Introduction
Increasing evidence suggests that the migration of
tumor cells is not solely a consequence of genetic alterations, but
is regulated by various epigenetic factors. Chemokines,
neurotransmitters, and other structurally non-related ligands of
serpentine receptors are known to be significant initiators of
migratory activity (1). With the
development of neurobiology, research has revealed that
neurotransmitters are involved in the growth and development of
carcinoma (2,3). γ-aminobutyric acid (GABA) was
originally identified as a principal inhibitory neurotransmitter in
the adult mammalian brain. It has, however, become clear that GABA
and GABA receptors exist in a number of non-neuronal peripheral
tissues (4,5), including the biliary tract system
(6). Moreover, findings of previous
studies showed that the expression of GABA and its synthetic
enzyme, GAD, were significantly increased in neoplastic tissues,
such as colorectal carcinoma, breast cancer and gastric cancer, as
compared with normal tissues (4,7–9).
Furthermore, GABA was found to be related to the invasion and
metastasis of cancer cells (10),
although the effect is different among various tumor types; this
effect involves promotion in prostate cancer (11) and inhibition in colon carcinoma
(12). Previously, we reported on
the inhibition of proliferation of cholangiocarcinoma cells by GABA
(13). Since the manner in which
GABA affects the invasion and metastasis of cholangiocarcinoma
cells remains to be determined, this study aimed to investigate the
effect of GABA on the regulation of cholangiocarcinoma cell
migration and to describe its mechanism of action. A secondary aim
was to determine whether the disposition played any role in the
progression of the disease.
Materials and methods
Materials
Cholangiocarcinoma QBC939 cells (14) were provided by Dr Shu-Guang Wang
(Hepatobiliary Surgery Institute of Chinese PLA, The Third Military
Medical University, Chongqing, China). GABA was produced by Sigma
Company (St. Louis, MO, USA), and it was diluted to concentrations
of 1, 10, 100 and 1000 μmol/l, respectively.
Methods
Cell culture
Cholangiocarcinoma QBC939 cells were cultured at
37°C in RPMI-1640 supplemented with fetal calf serum (100 ml/l),
penicillin (100×103 U/l), streptomycin (100 mg/l) and
L-glutamine (2 mmol/l). The pH was maintained at 7.2–7.4 by
equilibration with 5% CO2. The QBC939 cells were
subcultured with EDTA, and the cells used in the experiment were in
the exponential growth phase.
Cell adhesion assay
Cells in 96-well plates were incubated at 37°C with
Matrigel for 1 h and then blocked with phosphate-buffered saline
(PBS) containing 10 g/l bovine serum albumin (BSA) for another 1 h
at the same temperature. The QBC939 cells were exposed to different
concentrations of GABA (1, 10, 100 and 1000 μmol/l) for 24 h and
were suspended in serum-free medium at a density of
1×105 cells/ml. Then, 0.1 ml of the QBC939 cell
suspension was added to each well, and incubation was carried out
at 37°C for 1 h. The plates were washed three times with PBS to
remove the unattached cells. The remaining QBC939 cells in the
96-well plates were reacted with MTT assay for 4 h at 37°C,
solubilized with DMSO, and the absorbance of each well was measured
at 492 nm with a Biocell HT1 microplate reader. Results were
expressed as the percentage of total cells, assuming that the
adhesion of cells in the control represented 100%.
In vitro invasion assay
An invasion assay assessing the ability of cells to
invade a synthetic basement membrane was performed in transwell
chambers (Costar Co., USA) with a polycarbonate filter (6.5-mm pore
size) separating the upper and lower chambers. The top surface of
the polycarbonate filter was coated with Matrigel, and the bottom
with fibronectin. QBC939 cells (1×105) treated with
different concentrations of GABA (1, 10, 100 and 1000 μmol/l) for
24 h were added to the upper transwell chamber in 100 ml of
serum-free RPMI-1640 containing 0.1% BSA, while 600 ml of
serum-free BSA-RPMI-1640 was added to the lower chamber. After 4 h,
the filters were fixed in methanol and stained with hematoxylin and
eosin. The non-invading cells on the top surface of the filter
membrane were removed with a cotton swab. Cells on the bottom
surface of the filter were counted, and the mean number of cells
was determined from five high-power fields under a light
microscope. The inhibitory rate (IR) was calculated as: IR (%) =
number of invasive cells in the negative control group - number of
invasive cells in the test groups/number of invasive cells in the
negative control group × 100.
