h, and then harvested.
PBMCs were pre-incubated with 10 µg/ml L. pentosus S-PT84
for 24 h. After staining with JC-1, the cells were subjected to
apoptosis analysis using FACSCalibur. The top gate indicates normal
mitochondria (healthy cells, red fluorescence), whereas the bottom
gate indicates depolarized mitochondria (apoptotic cells, green
fluorescence). Data are presented as the mean ± standard deviation
of three experiments. *P<0.05 and **P<0.01, as indicated.
SFN, Sulforaphane; PBMCs, peripheral blood mononuclear cells. |
Sulforaphane enhances apoptosis
induced by Lactobacillus in human colon cancer cells via the TNFα
pathway
Three death factors (TNFα, FasL, and TRAIL) and
their receptors have been identified (38). Furthermore, cytotoxic lymphocytes
induce apoptosis in target cells by these death factors and/or
perforin and granzyme B (39,40). To
investigate the apoptosis pathway induced by sulforaphane and
co-culture with Lactobacillus-treated PBMCs, we used a
TNFR/Fc chimera, DR5/Fc chimera, Fas/Fc chimera, Granzyme B
inhibitor I, and pan-caspase inhibitor. Apoptosis was markedly
blocked by the pan-caspase inhibitor zVAD-fmk, which indicates that
apoptosis was caspase-dependent in HCT116 and SW480 cells (Fig. 3). Furthermore, the TNFR/Fc chimera
partially inhibited apoptosis in both cells (Fig. 3). On the other hand, the DR5/Fc
chimera, Fas/Fc chimera, and Granzyme B inhibitor I did not affect
apoptosis. These results suggest that the combination of
sulforaphane and co-culture with Lactobacillus-treated PBMCs
