Introduction
Acute myeloid leukemia (AML) is one of the most
common hematological malignancies worldwide (1). Multiple stress factors can damage cells
and induce carcinogenesis (2), and
apoptosis may eliminate malignant cells (3). However, malignant cells are capable of
evading apoptosis, which makes tumors, including AML, difficult to
treat (4). The aberrant upregulation
of BCL-2, an anti-apoptotic protein of the BCL-2 family, is
associated with carcinogenesis and drug resistance (5). BCL-XL, another BCL-2 family
anti-apoptotic protein, has been demonstrated to be expressed in
eight head and neck squamous cell carcinoma (HNSCC) cell lines
(6). Reportedly, AML tumorigenesis
and drug resistance are associated with myeloid cell leukemia 1
(MCL-1), which also belongs to the BCL-2 family of anti-apoptotic
proteins (7,8). The aforementioned anti-apoptotic
proteins, including MCL-1, BCL-2 and BCL-XL, can bind and sequester
BAX, BCL2 antagonist/killer 1, BCL-2-like protein 11 or BAD, which
are their pro-apoptotic counterparts in the evasion of apoptosis
(9).
BCL-2 anti-apoptotic antagonists, including BCL-2
homology 3 (BH3) mimic small molecules, have been developed
(10). Hitherto, Abbott Laboratories
have developed BH3 mimetics, including ABT-737 (BCL-2/BCL-XL
antagonist) and ABT-199 (BCL-2-selective antagonist) (11). ABT-737 has a high affinity for
BCL-2/BCL-XL and can promote apoptosis of malignant cells (12,13). The
upregulation of anti-apoptotic BCL-2 family proteins is associated
with multiple different types of tumor (14), including non-small cell lung cancer
(15), AML (7,8,13), lymphoma (4,16),
multiple myeloma (12,16), neuroblastoma (17), HNSCC (6), hepatocellular carcinoma (10) and esophageal squamous cell carcinoma
(ESCC) (18).
It has been reported that upregulation of MCL-1 is
associated with drug resistance (19,20);
however, ABT-737 or other ABT BH3 mimetics have low affinities for
MCL-1, thus, malignant cells with high MCL-1 expression are
resistant to ABT compounds (21). In
addition, MCL-1 amplification and upregulation are frequently
associated with poor prognosis in multiple different types of tumor
(22). For example, high MCL-1
expression in breast tumors is associated with high tumor grade and
poor patient prognosis (23). It has
been reported that MCL-1 small interfering RNA knockdown restores
ABT-737 sensitivity, indicating that MCL-1 serves a critical role
in ABT-737 resistance in leukemic cells (13,24).
A variety of approaches have been developed,
including BH3 mimetics, which bind and antagonize MCL-1 or other
BCL-2 family anti-apoptotic proteins (25). Furthermore, it has previously been
reported that BH3 mimetic MCL-1-selective antagonist could inhibit
the expression of MCL-1 (26,27).
A-1210477, which binds to MCL-1 with high affinity, was the first
MCL-1-BH3-only antagonist (6,18).
The present study assessed the efficacy of
MCL-1-selective antagonist A-1210477 and/or BCL-2/BCL-XL antagonist
ABT-737 in AML cell lines and mouse models. A-1210477 exhibited a
high affinity for anti-apoptotic protein MCL-1. In the present
study, the potential therapeutic effects of A-1210477 were
evaluated in vitro and in vivo, and the results
indicated that ABT-resistant AML cell lines or mouse models could
be inhibited by A-1210477, which rendered it a potential targeted
therapy that could be beneficial to patients with hematological
malignancies or solid tumors.
Materials and methods
Cell lines
Human leukemia MV4-11 and HL-60 cell lines were
obtained from the American Type Culture Collection. Human leukemia
MOLM13 and OCI-AML3 cell lines were purchased from Shanghai Bioleaf
Biotech Co., Ltd., and cultured in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.), 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C, according to the manufacturer's
protocol.
Mouse strains
NSG-SGM3 mice were obtained from Jackson Laboratory,
and housed in a specific pathogen-free facility at 25°C,
(relatively humidity 50%, 12 h light/12 h dark). Sterile water and
feed were delivered aseptically. The mice were allowed free access
to food and water, The Institutional Animal Ethics Committee of
Xinhua Hospital (Shanghai, China) approved all mouse experiments of
the present study. A total of 1×106 cells, including
MOLM-13, MV4-11, HL-60 and OCI-AML3 cells, were injected into
8-week-old mice through the tail vein. In the present study, a
total 192 of mice were used (96 male and 96 female), and the weight
of the mice ranged from 20–22 g.