Measurement of matrix
metalloproteinases (MMPs) by reverse transcription-polymerase chain
reaction (RT-PCR)
QBC939 cells were treated with different
concentrations of GABA (1, 10, 100 and 1000 μmol/l) for 24 h and
collected by centrifugation. Total RNA was isolated using TRIzol
reagent according to the manufacturer’s instructions. The
concentrations and purity of the total RNA were determined using
the DUR 640 nucleic acid and protein analyzer (Beckman, Coulter,
Fullerton, CA, USA). The first-strand cDNA was synthesized from 5
μg of total RNA using 50 pmol of oligo (dT) primers, 10 units of
AMV reverse transcriptase XL, 20 units RNase inhibitor, 5X buffer
and 10 mmol/l each dNTP in a total volume of 20 μl. PCRs were
performed using the respective primers for MMP-2, MMP-9 and
β-actin. The primer sequences which were obtained from GenBank are
listed in Table I. PCR was carried
out in a 25-μl volume containing 4 μl of CDNA template, 10X PCR
buffer, 20 μmol/l of each primer, 2.5 mmol/l dNTP mixture, and 2.5
units of Taq polymerase. Following denaturation at 96°C for 3 min,
the reaction mixtures were subjected to 35 cycles of PCR
amplification in a PCT-100™ programmable thermal controller. Each
cycle consisted of 45 sec of denaturation at 94°C, a primer
specific annealing temperature and period (at 5°C for 45 sec) and
extension at 72°C (1 min).
 | Table IPrimer sequences and sizes of the
expected PCR products. |
Table I
Primer sequences and sizes of the
expected PCR products.
| Primer | Sequence | Length (bp) |
|---|
| MMP-2 | Sense |
5′-CCACGTGACAAGCCCATGGGGCCCC-3′ | 480 |
| Antisense |
5′-GCAGCCTAGCCAGTCGGATTTGATG-3′ | |
| MMP-9 | Sense |
5′-GCCACTTGTCGGCGATAAGG-3′ | 243 |
| Antisense |
5′-CACTGTCCACCCCTCAGAGC-3′ | |
| β-actin | Sense |
5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ | 610 |
| Antisense |
5′-CTAGAAGCATTTGCGGTGGACAATGGAGGG-3′ | |
The amplified products were separated in 20 g/l
agarose gel and stained with ethidium bromide. Following
electrophoresis, the gel was observed, and images were captured
under an ultraviolet reflector. The density and area of each band
were analyzed using ChemiImager™ 4000 digital system (Alpha
Innotech, San Leandro, CA, USA).
Zymography
Zymography was used for the analysis of MMP activity
secreted into the culture medium of the cell line, as previously
described (15). QBC939 cells were
seeded at a density of 5×104 cells/pore in 24-well
plates and maintained in 400 μl of serum-free medium containing
different concentrations of GABA (1, 10, 100, and 1000 μmol/l) for
24 h. Following centrifugation at 500 g for 10 min, the supernatant
was collected and stored at −20°C. SDS-PAGE was performed on
gradient gels that contained 1.0 g/l gelatin and run for 3–4 h
under non-denaturing conditions. Following electrophoresis, the
gels were incubated in 2.5% Triton X-100 for 1 h and then incubated
in substrate buffer [50 μmol/l Tris (pH 7.5), 10 mmol/l
CaCl2, 200 mmol/l NaCl and 1 μmol/l ZnCl2]
for 18 h at 37°C. Following incubation, the gels were stained in a
solution containing 1 g/l Coomassie blue R250 for 4 h and destained
with 45% methanol and 10% acetic acid until clear bands were
noted.
Statistical analysis
Data were expressed as the mean ± SD.Data analysis
was performed using the one-way ANOVA and the Student’s t-test.
P<0.05 was considered to be statistically significant.
