Introduction
Colorectal cancer (CRC) is one of the leading causes
of morbidity and mortality both in the United States and worldwide.
In 2009, the estimated new cases of colon and rectum cancers were
106,100 and an estimated 49,920 patients succumbed in the United
States alone (1). Approximately
850,000 people develop CRC annually and 500,000 succumb to the
disease (2). The prevention and
treatment of CRC thus remains a global health issue.
Apigenin is a flavonoid belonging to the flavone
structural class and is chemically known as
4′,5,7,-trihydroxyflavone (3).
Apigenin has been shown to possess significant anti- inflammatory,
antioxidant and anti-carcinogenic properties (4). Pre-treatment with apigenin induces the
apoptosis of HCT-116 colon cancer cells (5) and inhibits the growth of colon
carcinoma cell lines, including SW480, HT-29 and Caco-2 (6). A previous study showed that apigenin
enhanced apoptosis in HT29 cells with wild-type adenomatous
polyposis coli (7). In vivo
studies showed that apigenin moderately reduces
azoxymethane-induced colon carcinogenesis and inhibits the growth
of colorectal cancer (8). However,
to the best of our knowledge no published data are currently
available regarding the in vivo study of apigenin on the
growth of CRC in mice. Therefore, an in vivo study using
fluorescence imaging may aid in a detailed analysis of the
effectiveness of apigenin on xenograft tumors derived from SW480, a
cell line established from a primary adenocarcinoma of the colon
(9).
Materials and methods
Cell culture
SW480/enhanced green fluorescent protein (eGFP) was
donated by Dr Zhao, Department of Pathology, Southern Medical
University, China. Tumor cells were cultured in RPMI-1640 medium
(Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine
serum (Hyclone, Logan, UT, USA), 100 U/ml penicillin/streptomycin
(Gibco) and 500 μg/ml G418, and incubated in a humidified chamber
with 5% CO2 at 37˚C.
Mice and xenograft tumors
Male BALB/c-nu mice (specific pathogen-free) were
obtained from the Laboratory Animal Center of Southern Medical
University. The animals were maintained at a constant room
temperature with a 12-h light cycle in a conventional animal
colony. The mice were between 6 and 8 weeks old at the beginning of
the experiments, and weighed 15–20 g. Tumor xenografts were
generated by subcutaneous injection of 5×106 cells on
the right inguen of the nude mice. The procedures were performed
using approved protocols, in accordance with the guidelines of the
Animal Care and Use Committee of Southern Medical University. Three
days following the injection, the fluorescence emitted by the tumor
cells was imaged by a whole-body GFP imaging system (Imagestation
2000MM; Kodak, USA) to visualize the formation of the tumor. A
total of 16 tumor-bearing mice were randomly divided into two
groups, the apigenin and control groups. Apigenin (Sigma-Aldrich,
USA) was dissolved in dimethyl sulfoxide (DMSO) and diluted in
phosphate-buffered saline (PBS). Each mouse in the apigenin group
was injected intraperitoneally with 20 mg/kg of apigenin, while in
the control group, each mouse was injected with the same amount of
vehicle solution (DMSO-PBS). Images of tumors were obtained every 6
days and analyzed to calculate the tumor area and tumor growth
using Molecular Imaging Software Version 4.0 provided by Kodak
2000MM System.
Terminal deoxynucleotidyl transferase
dUTP nick end-labeling (TUNEL) assay
A TUNEL apoptosis assay kit for paraffin section was
purchased from Nanjing KeyGen Biotech. Inc., China. The procedures
were performed in accordance with the manufacturer's instructions.
Through microscopic observation, cells were defined as apoptotic if
the nuclei were positively stained. The positive cells were counted
in three high-power fields. The apoptotic index (AI) was calculated
as the percentage of positively-stained cells: AI = (number of
positive cells/total number of nucleated cells) × 100%.
Real-time quantative RT-PCR
The primer sequences are shown in Table I. cDNA was synthesized by oligo dT
primed reverse transcription from 2 μg of total RNA using an access
RT system (Takara). Real-time PCR was performed using the Mx3005P
real-time PCR system (Stratagene) and Stratagene's Brilliant
SYBR-Green QPCR Master Mix kit, following the manufacturer's
instructions. Thermal cycling conditions were: 95˚C for 5 min and
40 cycles at 95˚C for 10 sec, followed by 60˚C for 30 sec. Human
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was amplified
as an internal control. The target and GAPDH genes were amplified
in the same reaction. The relative quantification was determined
using the 2-ΔΔCt method.
 | Table IPrimer sequence information. |
Table I
Primer sequence information.