Assessment of cell viability
ABT-737 was the selective BCL-2/BCL-XL antagonist
(Active Biochem Ltd.), A-1210477 was the MCL-1-selective antagonist
(MedChemExpress) were solubilized in DMSO at different
concentrations (0.1, 1.0, 5.0 and 10.0 µM). HL60, MOLM13, MV4-11
and OCI-AML3 cell lines were treated for 72 h at 37°C with
A-1210477 and/or ABT-737. DMSO was used as a control at a
concentration of 0.001%. A Real-Time-Glo™ MT assay (Promega
Corporation) was used to assess the cell viability according to the
manufacturer's protocol. The present study also used a fluorescence
microscope to visualize the cell luminescence.
MCL-1 expression in AML cell
lines
Total proteins from AML cell lines were extracted
using mammalian protein extraction reagent according to the
manufacturer's protocol (Thermo Fisher Scientific, Inc.). A Pierce
BCA Protein Assay kit (Thermo Fisher Scientific, Inc.) was used to
detect total protein concentration. The MCL-1 levels in the cell
lines were detected via an MCL-1 ELISA kit (cat. no. LM-MCL1-Hu;
LMAI Bio). The content of total protein or MCL-1 protein was
detected from each AML line, respectively. Subsequently, the ratio
of MCL-1/total protein was calculated in order to normalize the
different total proteins in multiple AML cell lines. In the present
study, the ratio of MCL-1/total protein <0.02 was defined as low
MCL-1 level, the ratio of MCL-1/total protein ranged from 0.02 to
0.1 was defined as intermediate MCL-1 level. The ratio of
MCL-1/total protein >0.1 was defined as high MCL-1 level.
Animal study
The 8-week-old mice (Jackson Laboratory) were
irradiated (100 cGy). Subsequently, 1×106 MOLM-13,
MV4-11, HL-60 or OCI-AML3 cells were injected into the mice through
the tail vein. After 7 days, the mice were administered ABT-737
and/or A-1210477 at the dosages of 50, 75 or 100 mg/kg three times
per week (a total of 15 injections in 35 days) via intraperitoneal
injection. The vehicle control mice were also injected with
1×106 MOLM-13, MV4-11, HL-60 or OCI-AML3 cells,
respectively, and were treated with DMSO at a concentration of
0.001%.
Murine bone marrow (BM)
preparation
BM cells were isolated from each mouse (treated and
control). In addition, APC/Cy7 anti-human CD45 (1:1,000; cat. no.
368516; BioLegend) and PE anti-mouse CD45 antibodies (1:1,000; cat.
no. 103106; BioLegend) were used to stain BM cells and were
incubated at room temperature for 20 min. Flow cytometry (BD™
Digital Flow Cytometers, BD™ LSR II flow cytometer, BD FACSDiva™
software; version 4.1; BD Biosciences) was performed to analyze the
chimera rates of hCD45(+) BM cells from treated and control mice.
The examples were analyzed using a BD™ LSR II flow cytometer with
BD FACSDiva™ software (BD Biosciences; version 4.1) using a
two-laser, 6-color configuration.
Immunohistochemistry (IHC)
IHC analysis was performed in the treated and
control mice. Numerous tissue samples (liver, spleen and lung) were
obtained from each mouse to evaluate the chimera rate of the
hCD45(+) cells. The 5 µm-thick sections were fixed with acetone for
10 min at 4°C and treated with 0.3% Triton X-100 in PBS for 15 min
at room temperature. These sections were blocked with 10% goat
serum (YEASEN) for 30 min at room temperature, and then stained
with mouse-anti-hCD45 primary antibody (1:100; cat. no. MBS438093;
Mybiosource) at 4°C overnight. The following day the sections
continued to be incubated at 37°C for 60 min, and washed with PBS
three times. The sections were then stained with secondary
antibodies, namely, goat-anti-mouse IgG antibodies conjugated to
Alexa Fluor 488 (1:100; cat. no. A11001; Invitrogen; Thermo Fisher
Scientific, Inc.), incubated at 37°C for 30 min, and washed with
PBS three times. A fluorescence microscope was used to examine the
aforementioned sections and acquired images (Leica Microsystems,
Inc.) at ×200 magnification ×200 for the liver samples and ×400
magnification for the spleen and lung samples.
Statistical analysis
The data are presented as the mean ± standard
deviation. The experiments were performed in triplicate.