Results
Effect of GABA on the attachment ability
of QBC939 cells
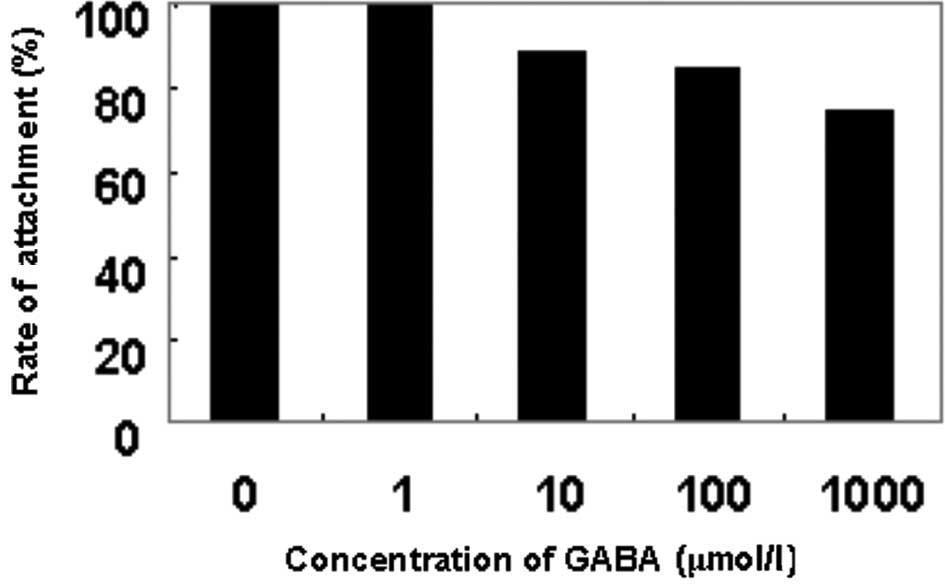
As shown in Fig. 1,
the rate of attachment to Matrigel was decreased as the
concentration of GABA was increased. At the concentration of 10
μmol/l GABA, the rate of cell attachment was significantly lower
than that of the negative control group (P<0.05), and the
inhibitory rate was 10.9%. The inhibitory effect was positively
correlated with the concentration of GABA.
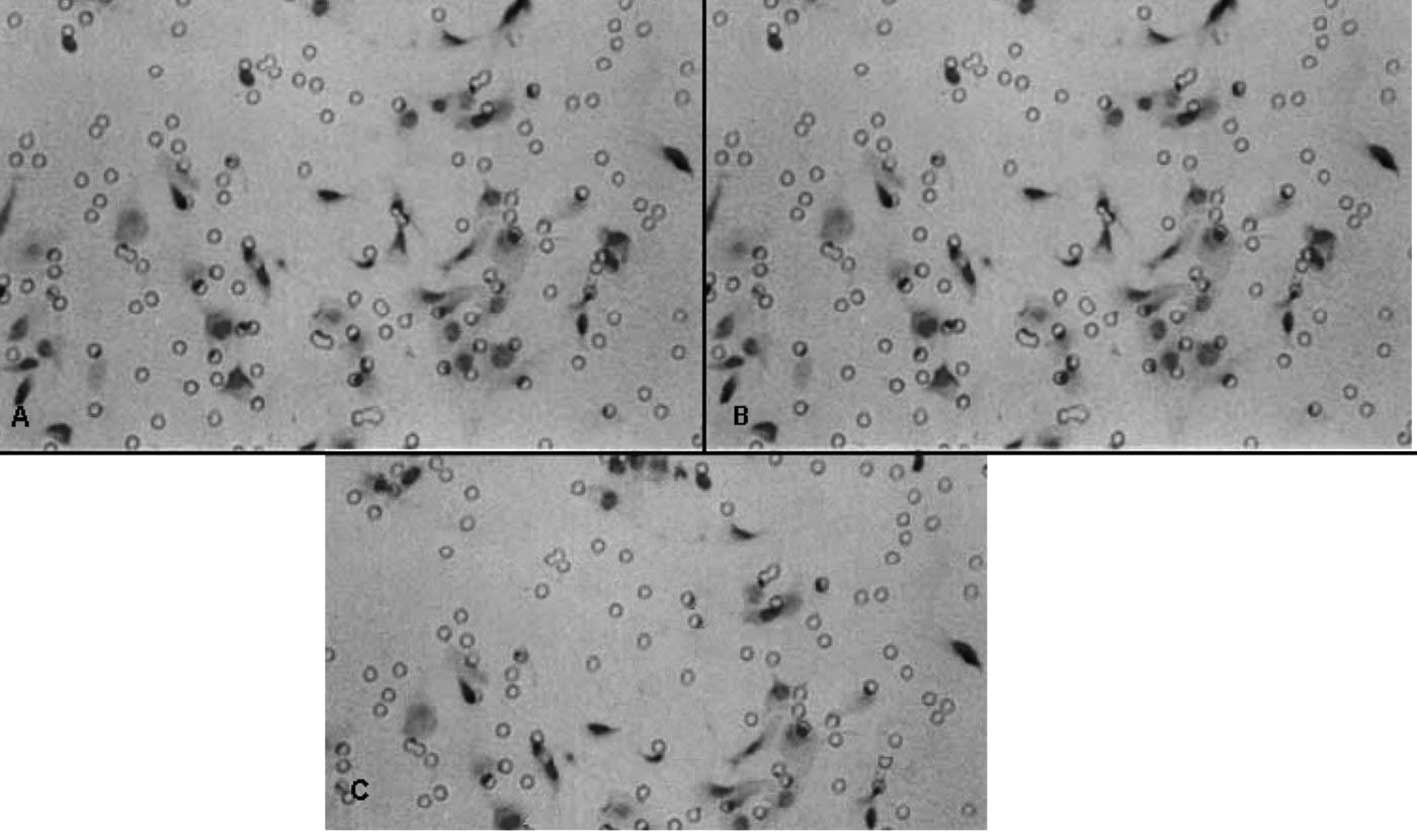
Effect of GABA on the invasive ability of
QBC939 cells
Table II shows that
the invasive abilities of the QBC939 cells treated with 100 and
1000 μmol/l of GABA were significantly lower than that of the
negative control group (P<0.01), and the inhibitory rates were
28.88 and 34.45%, respectively. At 10 μmol/l GABA, the inhibitory
rate was 16.12%, but no statistical significance was noted when
compared to the negative control group (P>0.05). The effect of
GABA on the invasive ability of QBC939 cells is shown in Fig. 2.