induced caspase-dependent apoptosis by the TNFα-TNFR pathway.
 | Figure 3.SFN enhances apoptosis induced by
Lactobacillus in human colon cancer cells via the TNFα
pathway. PBMC (2×106 /ml) were pre-incubated with 10
µg/ml L. pentosus S-PT84 for 24 h. HCT116 and SW480 cells
were co-incubated with PBMCs under treatment of SFN with or without
1 µg/ml TNFR/Fc chimera, 1 µg/ml DR5/Fc chimera, 1 µg/ml Fas/Fc
chimera, 20 µmol/l Granzyme B inhibitor I, or 20 µmol/l zVAD-fmk
for 48 h, and then harvested. After fixation and staining with
propidium iodide, (A) HCT116 or (B) SW480 cells were subjected to
cell cycle analysis using FACSCalibur. Data are presented as the
mean ± standard deviation of three experiments. *P<0.05 and
**P<0.01, as indicated. SFN, Sulforaphane; PBMC, peripheral
blood mononuclear cells; TNFR, tumor necrosis factor receptor. |
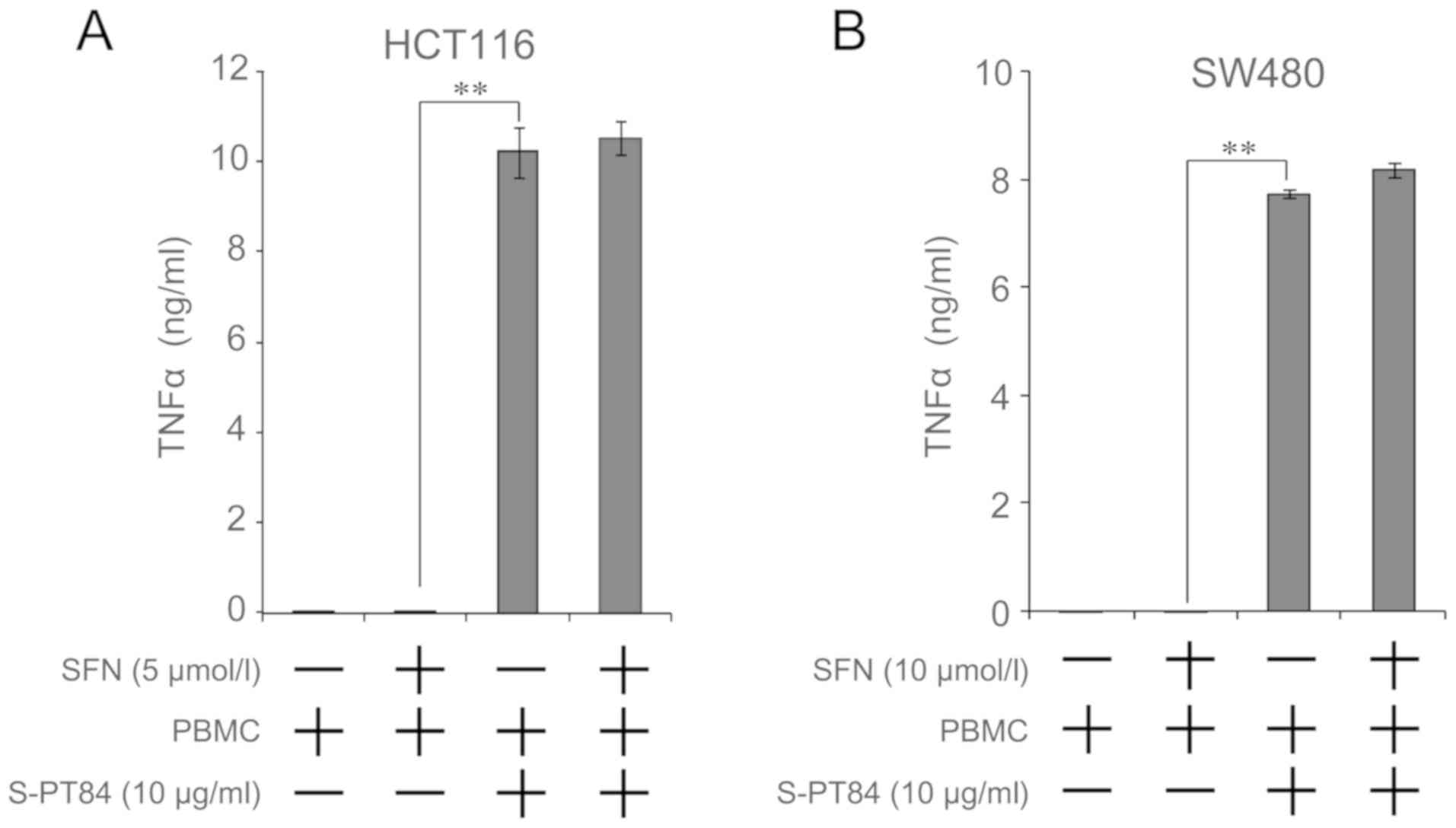
Secretion of TNFα is increased by
addition of Lactobacillus
We found that TNFα was involved in apoptosis by a
combination of sulforaphane and co-culture with
Lactobacillus-treated PBMCs. Microbial stimuli increases the
secretion of various cytokines, including TNFα, from PBMCs
(26). Therefore, we evaluated the
amount of TNFα secretion from the combination of sulforaphane and
co-culture with Lactobacillus-treated PBMCs using culture
supernatant by ELISA. As shown in Fig.
4, TNFα concentration markedly increased by co-culture with
Lactobacillus-treated PBMCs, regardless of sulforaphane
treatment, in both cells.