Differences among groups were determined using ANOVA followed by a
Newman-Keul's post hoc test using SPSS (version 25.0; SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
AML cells with high MCL-1 levels are
resistant to ABT-737
The effects of ABT-737 on MOLM-13, MV4-11, HL-60 and
OCI-AML3 cells were tested at 0.1, 1.0, 5.0 or 10 µM. As presented
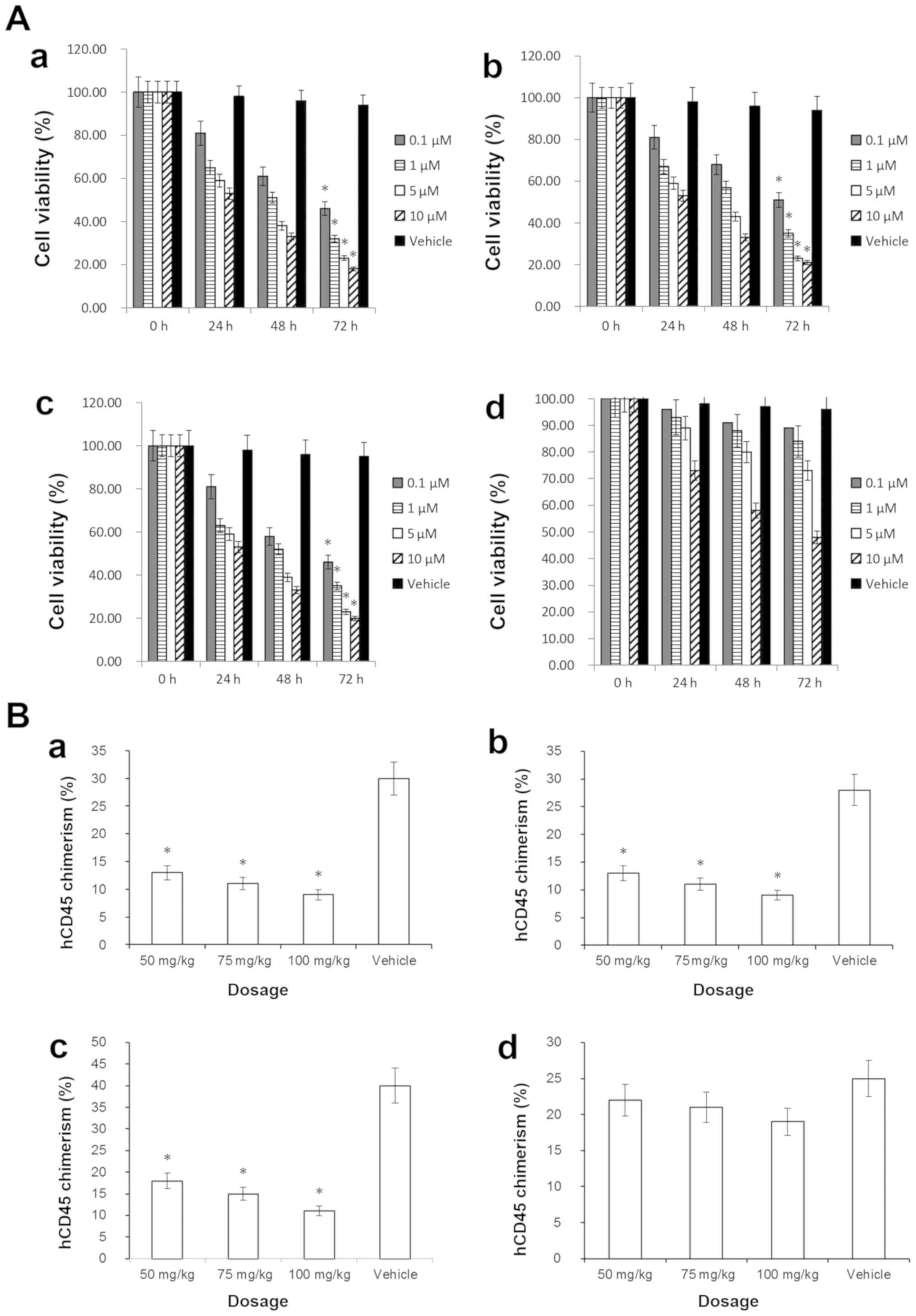
in Fig. 1A, following treatment with
0.1 µM ABT-737 for 72 h, the viability of HL-60 (46%), MOLM-13
(51%) and MV4-11 (46%) cells was decreased significantly
(P<0.05; Fig. 1A-a-c), compared
with the vehicle control. MCL-1 serves a critical role in
resistance to ABT-737 (7,9,11). In
this study, we used an MCL-1 ELISA kit to analyze the MCL-1 protein
levels of the aforementioned AML cell lines. The results indicated
that the MCL-1 level in HL-60 cell line was low, MOLM-13 cell line
was intermediate, MV4-11 cell line was intermediate, and the MCL-1
level in OCI-AML3 cell line was high. The cell viability assessment
indicated that the OCI-AML3 cell line did not exhibit a
statistically significant decrease in cell viability following
ABT-737 single-agent treatment (P>0.05; Fig. 1A-d), suggesting that ABT-737
resistance of AML could be associated with high expression levels
of MCL-1. These results are consistent with those of a previous
study (28).