| Gene name | Primers | Product (bp) |
|---|
| BAG-1 | Sense 5′ -
ACTGTCACCCACAGCAATGA-3′
Antisense 5′ - TGTGGAACCCCTATGACCTC-3′ | 116 |
| Bcl-2 | Sense 5′ -
ATGTGTGTGGAGAGCGTCAA-3′
Antisense 5′ - ACAGTTCCACAAAGGCATCC-3′ | 136 |
| CCND1 | Sense 5′ -
GATCAAGTGTGACCCGGACT-3′
Antisense 5′ - TCCTCCTCTTCCTCCTCCTC-3′ | 126 |
| FADD | Sense 5′ -
CCTGTGTGCAGCATTTAACG-3′
Antisense 5′ - GTCCTCGATGCTGTCGATCT-3′ | 106 |
| yrdC | Sense 5′ -
CGGATTCCTGATCATGCTTT-3′
Antisense 5′ - AGGACAACTGAGGCCAGAGA-3′ | 139 |
| GAPDH | Sense 5′ -
ACAGTCAGCCGCATCTTCTT -3′
Antisense 5′ - ACGACCAAATCCGTTGACTC -3′ | 94 |
Immunohistochemistry assay
The tissues were embedded and cut into 4-μm
sections. Immunohistochemistry was performed according to the
protocol as previously described (10). The sections were incubated with the
primary antibodies [rabbit anti-Fas-associated protein with death
domain (FADD) antibody, Cell signaling technology (CST)] and
biotinylated secondary antibody (Zymed Laboratories Inc., South San
Francisco, CA, USA). Nuclear counterstaining was performed using
haematoxylin. Brown stains in the immunostained regions was
considered to be FADD-positive staining. The scoring approach in
the assessment of immunohistochemically-stained tissue sections was
based on a simple and reproducible protocol as previously described
(10).
Western blotting
The protein lysates were loaded onto 10%
SDS-polyacrylamide gel, electrotransferred to polyvinylidene
fluoride (PVDF) (Immobilon P; Millipore, Bedford, MA, USA)
membranes and blocked with 5% non-fat dry milk in Tris-buffered
saline (TBST, 100 mM NaCl, 50 mM Tris, 0.1% Tween-20; pH 7.5). The
membranes were incubated with FADD, anti-phospho-FADD (Ser 194)
(CST) polyclonal antibodies or anti-GAPDH monoclonal antibody (CST)
overnight at 4˚C, respectively, followed by 1-h incubation with
horseradish peroxidase (HRP)-conjugated secondary antibody. The
signal was detected using an enhanced chemiluminescence system
(ECL; CST) and documented with a charge-coupled device system
(Imagestation 2000MM; Kodak). Data analysis was performed using the
Molecular Imaging Software Version 4.0. The protein level in each
sample was normalized to the level of GAPDH for the corresponding
sample.
Statistical analysis
The experiments were repeated a minimum of three
times. The data were expressed as means ± SD. The data were
analyzed with the Statistical Package (version 13.0; SPSS).
Differences between the two groups were analyzed using Student's
t-test. P<0.05 was considered to be statistically
significant.
Results
Effects of apigenin on the growth of
tumor xenografts
The tumor areas increased rapidly from 1525.88 to
3991.38 pixels in the control group, while the tumor areas
increased from 1496.13 to 2963.38 pixels in the apigenin group
after 27 days. Apigenin treatment inhibited the growth of
colorectal cancer xenografts after 21 (P<0.05) and 27 days
(P<0.01) compared to the control group (Fig. 1).