Effects of sulforaphane and/or
co-culture with Lactobacillus-treated PBMCs on the expression of
various intracellular regulators of apoptosis on human colon cancer
cells
To investigate the extrinsic and intrinsic pathways
of apoptosis induced by the combination of sulforaphane and
co-culture with Lactobacillus-treated PBMCs, we examined the
expression of apoptosis-related proteins using western blotting
(Fig. 5), and normalized histograms
were shown in Fig. S2. As shown in
these data, cIAP-1 and cIAP-2, anti-apoptotic IAP family proteins,
were significantly upregulated by co-culture with
Lactobacillus-treated PBMCs in both cancer cells. However,
sulforaphane treatment suppressed the induction of cIAP-1 and
cIAP-2 (Figs. 5A, B and S2). Co-culture with
Lactobacillus-treated PBMCs slightly induced the expression
of TNFR1, a TNFα receptor, in HCT116 cells (Figs. 5A and S2). Additionally, sulforaphane further
increased the induction of TNFR1 in HCT116 cells (Figs. 5A, B and S2). XIAP was markedly downregulated by
sulforaphane in SW480 cells with or without co-culture with
Lactobacillus-treated PBMCs (Figs. 5B and S2). The expression of Bcl-2 was slightly
induced by sulforaphane with Lactobacillus-treated PBMCs in
HCT116 cells (Figs. 5A and S2). Co-culture with
Lactobacillus-treated PBMCs increased Bax in SW480 cells
with or without sulforaphane treatment (Figs. 5B and S2). The expression of Bim, Bcl-xL and
Bcl-2 did not significantly change in SW480 cells (Figs. 5B and S2). These results raise the possibility
that TNFR1 in the extrinsic pathway and cIAP-1, cIAP-2 and Bax in
the intrinsic pathway were involved in apoptosis by the combination
of sulforaphane and co-culture with Lactobacillus-treated
PBMCs in human colon cancer cells.
Discussion
Combinations of different chemopreventive agents may
synergistically enhance their preventive effects, and
‘combination-oriented molecular-targeting prevention’ of cancer is
a practical approach for the chemoprevention of human malignant
tumors (32).
Sulforaphane induces apoptosis in several types of
cancer cells (9–13), and Lactobacilli also induce
apoptosis in cancer cells by activating immune cells (25,41). We
found that sulforaphane enhanced apoptosis in human colon carcinoma
cells by co-culture with Lactobacillus-treated PBMCs.
Lactobacillus strains upregulate TRAIL
production in human immune cells (25), and sulforaphane induces the
expression of DR5, a TRAIL receptor, in human cancer cells
(35,36). Therefore, apoptosis induced by a
combination of sulforaphane and the Lactobacillus strain
L. pentosus S-PT84 may depend on the TRAIL pathway. However,
we found that apoptosis was TRAIL-independent because exogenous
addition of a soluble human recombinant DR5/Fc chimera, which binds
soluble TRAIL and prevents TRAIL binding to its receptor, failed to
prevent apoptosis. Furthermore, the concentration of TRAIL in the
culture supernatant was low from the combined treatment of
sulforaphane and co-culture with Lactobacillus-treated PBMCs
(data not shown). Instead, apoptosis was likely caused by TNFα
because the TNFR/Fc chimera significantly inhibited apoptosis.
Moreover, soluble human recombinant Fas/Fc chimera and a granzyme B
inhibitor did not attenuate apoptosis by the combination of
sulforaphane and co-culture with Lactobacillus-treated PBMCs
in HCT116 and SW480 cells. On the other hand, apoptosis was
markedly inhibited by zVAD-fmk, a pan-caspase inhibitor, and
induced mitochondria membrane depolarization. Sulforaphane induces
apoptosis in various tumor cells via a mitochondria-dependent
pathway by inducing reactive oxygen species (ROS) (42–44).
These results suggest that this combination was dependent on both
the extrinsic and intrinsic pathways of apoptosis. Sulforaphane
suppresses LPS-induced cell signaling, including TNFα (45), but we found that sulforaphane did not
inhibit TNFα production induced by L. pentosus S-PT84 in
both cell types. Sulforaphane acts as a histone deacetylase (HDAC)
inhibitor (46,47), and several HDAC inhibitors suppress
the activities of immune cells (48–50).