In addition, the aforementioned AML cell lines were
injected into transgenic NSG-SGM3 mice via the tail vein. Following
treatment with ABT-737 alone, BM cells were isolated from each
mouse. CD45 antigen (leukocyte common antigen), which is a unique
and ubiquitous membrane glycoprotein with a molecular mass of ~200
kDa, is expressed on almost all leukocyte cells (29). Human AML cell lines were hCD45(+),
which could engraft in the BM of the recipient mice. Following
single-agent ABT-737 treatment, BM cells were isolated from treated
and control mice. ‘Chimera’ in this context referred to the
proportion of engrafted human AML cells that were hCD45(+) in the
HL-60, MV4-11, OCI-AML3 or MOLM13 recipient mice. FCM was used to
detect the positive rate of hCD45(+) cells in the BM of recipient
mice. Fig. S1A indicates that in
the human AML cell line MV4-11, the anti-hCD45(+) rate was 99.9%.
Fig. S1B indicates that in blank
control mice (without the injection of human AML cell lines), the
anti-hCD45(+) rate was 0.23%. These data indicated that normal
murine BM cells did not express hCD45, suggesting that the hCD45
expression in the recipient mice was due to the engraftment of
injected human AML cells.
Fig. 1B-a-c
demonstrates that following ABT-737 single-agent treatment, (at 50,
75 and 100 mg/kg), the hCD45(+) chimera rates of each recipient
mouse injected with HL-60 (9%), MV4-11 (11%) or MOLM-13 cells (9%),
were decreased significantly (P<0.05) compared with the vehicle
control. Fig. S1C demonstrates a
representative FCM plot of OCI-AML3 mice following treatment with
ABT-737 alone. Fig. S1D presents
data for the vehicle control mice that were injected with
1×106 OCI-AML3 cells and treated with DMSO at a
concentration of 0.001%. The hCD45(+) chimera rate of the BM cells
from OCI-AML3-injected mice was not significantly decreased
(P>0.05) compared with the vehicle control, indicating that the
OCI-AML3 mouse model possessed ABT-737 resistance (Fig. 1B-d). Overall, the data suggested that
AML cells with high MCL-1 expression levels may exhibit resistance
to ABT-737.
A-1210477 inhibits viability of AML
cell lines irrespective of their resistance to ABT-737
HL-60, MOLM-13, MV4-11, and OCI-AML3 cell lines were
treated with A-1210477 at 0.1, 1.0, 5.0 and 10 µM, in order to
evaluate the inhibitory effects of A-1210477. Fig. 2A-a-d demonstrates that following
treatment with A-1210477 (0.1 µM) for 72 h, the viability of HL-60
(47%), MOLM-13 (46%), MV4-11 (38%) and OCI-AML3 cells (43%) were
decreased significantly compared with the corresponding vehicle
control (HL-60, 93%; MOLM-13, 95%; MV4-11, 93%; OCI-AML3, 95%; all
P<0.05). The aforementioned data suggested that AML cell lines,
including OCI-AML3, possessed A-1210477 sensitivity, indicating
that A-1210477 inhibited AML cells as a single agent irrespective
of their resistance to ABT-737.
Furthermore, the aforementioned AML cell lines were
injected into NSG-SGM3 mice via the tail vein. Following treatment
with A-1210477 alone, BM cells were isolated from each experimental
and control mouse. Human AML cell lines were hCD45(+), which could
engraft in the BM of a recipient mouse. FCM was used to detect the
hCD45(+) chimera rate. Fig. 2B-a-d
demonstrates that following A-1210477 single-agent treatment (at
50, 75 and 100 mg/kg,), the hCD45(+) chimera rates of mice injected
with HL-60 (8%), MV4-11 (10%), MOLM-13 (9%) or OCI-AML3 cells (9%)
were decreased significantly compared with the corresponding
vehicle control mice (HL-60, 30%; MV4-11, 40%; MOLM-13, 28%;
OCI-AML3, 25%, respectively; all P<0.05). These results
indicated that A-1210477 could counteract ABT-737 resistance in
vivo.
IHC was performed in different mouse groups that
were treated with either A-1210477 or ABT-737. Tissues (liver,
spleen and lung) were collected from these mice for the evaluation
of engrafted AML cells. As aforementioned, the in vitro
results indicated that HL-60, MV4-11 or MOLM-13 cells were
sensitive to ABT-737. As presented in Fig. 1B-a-c after ABT-737 single-agent
treatment, the in vivo data also suggested that following
ABT-737 treatment alone, engrafted hCD45(+) cells in HL-60, MV4-11
or MOLM-13 AML mouse models were decreased compared with vehicle
control mice, The IHC of HL-60, MV4-11 or MOLM-13 AML mouse models
exhibited similar results to that in Fig. 1B-a-c, indicating that the engrafted
hCD45(+) cells in HL-60, MV4-11 or MOLM-13 AML mouse models were
decreased compared with vehicle control mice (data not shown).