Effects of apigenin on the apoptosis of
tumor and expression of FADD
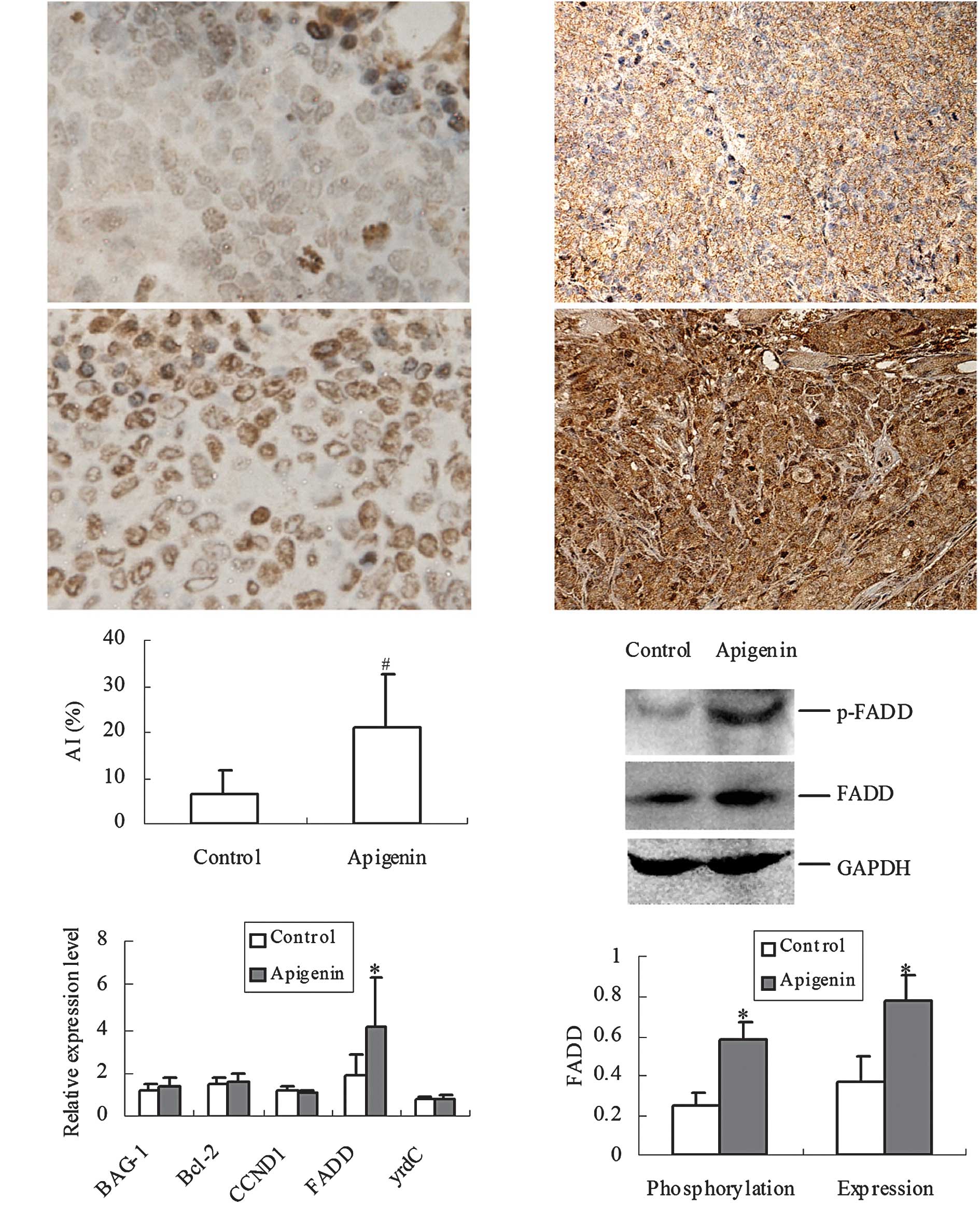
Based on TUNEL staining, a higher number of
brown-stained cells were found in the apigenin group compared to
those of the control group (P<0.01) (Fig. 2A–C). Five genes that related to the
proliferation and apoptosis of CRC, i.e., cyclin D1 (CCND1), BAG-1,
Bcl-2, yrdC and FADD were screened, and it was found that FADD was
up-regulated by apigenin (Fig.
2D).
Immunological staining showed that the expression of
FADD was mainly present in the cytoplasm of CRC cells in the
control group, while the overexpression of FADD was observed in
both the cytoplasm and nucleus (Fig.
2E–F) in the apigenin pre-treated mice. Western blotting showed
that the phosphorylation (P<0.05) and expression (P<0.05) of
FADD increased significantly in the apigenin group compared to the
control group (Fig. 2G–H).
Discussion
The real-time in vivo imaging system utilizes
light emitted by a bioluminescent or fluorescent reporter gene (or
fluorescent molecule, such as a dye or quantum dot) expressed in a
living organism and analyzes the source and strength of that
bioluminescent or fluorescent signal non-invasively, allowing for
extensive longitudinal modeling in the same live animal (11). In our study, a CRC cell line stably
expressing eGFP was selected since cancer growth is observed
directly in live animals by epifluorescence. A high correlation was
found between the fluorescence intensity of the cancer and the mass
of cancer cells. Regarding CRC tumors, an imaging system would be
more reliable for sizing, due to the difficulty experienced in
compressing the CRC tumor with the caliper (12). Our study using in vivo
imaging shows that apigenin decreases the region of colon cancer
derived from SW480 by increasing AI. Apigenin inhibits the
proliferation of the human anaplastic thyroid carcinoma cell line
(ARO). The inhibitory effect is associated with the increase of
programmed cell death (13).