Therefore, sulforaphane may potentially suppress immune activities
by inhibiting HDAC. We investigated the effect of sulforaphane on
histone acetylation by western blotting, but acetylation of histone
H4 was not changed by 5 and 10 µM sulforaphane in PBMCs.
The expression of cIAP-1 and cIAP-2 was also
markedly induced by co-culture with Lactobacillus-treated
PBMCs in both cells. The induction of cIAP-1 and cIAP-2 may
contribute to the prevention of apoptosis in colon cancer cells in
co-culture with Lactobacillus-treated PBMCs despite
upregulation of TNFα. Interestingly, sulforaphane suppressed the
expression of cIAP-1 and cIAP-2 induced by co-culture with
Lactobacillus-treated PBMCs. Sulforaphane downregulates
cIAP-1 and cIAP-2 in human prostate cancer cells (14). Proteins in the IAP family are widely
and highly expressed in many cancers and are an important target
for anticancer therapy (51).
We found that sulforaphane enhanced apoptosis in
human colon cancer cells under co-culture with
Lactobacillus-treated PBMCs. Production of TNFα from PBMCs
was induced by co-culture with Lactobacillus, and
sulforaphane suppressed the expression of cIAP-1 and cIAP-2 in
colon cancer cells. Furthermore, to verify the preventive effect of
the combination for colon carcinogenesis, the verification by
long-term experiments with exposure to an exogenous chemical
carcinogen are needed.
Sulforaphane and Lactobacillus are contained
in a wide variety of foods; therefore, consumption of sulforaphane
and Lactobacillus may have a protective effect against colon
cancer.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mr. Takashi Yasuda,
Dr Hiroyuki Hoshiko, Dr Takeshi Kanamori and Dr Keiichi Abe
(Research Institute, Suntory Global Innovation Center) for
supplying Lactobacillus pentosus S-PT84 and their insightful
comments and suggestions.
Funding
The present study was supported in part by JSPS
KAKENHI (grant. no. JP25860466) and by a grant from the Suntory
Co., Japan (grant. no. J142001069).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY, MH and TS conceived and designed the study, and
wrote the manuscript. SY and MH conducted the experiments, and
performed the data analysis. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
PBMCs were acquired from healthy volunteers after
obtaining written informed consent. The present study was approved
by the Kyoto Prefectural University of Medicine Research Ethics
Committee (permission. no. RBMR-C-919).
Patient consent for publication
Not applicable.
Competing interests
Toshiyuki Sakai received funding from Suntory Co.
(Japan) who also supplied the Lactobacillus pentosus strain
S-PT84 used in the study.
Glossary
Abbreviations
Abbreviations:
|
PBMCs
|
peripheral blood mononuclear cells
|
|
TNF
|
tumor necrosis factor
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terry P, Giovannucci E, Michels KB,
Bergkvist L, Hansen H, Holmberg L and Wolk A: Fruit, vegetables,
dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst.
93:525–533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sato Y, Tsubono Y, Nakaya N, Ogawa K,
Kurashima K, Kuriyama S, Hazawa A, Nishino Y, Shibuya D and Tsuji
I: Fruit and vegetable consumption and risk of colorectal cancer in
Japan: The Miyagi Cohort Study. Public Health Nutr. 8:309–314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aoyama N, Kawado M, Yamada H, Hashimoto S,
Suzuki K, Wakai K, Suuki S, Watanabe Y and Tamakoshi A: Low intake
of vegetables and fruits and risk of colorectal cancer: The Japan
collaborative cohort study. J Epidemiol. 24:353–360. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shemesh N and Arber N: Curcumin alone and
in combination for prevention of colorectal cancer. Curr Colorectal
Cancer Rep. 10:62–67. 2014. View Article : Google Scholar
|
|
6
|
Sharmila G, Bhat FA, Arunkumar R, Elumalai
P, Raja Singh P, Senthilkumar K and Arunakaran J: Chemopreventive
effect of quercetin, a natural dietary flavonoid on prostate cancer
in in vivo model. Clin Nutr. 33:718–726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ullah MF, Bhat SH, Husain E, Abu-Duhier F,
Hadi SM, Sarkar FH and Ahmad A: Cancer chemopreventive pharmacology
of phytochemicals derived from plants of dietary and non-dietary
origin: Implication for alternative and complementary approaches.