Whereas, hCD45(+) chimera rate of the BM cells from
OCI-AML3-injected mice was not significantly decreased (P>0.05)
compared with the vehicle control, indicating that the OCI-AML3
mouse model possessed ABT-737 resistance (Fig. 1B-d). However, Fig. 2B-d demonstrated that following
A-1210477 single-agent treatment, the hCD45(+) chimera rates of
mice injected with OCI-AML3 cells (9%) were decreased significantly
compared with vehicle control mice (25%; P<0.05). Furthermore,
the IHC results indicated that even at the dosage of 100 mg/kg,
ABT-737 only exerted little effects on engrafted hCD45 cells in
OCI-AML3 mice (Fig. 3), suggesting
that OCI-AML3 cells were resistant to ABT-737. By contrast,
following treatment with A-1210477 alone, the engrafted hCD45 cells
of OCI-AML3 AML mouse models (Fig.
3) decreased compared with the vehicle control. These results
further indicated that A-1210477 could counteract ABT-737
resistance in vivo.
A-1210477 may exert a combined action
with ABT-737 on AML cells
The combination effect of A-1210477 and ABT-737 was
tested in AML cells. Following combination treatment of A-1210477
and ABT-737 (both at 1 µM) for 72 h, the cell viability was tested
in the aforementioned cell lines. Fig.
4A-C demonstrates that the viability of MOLM-13 (11%), MV4-11
(10%) and HL-60 (13%) cells was decreased significantly following
combined treatment compared with treatment with ABT-737 alone
(MOLM-13 cells, 35%; MV4-11, 33%; HL-60, 32%; all P<0.05), or
A-1210477 alone (MOLM-13, 28%; MV4-11, 27%; HL-60, 29%; all
P<0.05). Fig. 4D demonstrates
that, following combination treatment, the viability of OCI-AML3
cells that were resistant to ABT-737 were not significantly
decreased compared with ABT-737 or A-1210477 single-agent
(P>0.05). Furthermore, after the aforementioned mouse models
received combination treatment of A-1210477 and ABT-737 (both at a
dosage of 75 mg/kg), the hCD45 chimera rate of BM was detected via
FCM in AML cell-injected mice. Fig.
5A-C demonstrated that the hCD45(+) chimera rates of BM from
AML-cell-injected mice following combined treatment (MOLM-13, 6%;
MV4-11, 7%; HL-60, 5%), were significantly decreased compared with
those of ABT-737 alone (MOLM-13, 11%; MV4-11, 15%; HL-60, 11%; all
P<0.05), or A-1210477 alone (MOLM-13, 10%; MV4-11, 13%; and
HL-60, 10%; all P<0.05). Fig. 5D
indicated that after combination treatment, BM hCD45 chimera rate
of OCI-AML3 mice did not decrease significantly compared with
ABT-737 or A-1210477 single-agent, which was consistent with the
in vitro results. Therefore, it was speculated that
A-1210477 may exert a combined action with ABT-737 in AML on a
BCL-2/MCL-1 manner.
Discussion
Over the past decades, the treatment of AML relied
on conventional chemotherapies. At present, novel targeted
therapies have started to emerge. Both targeted therapy or
chemotherapy could result in apoptotic cell death (30,31).
Jilg et al (32) demonstrated
that in patients with high-risk myelodysplastic syndrome (MDS) or
secondary (s)AML, antagonizing BCL-2 family anti-apoptotic proteins
can promote apoptosis. ABT-199 and ABT-737, which are BH3 mimic
small-molecules, inhibit multiple different types of tumor, such as
non-small cell lung cancer (15),
AML (7,8,13),
lymphoma (4,16), multiple myeloma (12,16),
neuroblastoma (17), HNSCC (6), hepatocellular carcinoma (10) and ESCC (18), in a BCL-2 and/or BCL-XL-dependent
manner (13). Furthermore,
researchers have developed BCL-XL-selective antagonists (33).
Abnormally high expression levels of MCL-1 may
result in resistance to ABT compounds (28). It has previously been reported that
inhibition of MCL-1 can eliminate AML cells, which suggests that
MCL-1 could serve a critical role in AML (7,8). It has
been reported that MCL-1 removal, without antagonizing BCL-2 or
BCL-XL, can inhibit AML cells, thereby curing mice with AML
(34). Therefore, targeting MCL-1
may be a potential therapy option for AML. A-1210477, which has a
high affinity and selectivity for MCL-1, was the first BH3 mimic
MCL-1-selective antagonist (6).