Treatment with apigenin significantly inhibited the cell
proliferation in MDA-MB-453 cells that were resistant to
5-fluorouracil. This inhibition resulted in apoptosis, as evidenced
by the increased number of apoptotic cells and the activation of
caspase-3 (14). Taken together,
the results suggest that apigenin inhibits the proliferation of
cancer cells by up-regulating the cell apoptosis rate.
To establish why apigenin inhibits the growth of
CRC, five genes were selected to determine the effect of apigenin
on the proliferation and apoptosis of CRC. CCND1 (15) and BAG-1 (16) overexpression in colorectal tumor
cells contribute to abnormal growth and tumorigenicity, and the
expression of yrdC promotes the proliferation of SW-480 (17). A decreased expression of Bcl-2 in
tumor cells significantly relates to increased tumor size (18). Transfection of a dominant negative
FADD prevents the induction of apoptosis (19). Therefore, the five genes were
screened by real-time quantative RT-PCR and only FADD was found to
be up-regulated by apigenin.
Fas is a death receptor, transducing cell death
signaling upon stimulation by Fas ligand. During Fas-initiated cell
death signaling, the formation of Fas-death-inducing signaling
complex (Fas-DISC) is the initial step. Previous observations
showed that FADD is an essential component of DISC, which contains
a death domain and induces apoptosis when overexpressed in multiple
cell types (20–23). Our results show that FADD expression
is up-regulated in CRC cells with a high AI, which is consistent
with the observation that transfection of a dominant-negative
mutant of FADD decreases the apoptotic response of SW480 cells to
resveratrol (24). These results
suggest that cells reprogrammed to increase the abundance of FADD
may promote apoptosis in cells doomed to die. Since quantitatively
phosphorylated FADD plays an essential role in Burkitt lymphoma
BJAB cell cycle arrest at the G2/M phase (25), the up-regulated phosphorylation of
FADD induced by apigenin may be involved in the regulation of
proliferation of CRC cells. Thus, we propose that apigenin
suppresses the growth of CRC by enhancing the expression of and
inducing the phosphorylation of FADD, which mediates the apoptosis
of tumor cells and inhibits cell proliferation. However, further
studies should be conducted as to which mechanisms of
phosphorylation and translocation of FADD to nucleus are involved
in order to elucidate the preventive effects of apigenin on the
growth of CRC.
Acknowledgements
This study was supported by grants from the National
Natural Sciences Foundation of China (No. 30670580).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
Benson AB III: Epidemiology, disease
progression, and economic burden of colorectal cancer. J Manag Care
Pharm. 13:S5–S18. 2007.PubMed/NCBI
|
|
3
|
Noh HJ, Sung EG, Kim JY, Lee TJ and Song
IH: Suppression of phorbol-12-myristate-13-acetate-induced tumor
cell invasion by apigenin via the inhibition of p38
mitogen-activated protein kinase-dependent matrix
metalloproteinase-9 expression. Oncol Rep. 24:277–283. 2010.
|
|
4
|
Patel D, Shukla S and Gupta S: Apigenin
and cancer chemoprevention: Progress, potential and promise
(review). Int J Oncol. 30:233–245. 2007.PubMed/NCBI
|
|
5
|
Farah M, Parhar K, Moussavi M, Eivemark S
and Salh B: 5,6-Dichloro-ribifuranosylbenzimidazole- and
apigenin-induced sensitization of colon cancer cells to
TNF-alpha-mediated apoptosis. Am J Physiol Gastrointest Liver
Physiol. 285:G919–G928. 2003. View Article : Google Scholar
|
|
6
|
Wang W, Heideman L, Chung CS, Pelling JC,
Koehler KJ and Birt DF: Cell-cycle arrest at G2/M and growth
inhibition by apigenin in human colon carcinoma cell lines. Mol
Carcinog. 28:102–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung CS, Jiang Y, Cheng D and Birt DF:
Impact of adenomatous polyposis coli (APC) tumor supressor gene in
human colon cancer cell lines on cell cycle arrest by apigenin. Mol
Carcinog. 46:773–782. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Au A, Li B, Wang W, Roy H, Koehler K and
Birt D: Effect of dietary apigenin on colonic ornithine
decarboxylase activity, aberrant crypt foci formation, and
tumorigenesis in different experimental models. Nutr Cancer.
54:243–251. 2006. View Article : Google Scholar
|
|
9
|
Leibovitz A, Stinson JC, McCombs WB III,
McCoy CE, Mazur KC and Mabry ND: Classification of human colorectal
adenocarcinoma cell lines. Cancer Res. 36:4562–4569.