 | Table IIEffect of γ-aminobutyric acid on the
invasive ability of QBC939 cells. |
Table II
Effect of γ-aminobutyric acid on the
invasive ability of QBC939 cells.
| Groups | Cell number (mean ±
SD) | Inhibitory frequency
(%) |
|---|
| Negative control | 60.00±9.54 | 0 |
| 1 μmol/l GABA | 58.33±4.04 | 2.78 |
| 10 μmol/l | 50.33±5.51 | 16.12 |
| 100 μmol/l | 42.67±4.04a | 28.88 |
| 1000 μmol/l | 39.33±3.52a | 34.45 |
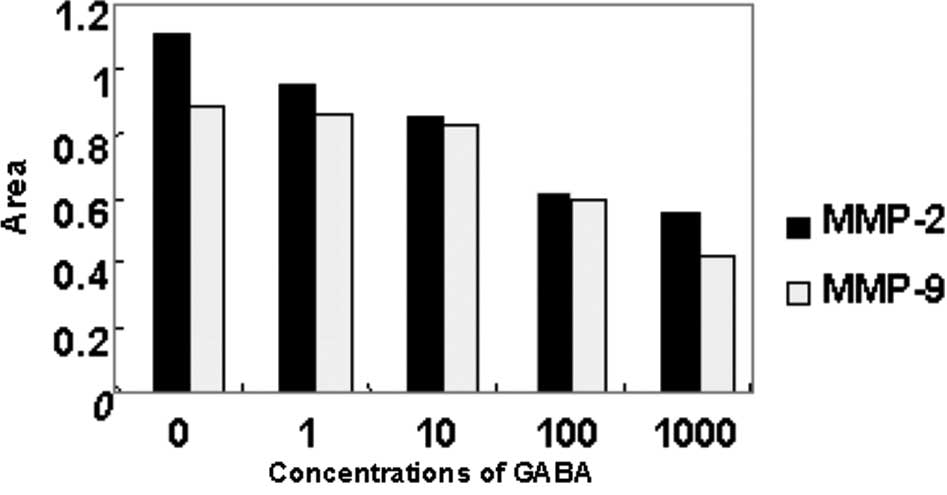
Effect of GABA on collagenase activity in
QBC939 cells
As shown in Figs. 5
and 6, MMP-2 and MMP-9 expression
was detected in the serum-free medium supernatant of the QBC939
cells. Moreover, the activity of MMP-2 was stronger than that of
MMP-9. At concentrations of 10, 100 and 1000 μmol/l, GABA
significantly reduced MMP-9 (92 kDa type IV collagenase) and MMP-2
(72-kDa type II collagenase) activity in a dose-dependent manner.
However, at 1 μmol/l GABA, the collagenase activity did not differ
from that in the negative control group (Figs. 3 and 4).