A-1210477 specifically binds MCL-1 and promotes apoptosis of cancer
cells in an MCL-1-dependent manner (35). Lin et al (18) revealed that A-1210477 treatment
decreases ESCC formation and animal weight loss in a dose-dependent
manner. In addition, the authors of this study demonstrated that
A-1210477 treatment increases the number of apoptotic cells in ESCC
tissues, which provides evidence towards the contribution of MCL-1
to ESCC development by promoting cell proliferation and inhibition
of apoptosis, providing a potential therapy option for
MCL-1-selective antagonist in treating ESCC (18).
It has been reported that in high-risk MDS or sAML,
high expression levels of MCL-1 result in resistance to ABT-199 and
ABT-737 (32). In the present study,
the efficacy of A-1210477 and/or ABT-737 was evaluated in AML cell
lines. In addition, following treatment with ABT-737 alone, the
cell viability of MOLM-13, MV4-11 and HL-60 cells was significantly
decreased (P<0.05); however, ABT-737 had little effect on the
OCI-AML3 cell line with high expression levels of MCL-1. By
contrast, following treatment with A-1210477, the viability of
HL-60, MV4-11, MOLM-13 and OCI-AML3 cells was significantly
decreased (P<0.05) irrespective of ABT-737 resistance.
Furthermore, the results indicated that hCD45(+) chimeras of BM
from MOLM-13, MV4-11 and HL-60 AML mouse models significantly
decreased following treatment with ABT-737 alone (P<0.05).
However, AML mice that exhibited high MCL-1 expression, including
OCI-AML3-injected mice, were resistant to ABT-737. The data from
the aforementioned AML mouse models indicated sensitivity to
A-1210477, suggesting that MCL-1-selective antagonist could
overcome ABT-737 resistance.
It has previously been reported that in the samples
of 577 patients with AML, MCL-1, BCL-2 and BCL-XL were expressed
heterogeneously, and their expression overlapped (36). To evade apoptosis, cancer cells use
anti-apoptotic BCL-2 family proteins to bind and neutralize
apoptotic activators (35). Since
the overlapping of anti-apoptotic proteins in the BCL-2 family
serves an important role in therapeutic resistance, the
simultaneous targeting of the anti-apoptotic proteins of BCL-2
family could overcome drug resistance (37,38). Lin
et al (38) revealed that
resistant AML cell lines could be resensitized to BCL-2-selective
inhibitors by targeting MCL-1 and BCL-XL, and by preemptively
targeting MCL-1 and/or BCL-XL, alongside the administration of
BCL-2-selective antagonist ABT-199, which is capable of delaying
the acquisition of drug resistance (38).
Luedtke et al (39) evaluated the effect of BCL-2-selective
antagonist ABT-199 or A-1210477 alone, and in combination with
ABT-199-resistant AML cell lines U937 and THP-1. Synergy has been
observed between these two drugs for THP-1 (CI<0.30) and U937
(CI<0.70) cell lines. Combination treatment of A-1210477 and
ABT-199 has also been performed in an ABT-199-sensitive cell line
(MOLM-13), and the results indicated that A-1210477 synergizes with
ABT-199 (CI<0.16), which suggests that A-1210477 could synergize
with ABT-199 regardless of ABT-199 sensitivity (39). In the present study, combination
treatment of A-1210477 and ABT-737 was evaluated in AML. The data
suggested that, following combination treatment with ABT-737 and
A-1210477, viability of AML cell lines, including MOLM-13, MV4-11
and HL-60, was significantly decreased (P<0.05) compared with
that following ABT-737 or A-1210477 treatment alone. Furthermore,
the results indicated that, following combination treatment with
ABT-737 and A-1210477, the BM hCD45(+) chimera rates of MOLM-13,
MV4-11 and HL-60 AML mice were decreased significantly (P<0.05)
compared with those of ABT-737 or A-1210477 alone. Therefore, it
was speculated that A-1210477 may exert a combined action with
ABT-737 on AML cells, which depends on the anti-apoptotic proteins
of the BCL-2 family in a MCL-1/BCL-2/BCL-XL-dependent manner.