Phytochem Rev. 13:811–833. 2014. View Article : Google Scholar
|
|
8
|
Zhang Y, Talalay P, Cho CG and Posner GH:
A major inducer of anticarcinogenic protective enzymes from
broccoli: Isolation and elucidation of structure. Proc Natl Acad
Sci USA. 89:2399–2403. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fahey JW, Zhang Y and Talalay P: Broccoli
sprouts: An exceptionally rich source of inducers of enzymes that
protect against chemical carcinogens. Proc Natl Acad Sci USA.
94:10367–10372. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gamet-Payrastre L, Li P, Lumeau S, Cassar
G, Dupont MA, Chevolleau S, Gasc N, Tulliez J and Terce F:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.PubMed/NCBI
|
|
11
|
Singh AV, Xiao D, Lew KL, Dhir R and Singh
SV: Sulforaphane induces caspase-mediated apoptosis in cultured
PC-3 human prostate cancer cells and retards growth of PC-3
×enografts in vivo. Carcinogenesis. 25:83–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pledgie-Tracy A, Sobolewski MD and
Davidson NE: Sulforaphane induces cell type-specific apoptosis in
human breast cancer cell lines. Mol Cancer Ther. 6:1013–1021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh SV, Srivastava SK, Choi S, Lew KL,
Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, et
al: Sulforaphane-induced cell death in human prostate cancer cells
is initiated by reactive oxygen species. J Biol Chem.
280:19911–19924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi S, Lew KL, Xiao H, Herman-Antosiewicz
A, Xiao D, Brown CK and Singh SV: D,L-Sulforaphane-induced cell
death in human prostate cancer cells is regulated by inhibitor of
apoptosis family proteins and Apaf-1. Carcinogenesis. 28:151–162.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fimognari C and Hrelia P: Sulforaphane as
a promising molecule for fighting cancer. Mutat Res. 635:90–104.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Myzak MC and Dashwood RH: Chemoprotection
by sulforaphane: Keep one eye beyond Keap1. Cancer Lett.
233:208–218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clarke JD, Dashwood RH and Ho E:
Multi-targeted prevention of cancer by sulforaphane. Cancer Lett.
269:291–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Juge N, Mithen RF and Traka M: Molecular
basis for chemoprevention by sulforaphane: A comprehensive review.
Cell Mol Life Sci. 64:1105–1127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yeh CT and Yen GC: Effect of sulforaphane
on metallothionein expression and induction of apoptosis in human
hepatoma HepG2 cells. Carcinogenesis. 26:2138–2148. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takeda K and Okumura K: Effects of a
fermented milk drink containing Lactobacillus casei strain
Shirota on the human NK-cell activity. J Nutr. 137((3 Suppl 2)):
S791–S793. 2007. View Article : Google Scholar
|
|
21
|
Takagi A, Matsuzaki T, Sato M, Nomoto K,
Morotomi M and Yokokura T: Inhibitory effect of oral administration
of Lactobacillus casei on 3-methylcholanthrene-induced
carcinogenesis in mice. Med Microbiol Immunol. 188:111–116. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Moreno de LeBlanc A, Matar C, LeBlanc N
and Perdigón G: Effects of milk fermented by Lactobacillus
helveticus R389 on a murine breast cancer model. Breast Cancer
Res. 7:R477–R486. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orland A, Linsalata M and Russo F:
Antiproliferative effects on colon adenocarcinoma cells induced by
co-administration of vitamin K1 and Lactobacillus rhamnosus
GG. Int J Oncol. 48:2629–2638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishikawa H, Akedo I, Otani T, Suzuki T,
Nakamura T, Takeyama I, Ishiguro S, Miyaoka E, Sobue T and Kakizoe
T: Randomized trial of dietary fiber and Lactobacillus casei
administration for prevention of colorectal tumors. Int J Cancer.