1976.PubMed/NCBI
|
|
10
|
Zhao L, Wang H, Li J, Liu Y and Ding Y:
Overexpression of Rho GDP-dissociation inhibitor alpha is
associated with tumor progression and poor prognosis of colorectal
cancer. J Proteome Res. 7:3994–4003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Zhang QL, Jiang HY and Ding YQ:
Establishment of a whole-body visualization model of orthotopically
implanted colorectal carcinoma and metastasis model in nude mice.
Nan Fang Yi Ke Da Xue Xue Bao. 27:1161–1163. 11662007.PubMed/NCBI
|
|
12
|
Cemazar M, Golzio M, Escoffre JM, Couderc
B, Sersa G and Teissié J: In vivo imaging of tumor growth after
electrochemotherapy with cisplatin. Biochem Biophys Res Commun.
348:997–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin F, Giuliano AE and van Herle AJ:
Signal pathways involved in apigenin inhibition of growth and
induction of apoptosis of human anaplastic thyroid cancer cells
(ARO). Anticancer Res. 19:4297–4303. 1999.PubMed/NCBI
|
|
14
|
Choi EJ and Kim GH: 5-Fluorouracil
combined with apigenin enhances anticancer activity through
induction of apoptosis in human breast cancer MDA-MB-453 cells.
Oncol Rep. 22:1533–1537. 2009.PubMed/NCBI
|
|
15
|
Arber N, Doki Y, Han EK, Sgambato A, Zhou
P, Kim NH, Delohery T, Klein MG, Holt PR and Weinstein IB:
Antisense to cyclin D1 inhibits the growth and tumorigenicity of
human colon cancer cells. Cancer Res. 57:1569–1574. 1997.PubMed/NCBI
|
|
16
|
Barnes JD, Arhel NJ, Lee SS, Sharp A,
Al-Okail M, Packham G, Hague A, Paraskeva C and Williams AC:
Nuclear BAG-1 expression inhibits apoptosis in colorectal
adenoma-derived epithelial cells. Apoptosis. 10:301–311. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tao GQ, Yang M, Jiang HH, Yan YQ and Wang
XH: The experiment on expression of human yrdC promotes
proliferation of colon cancer SW-480 cells. Shanghai Med J.
31:596–598. 2008.
|
|
18
|
Ofner D, Riehemann K, Maier H, Riedmann B,
Nehoda H, Tötsch M, Böcker W, Jasani B and Schmid KW:
Immunohistochemically detectable bcl-2 expression in colorectal
carcinoma: correlation with tumor stage and patient survival. Br J
Cancer. 72:981–985. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chinnaiyan AM, Tepper CG, Seldin MF,
O'Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME and
Dixit VM: FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and
tumor necrosis factor receptor-induced apoptosis. J Biol Chem.
271:4961–4965. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boldin MP, Varfolomeev EE, Pancer Z, Mett
IL, Camonis JH and Wallach D: A novel protein that interacts with
the death domain of Fas/APO1 contains a sequence motif related to
the death domain. J Biol Chem. 270:7795–7798. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chinnaiyan AM, O'Rourke K, Tewari M and
Dixit VM: FADD, a novel death domain-containing protein, interacts
with the death domain of Fas and initiates apoptosis. Cell.
81:505–512. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yanase N, Kanetaka Y and Mizuguchi J:
Interferon-α-induced apoptosis via tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-dependent and -independent
manner. Oncol Rep. 18:1031–1038. 2007.
|
|
23
|
Lu HF, Lai KC, Hsu SC, Lin HJ, Yang MD,
Chen YL, Fan MJ, Yang JS, Cheng PY, Kuo CL and Chung JG: Curcumin
induces apoptosis through FAS and FADD, in caspase-3-dependent and
-independent pathways in the N18 mouse-rat hybrid retina ganglion
cells. Oncol Rep. 22:97–104. 2009.PubMed/NCBI
|
|
24
|
Delmas D, Rébé C, Lacour S, Filomenko R,
Athias A, Gambert P, Cherkaoui-Malki M, Jannin B, Dubrez-Daloz L,
Latruffe N and Solary E: Resveratrol-induced apoptosis is
associated with Fas redistribution in the rafts and the formation
of a death-inducing signaling complex in colon cancer cells. J Biol
Chem. 278:41482–41490. 2003. View Article : Google Scholar
|
|
25
|
Scaffidi C, Volkland J, Blomberg I,
Hoffmann I, Krammer PH and Peter ME: Phosphorylation of FADD/MORT1
at serine 194 and association with a 70-kDa cell cycle-regulated
protein kinase. J Immunol. 164:1236–1242. 2000. View Article : Google Scholar : PubMed/NCBI
|