Effect of GABA on the expression of MMP-2
and MMP-9 mRNA in QBC939 cells
The expression of MMP-2 and MMP-9 mRNA of QBC939
cells treated at different concentrations of GABA decreased in
comparison with that of the negative control group. As the
concentrations of GABA increased, the expression of MMP-2 and MMP-9
mRNA was down-regulated. Moreover, the decrease in MMP-2 mRNA
expression was more evident (Figs.
5 and 6).
Discussion
Our previous study (13) showed that GABA inhibits the
proliferation of cholangiocarcinoma QBC939 cells in vitro,
which is in accordance with its effects in other types of cancer.
However, the effect of GABA on cancer metastasis was found to vary
in different types of cancer. No report currently exists on the
effect of GABA in cholangiocarcinoma.
The present study is the first to prove that GABA
inhibits the metastasis of cholangiocarcinoma QBC939 cells in
vitro. Cancer metastasis involves a complex cascade of events
involving tumor dissemination from the primary site to distant
organs. Cancer cells must detach from the primary tumor, invade
stromal tissue, enter the circulation, extravasate and invade the
target organ, forming a metastatic colony. This increase or
decrease in heterotypic adhesion to a basement membrane has been
defined as the critical event of tumor invasion that signals the
initiation of the metastatic cascade (16,17).
In the present study, heterotypic adhesion of QBC939 cells to the
artificial basement membrane, Matrigel, was examined using an MTT
dye assay to stain adhered cells. Results showed that GABA
dose-dependently decreased the adhesive rate of QBC939 cells.
Moreover, we utilized an invasion assay using transwell chambers to
assess the ability of cells to invade a synthetic basement
membrane. The results showed that the invasive ability of the
QBC939 cells to the basement membrane was decreased in the two
experiments confirming that GABA inhibits the invasive ability of
QBC939 cells.
Proteolytic degradation of the extracellular matrix
is a key step in invasion, and MMPs are shown to be crucial
proteinases that enable tumor cells to permeate the basement
membrane and invade surrounding tissues. MMPs are a family of
highly homologous protein-degrading zinc-dependent enzyme
endopeptidases. This family currently includes more than 25 members
which can be divided into collagenases (MMP-1, -8 and -13),
gelatinases (MMP-2 and -9), stromelysins (MMP-3 and -10),
matrilysins (MMP-7 and -26) and the membrane-type MMPs (MMP-14 to
-17 and -24). In the present study, expression of MMP-2 mRNA and
MMP-9 mRNA was detected in the serum-free medium supernatant of
QBC939 cells although the activity of MMP-9 was lower than that of
MMP-2. Following treatment with GABA, the expression of MMP-2 and
MMP-9 mRNA of QBC939 cells was decreased as was their activity.
These results showed that GABA inhibits the invasion and metastasis
of cholangiocarcinoma cells in vitro, and the mechanism of
this effect was the inhibition of the expression and activity of
MMP-2 and MMP-9. Moreover, MMP-2 activity was a more important
factor in this effect. These findings indicate that GABA
stimulation promotes cancer cell invasion and that GABA-induced
cancer cell invasion is attributable to an increase in MMP-2 and
MMP-9.
Collectively, based on the findings of our study and
that of previous literature, GABA production was found to be
enhanced by an increase in diamines, polyamines and activity of
diamine oxidase. In the present study, an increase in GABA
secretion inhibited malignant cell proliferation and invasion.
These findings prove that the increase in content, production, and
secretion of GABA may constitute a cell response and an immune
defense mechanism against tumor development. However, even when
malignant cells produce high GABA concentrations, such levels are
much lower (18,19) than the doses of neuropeptides
required to inhibit cancer cell proliferation. Thus, we suggest
that the increased production of GABA by tumoral cells is
insufficient to block tumor growth, since inadequate concentrations
of the neuropeptide are achieved. These results also provided us
with theoretical proof that a good state of mind is helpful for the
rehabilition of disease or may slow the progression of disease,
particularly in cancer.
In conclusion, the present results indicate that
GABA inhibits cancer cell invasion, and a decrease in MMP-2 and
MMP-9 activity is the underlying mechanism of action. Although
findings of previous studies showed that inhibition of MMP activity
may not adequately prevent cancer growth or metastasis (20), further investigation is required.
Activation of the GABA-mediated pathway, possibly combined with MMP
inhibition, exhibits potential therapeutic value to prevent cancer
progression or metastasis and may warrant further attention,
particularly for the treatment of cancer patients with
metastasis.