The results of the present study indicated that
A-1210477 acted as a single agent to counteract resistance to
ABT-737 in AML. Therefore, MCL-1-selective antagonists may be
imperative for treatment of AML, making it a potential targeted
therapy option for patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW performed the experiments, analyzed the data and
wrote the article. SH designed the project, reviewed the data and
revised the article. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The Institutional Animal Ethics Committee of Xinhua
Hospital (Shanghai, China) approved all mouse experiments in the
present study (approval no. XHEC-F-2019-034).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamaguchi R, Lartigue L and Perkins G:
Targeting Mcl-1 and other Bcl-2 family member proteins in cancer
therapy. Pharmacol Ther. 195:13–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Juin P, Geneste O, Gautier F, Depil S and
Campone M: Decoding and unlocking the BCL-2 dependency of cancer
cells. Nat Rev Cancer. 13:455–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adams CM, Clark-Garvey S, Porcu P and
Eischen CM: Targeting the Bcl-2 family in B cell lymphoma. Front
Oncol. 8:6362019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhola PD and Letai A: Mitochondria-judges
and executioners of cell death sentences. Mol Cell. 61:695–704.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ow TJ, Fulcher CD, Thomas C, Broin PO,
López A, Reyna DE, Smith RV, Sarta C, Prystowsky MB, Schlecht NF,
et al: Optimal targeting of BCL-family proteins in head and neck
squamous cell carcinoma requires inhibition of both BCL-xL and
MCL-1. Oncotarget. 10:494–510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gores GJ and Kaufmann SH: Selectively
targeting Mcl-1 for the treatment of acute myelogenous leukemia and
solid tumors. Genes Dev. 26:305–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Glaser SP, Lee EF, Trounson E, Bouillet P,
Wei A, Fairlie WD, Izon DJ, Zuber J, Rappaport AR, Herold MJ, et
al: Anti-apoptotic Mcl-1 is essential for the development and
sustained growth of acute myeloid leukemia. Genes Dev. 26:120–125.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tutusaus A, Stefanovic M, Boix L, Cucarull
B, Zamora A, Blasco L, de Frutos PG, Reig M, Fernandez-Checa JC,
Marí M, et al: Antiapoptotic BCL-2 proteins determine
sorafenib/regorafenib resistance and BH3-mimetic efficacy in
hepatocellular carcinoma. Oncotarget. 9:16701–16717. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Souers AJ, Leverson JD, Boghaert ER,
Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH,
Fairbrother WJ, et al: ABT-199, a potent and selective BCL-2
inhibitor, achieves antitumor activity while sparing platelets. Nat
Med. 19:202–208. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta VA, Matulis SM, Conage-Pough JE,
Nooka AK, Kaufman JL, Lonial S and Boise LH: Bone marrow
microenvironment-derived signals induce Mcl-1 dependence in
multiple myeloma. Blood. 129:1969–1979. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Konopleva M, Milella M, Ruvolo P, Watts
JC, Ricciardi MR, Korchin B, McQueen T, Bornmann W, Tsao T, Bergamo
P, et al: MEK inhibition enhances ABT-737-induced leukemia cell
apoptosis via prevention of ERK-activated MCL-1 induction and
modulation of MCL-1/BIM complex. Leukemia. 26:778–787. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beroukhim R, Mermel CH, Porter D, Wei G,
Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J,
Urashima M, et al: The landscape of somatic copy-number alteration
across human cancers. Nature. 463:899–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Guttikonda S, Roberts L, Uziel T,
Semizarov D, Elmore SW, Leverson JD and Lam LT: Mcl-1 is critical
for survival in a subgroup of non-small-cell lung cancer cell
lines. Oncogene. 30:1963–1968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelly GL, Grabow S, Glaser SP, Fitzsimmons
L, Aubrey BJ, Okamoto T, Valente LJ, Robati M, Tai L, Fairlie WD,
et al: Targeting of MCL-1 kills MYC-driven mouse and human
lymphomas even when they bear mutations in p53. Genes Dev.
28:58–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bate-Eya LT, den Hartog IJ, van der Ploeg
I, Schild L, Koster J, Santo EE, Westerhout EM, Versteeg R, Caron
HN, Molenaar JJ and Dolman ME: High efficacy of the BCL-2 inhibitor
ABT199 (venetoclax) in BCL-2 high-expressing neuroblastoma cell
lines and xenografts and rational for combination with MCL-1
inhibition. Oncotarget. 7:27946–27958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin J, Fu D, Dai Y, Lin J and Xu T: Mcl-1
inhibitor suppresses tumor growth of esophageal squamous cell
carcinoma in a mouse model. Oncotarget. 8:114457–114462.
2017.PubMed/NCBI
|
|
19
|
Wertz IE, Kusam S, Lam C, Okamoto T,
Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akagi H, Higuchi H, Sumimoto H, Igarashi
T, Kabashima A, Mizuguchi H, Izumiya M, Sakai G, Adachi M,
Funakoshi S, et al: Suppression of myeloid cell leukemia-1 (Mcl-1)
enhances chemotherapy-associated apoptosis in gastric cancer cells.