116:762–767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horinaka M, Yoshida T, Kishi A, Akatani K,
Yausda T, Kouhara J, Wakada M and Sakai T: Lactobacillus
strains induce TRAIL production and facilitate natural killer
activity against cancer cells. FEBS Lett. 584:577–582. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haller D, Bode C, Hammes WP, Pfeifer AM,
Schiffrin EJ and Blum S: Non-pathogenic bacteria elicit a
differential cytokine response by intestinal epithelial
cell/leucocyte co-cultures. Gut. 47:79–87. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Donkor ON, Ravikumar M, Proudfoot O, Day
SL, Apostolopoulos V, Pukovics G, Vasiljevic T, Nutt SL and Gill H:
Cytokine profile and induction of T helper type 17 and regulatory T
cells by human peripheral mononuclear cells after microbial
exposure. Clin Exp Immunol. 167:282–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Izumo T, Maekawa T, Ida M, Noguchi A,
Kitagawa Y, Shibata H, Yasui H and Kiso Y: Effect of intranasal
administration of Lactobacillus pentosus S-PT84 on influenza
virus infection in mice. Int Immunopharmacol. 10:1101–1106. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Izumo T, Izumi F, Nakagawa I, Kitagawa Y,
Shibata H and Kiso Y: Influence of Lactobacillus pentosus
S-PT84 ingestion on the mucosal immunity of healthy and
Salmonella Typhimurium-infected mice. Biosci Microflora.
30:27–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayama K, Ishijima S, Ono Y, Izumo T, Ida
M, Shibata H and Abe S: Protective activity of S-PT84, a
heat-killed preparation of Lactobacillus pentosus, against
oral and gastric candidiasis in an experimental murine model. Med
Mycol J. 55:123–129. 2014.(In Japanese). View Article : Google Scholar
|
|
31
|
Izumo T, Maekawa T, Ida M, Kishi A,
Akatani K, Kitagawa Y and Kiso Y: Effect of Lactobacillus
pentosus S-PT84 ingestion on IFN-α production from plasmacytoid
dendritic cells by virus stimulation. Biosci Biotechnol Biochem.
75:370–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshida T, Horinaka M and Sakai T:
‘Combination-oriented molecular-targeting prevention’ of cancer: A
model involving the combination of TRAIL and a DR5 inducer. Environ
Health Prev Med. 15:203–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheung KL, Khor TO and Kong AN:
Synergistic effect of combination of phenethyl isothiocyanate and
sulforaphane or curcumin and sulforaphane in the inhibition of
inflammation. Pharm Res. 26:224–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nair S, Hebbar V, Shen G, Gopalakrishnan
A, Khor TO, Yu S, Xu C and Kong AN: Synergistic effects of a
combination of dietary factors sulforaphane and (−)
epigallocatechin-3-gallate in HT-29 AP-1 human colon carcinoma
cells. Pharm Res. 25:387–399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsui T, Sowa Y, Yoshida T, Murata H,
Horinaka M, Wakada M, Nakanishi R, Sakabe T, Kubo T and Sakai T:
Sulforaphane enhances TRAIL-induced apoptosis through the induction
of DR5 expression in human osteosarcoma cells. Carcinogenesis.