References
|
1
|
Entschladen F, Lang K, Drell TL, Joseph J
and Zanker KS: Neurotransmitters are regulators for the migration
of tumor cells and leukocytes. Cancer Immunol Immunother.
51:467–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li L, Gang C and Zhang GY: Effects of
neurotransmitters on the proliferation of human hepatocytes. Chin J
Bases Clin General Surg. 9:238–241. 2002.
|
|
3
|
Wu Fy, Ou ZL and Shao ZM: Classical
neurotransmitter and cancer metastasis. Foreign Medical Sciences
(Oncology section). 32:678–680. 2005.
|
|
4
|
Watanabe M, Maemura K, Kanbara K, Tamayama
T and Hayasaki H: GABA and GABA receptors in the central nervous
system and other organs. Int Rev Cytol. 213:1–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roberts E: Adventures with GABA: fifty
years on. GABA in the Nervous System. The View at Fifty Years.
Martin D and Olsen R: Lippincott Williams and Wilkins;
Philadelphia: pp. 1–24. 2000
|
|
6
|
Saito N, Taniyama K and Tanaka C: Uptake
and release of gamma-aminobutyric acid in guinea pig gallbladder.
Am J Physiol Gastrointest Liver Physiol. 249:G192–G196.
1985.PubMed/NCBI
|
|
7
|
Maemura K, Yamauchi H, Hayasaki H, et al:
Gamma-amino-butyric acid immunoreactivity in intramucosal colonic
tumors. J Gastroenterol Hepatol. 18:1089–1094. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kleinrok Z, Matuszek M, Jesipowicz J,
Matuszek B, Opolski A and Radzikowski C: GABA content and GAD
activity in colon tumors taken from patients with colon cancer or
from xenografted human colon cancer cells growing as s. c tumors in
athymic nu/nu mice. J Physiol Pharmacol. 49:303–310.
1998.PubMed/NCBI
|
|
9
|
Matuszek M, Jesipowicz M and Kleinrok Z:
GABA content and GAD activity in gastric cancer. Med Sci Monit.
7:377–381. 2001.PubMed/NCBI
|
|
10
|
Ortega A: A new role for GABA: inhibition
of tumor cell migration. Trends Pharmacol Sci. 24:151–154. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Azuma H, Inamoto T, Sakamoto T, et al:
Gamma-aminobutyric acid as a promoting factor of cancer metastasis;
induction of matrix metalloproteinase production is potentially its
underlying mechanism. Cancer Res. 63:8090–8096. 2003.
|
|
12
|
Joseph J, Niggemarm B, Zaenker KS, et al:
The neurotransmitter gamma-aminobutyric acid is an inhibitory
regulator for the migration of SW 480 colon carcinoma cells. Cancer
Res. 62:6467–6469. 2002.PubMed/NCBI
|
|
13
|
Liu CH, Huang Q and Wang C:
Gamma-aminobutyric acid-induced apoptosis and effect of telomerase
activity of cholangiocarcinoma cell line QBC939. Chin J
Hepatobiliary Surg. 15:41–44. 2009.
|
|
14
|
Wang SG, Han BL, Duan HC, et al:
Establishment of the characteristics of a cell line of extrahepatic
cholangiocarcinoma. Chin J Exp Surg. 14:67–70. 1997.
|
|
15
|
Ries C, Loher F, Zang C, Ismair MG and
Petrides PE: Matrix metalloproteinase production by bone marrow
mononuclear cells from normal individuals and patients with acute
and chronic myeloid leukemia or myelodysplastic syndromes. Clin
Cancer Res. 5:1115–1124. 1999.
|
|
16
|
Stetler-Stevenson WG and Kleiner:
Molecular biology of cancer: invasion and metastases. Cancer
Principles and Practice of Oncology. 6th edition. Devita VT,
Hellman S and Rosenberg SA: Lippincott Williams Willkins;
Philadelphia: pp. 123–136. 2001
|
|
17
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
18
|
Chanda R and Ganguly AK: Diamine-oxidase
activity and tissue di- and poly-amine contents of human ovarian,
cervical and endometrial carcinoma. Cancer Lett. 89:23–28. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicholson-Guthrie CS, Guthrie GD, Sutton
GP and Baenziger JC: Urine GABA levels in ovarian cancer patients:
elevated GABA in malignancy. Cancer Lett. 162:27–30. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|