Gastric Cancer. 16:100–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yecies D, Carlson NE, Deng J and Letai A:
Acquired resistance to ABT-737 in lymphoma cells that up-regulate
MCL-1 and BFL-1. Blood. 115:3304–3313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song L, Coppola D, Livingston S, Cress D
and Haura EB: Mcl-1 regulatessurvival and sensitivity to diverse
apoptotic stimuli inhuman non-small cell lung cancer cells. Cancer
Biol Ther. 4:267–276. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiang W, Yang CY and Bai L: MCL-1
inhibition in cancer treatment. Onco Targets Ther. 11:7301–7314.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H,
Dong XW, Hu YZ, Lin NM, He QJ and Yang B: Synergistic antitumor
activity of gemcitabine and ABT-737 in vitro and in vivo through
disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther.
10:1264–1275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stewart ML, Fire E, Keating AE and
Walensky LD: The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor
and apoptosis sensitizer. Nat Chem Biol. 6:595–601. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Placzek WJ, Sturlese M, Wu B, Cellitti JF,
Wei J and Pellecchia M: Identification of a novel Mcl-1 protein
binding motif. J Biol Chem. 286:39829–39835. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Muppidi A, Doi K, Edwardraja S, Drake EJ,
Gulick AM, Wang HG and Lin Q: Rational design of proteolytically
stable, cell-permeable peptide-based selective Mcl-1 inhibitors. J
Am Chem Soc. 134:14734–14737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan R, Ruvolo VR, Wei J, Konopleva M, Reed
JC, Pellecchia M, Andreeff M and Ruvolo PP: Inhibition of Mcl-1
with the pan-Bcl-2 family inhibitor (−)BI97D6 overcomes ABT-737
resistance in acute myeloid leukemia. Blood. 126:363–372. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rheinländer A, Schraven B and Bommhardt U:
CD45 in human physiology and clinical medicine. Immunol Lett.
196:22–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foight GW, Ryan JA, Gulla SV, Letai A and
Keating AE: Designed BH3 peptides with high affinity and
specificity for targeting Mcl-1 in cells. ACS Chem Biol.
9:1962–1968. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vo TT, Ryan J, Carrasco R, Neuberg D,
Rossi DJ, Stone RM, Deangelo DJ, Frattini MG and Letai A: Relative
mitochondrial priming of myeloblasts and normal HSCs determines
chemotherapeutic success in AML. Cell. 151:344–355. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jilg S, Reidel V, Muller-Thomas C, Konig
J, Schauwecker J, Hockendorf U, Huberle C, Gorka O, Schmidt B,
Burgkart R, et al: Blockade of BCL-2 proteins efficiently induces
apoptosis in progenitor cells of high-risk myelodysplastic
syndromes patients. Leukemia. 30:112–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tao ZF, Hasvold L, Wang L, Wang X, Petros
AM, Park CH, Boghaert ER, Catron ND, Chen J, Colman PM, et al:
Discovery of a potent and selective BCL-XL inhibitor with in vivo
activity. ACS Med Chem Lett. 5:1088–1093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bernardo PH, Sivaraman T, Wan KF, Xu J,
Krishnamoorthy J, Song CM, Tian L, Chin JS, Lim DS, Mok HY, et al:
Structural insights into the design of small molecule inhibitors
that selectively antagonize Mcl-1. J Med Chem. 53:2314–2318. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao Y, Nimmer P, Sheppard GS, Bruncko M,
Hessler P, Lu X, Roberts-Rapp L, Pappano WN, Elmore SW, Souers AJ,
et al: MCL-1 is a key determinant of breast cancer cell survival:
Validation of MCL-1 dependency utilizing a highly selective small
molecule inhibitor. Mol Cancer Ther. 14:1837–1847. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bogenberger JM, Kornblau SM, Pierceall WE,
Lena R, Chow D, Shi CX, Mantei J, Ahmann G, Gonzales IM, Choudhary
A, et al: BCL-2 family proteins as 5-Azacytidine-sensitizing
targets and determinants of response in myeloid malignancies.
Leukemia. 28:1657–1665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Williams MM, Lee L, Hicks DJ, Joly MM,
Elion D, Rahman B, McKernan C, Sanchez V, Balko JM, Stricker T, et
al: Key survival factor, Mcl-1, correlates with sensitivity to
combined Bcl-2/Bcl-xL blockade. Mol Cancer Res. 15:259–268. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin KH, Winter PS, Xie A, Roth C, Martz
CA, Stein EM, Anderson GR, Tingley JP and Wood KC: Targeting
MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in
acute myeloid leukemia. Sci Rep. 6:276962016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luedtke DA, Niu X, Pan Y, Zhao J, Liu S,
Edwards H, Chen K, Lin H, Taub JW and Ge Y: Inhibition of Mcl-1
enhances cell death induced by the Bcl-2-selective inhibitor
ABT-199 in acute myeloid leukemia cells. Signal Transduct Target
Ther. 2:170122017. View Article : Google Scholar : PubMed/NCBI
|