27:1768–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shankar S, Ganapathy S and Srivastava RK:
Sulforaphane enhances the therapeutic potential of TRAIL in
prostate cancer orthotopic model through regulation of apoptosis,
metastasis, and angiogenesis. Clin Cancer Res. 14:6855–6866. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nonaka Y, Izumo T, Izumi F, Maekawa T,
Shibata H, Nakano A, Kishi A, Akatani K and Kiso Y: Antiallergic
effects of Lactobacillus pentosus strain S-PT84 mediated by
modulation of Th1/Th2 immunobalance and induction of IL-10
production. Int Arch Allergy Immunol. 145:249–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vermijlen D, Froelich CJ, Luo D,
Suarez-Huerta N, Robaye B and Wisse E: Perforin and granzyme B
induce apoptosis in FasL-resistant colon carcinoma cells. Cancer
Immunol Immunother. 50:212–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Trapani JA and Smyth MJ: Functional
significance of the perforin/granzyme cell death pathway. Nat Rev
Immunol. 2:735–747. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rachid M, Matar C, Duarte J and Perdigon
G: Effect of milk fermented with a Lactobacillus helveticus
R389(+) proteolytic strain on the immune system and on the growth
of 4T1 breast cancer cells in mice. FEMS Immunol Med Microbiol.
47:242–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Choi WY, Choi BT, Lee WH and Choi YH:
Sulforaphane generates reactive oxygen species leading to
mitochondrial perturbation for apoptosis in human leukemia U937
cells. Biomed Pharmacother. 62:637–644. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jo GH, Kim GY, Kim WJ, Park KY and Choi
YH: Sulforaphane induces apoptosis in T24 human urinary bladder
cancer cells through a reactive oxygen species-mediated
mitochondrial pathway: The involvement of endoplasmic reticulum
stress and the Nrf2 signaling pathway. Int J Oncol. 45:1497–1506.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park HS, Han MH, Kim GY, Moon SK, Kim WJ,
Hwang HJ, Park KY and Choi YH: Sulforaphane induces reactive oxygen
species-mediated mitotic arrest and subsequent apoptosis in human
bladder cancer 5637 cells. Food Chem Toxicol. 64:157–165. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Folkard DL, Melchini A, Traka MH,
Al-Bakheit A, Saha S, Mulholland F, Watson A and Mithen RF:
Suppression of LPS-induced transcription and cytokine secretion by
the dietary isothiocyanate sulforaphane. Mol Nutr Food Res.
58:2286–2296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Myzak MC, Karplus PA, Chung FL and
Dashwood RH: A novel mechanism of chemoprotection by sulforaphane:
Inhibition of histone deacetylase. Cancer Res. 64:5767–5774. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dashwood RH, Myzak MC and Ho E: Dietary
HDAC inhibitors: Time to rethink weak ligands in cancer
chemoprevention? Carcinogenesis. 27:344–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Leoni F, Zaliani A, Bertolini G, Porro G,
Pagani P, Pozzi P, Donà G, Fossati G, Sozzani S, Azam T, et al: The
antitumor histone deacetylase inhibitor suberoylanilide hydroxamic
acid exhibits antiinflammatory properties via suppression of
cytokines. Proc Natl Acad Sci USA. 99:2995–3000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Leoni F, Fossati G, Lewis EC, Lee JK,
Porro G, Pagani P, Modena D, Moras ML, Pozzi P, Reznikov LL, et al:
The histone deacetylase inhibitor ITF2357 reduces production of
pro-inflammatory cytokines in vitro and systemic inflammation in
vivo. Mol Med. 11:1–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bridle BW, Chen L, Lemay CG, Diallo JS,
Pol J, Nguyen A, Capretta A, He R, Bramson JL, Bell JC, et al: HDAC
inhibition suppresses primary immune responses, enhances secondary
immune responses, and abrogates autoimmunity during tumor
immunotherapy. Mol Ther. 21:887–894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Saleem M, Qadir MI, Perveen N and Ahmad B,
Saleem U, Irshad T and Ahmad B: Inhibitors of apoptotic proteins:
New targets for anticancer therapy. Chem Biol Drug Des. 82:243–251.
2013. View Article : Google Scholar : PubMed/NCBI
|