Introduction
Oral cancer is one of the ten most common cancers in
the world, accounting for approximately 2% of all cancer types and
1% of all cancer-related deaths (1). Squamous cell carcinoma (SCC) is the
most common malignant tumor of the oral cavity, accounting for over
90% of the malignant neoplasms in this region (2). Despite recent advances in the
diagnosis and treatment modalities of surgery, radiotherapy and
chemotherapy for oral cancer, the 5-year survival rate has improved
only marginally (2). This result
indicates the limitations of these treatment modalities. Therefore,
additional treatment strategies, such as immunotherapy, are
required to improve the 5-year survival rate.
Cancer patients often suffer from immunodeficiency
and increased susceptibility to infection, resulting in death
(3). Macrophage activation in
phagocytosis and subsequent antigen presentation are involved in
immune development, and the capacity of macrophages to be activated
is indicative of host immune potential (4). Serum vitamin D3-binding protein (Gc
protein) is the precursor for the principal macrophage activating
factor (MAF). The MAF precursor activity of the serum Gc protein of
various cancer patients, including oral cancer patients, was lost
or reduced since the Gc protein is deglycosylated by serum
α-N-acetyl galactosaminidase (Nagalase) secreted from cancer cells
(5). Deglycosylated Gc protein
cannot be converted to MAF, leading to immunosuppression.
Administration of the Gc protein-derived MAF (GcMAF) that was
generated enzymatically in vitro from the Gc protein in
human serum, bypasses the impaired macrophage activation cascade
and efficiently activates macrophages. Highly activated macrophages
have been reported to have a tumoricidal potential (6–9). Pilot
studies have reported the efficacy of GcMAF-based immunotherapy of
metastatic cancer in animal and humans (10–13).
The aim of the present study was to investigate
whether or not GcMAF has an inhibitory effect on oral
carcinogenesis and tumor growth, using a
9,10-dimethyl-1,2-benzanthracene (DMBA)-induced hamster cheek pouch
carcinogenesis model. The cytocidal effect of GcMAF on its derived
squamous carcinoma cell line, HCPC-1, was also been examined, as
well as the possible combination of immunotherapy with GcMAF for
oral cancer.
Materials and methods
Preparation of GcMAF
Human serum was heat-inactivated at 60˚C for 1 h and
Gc protein fraction was precipitated by mixing with 30% saturated
ammonium (14).
The precipitate was dissolved in phosphate-buffered
saline (PBS) (pH 7.4) containing 0.5% Triton X-100 and 0.3%
tri-n-butyl phosphate, and was maintained overnight at room
temperature to resolve the lipid containing microbial contaminants.
The sample was precipitated by 30% saturated ammonium sulfate,
dissolved in 50 mM citrate buffer at pH 4.0 and maintained
overnight. Gc protein was purified using 25-hydroxyvitamin
D3-affinity chromatography (15).
Stepwise digestion of purified Gc protein with immobilized
β-galactosidase and sialidase yielded the most potent macrophage
activating factor (GcMAF) (16,17).
The immobilized enzymes were removed by
centrifugation. The final product, GcMAF, was filtered through a
low protein-binding filter, Millex-HV (Millipore Corp., Bedford,
MA, USA) for sterilization.
Animals, carcinogen treatment and GcMAF
administration
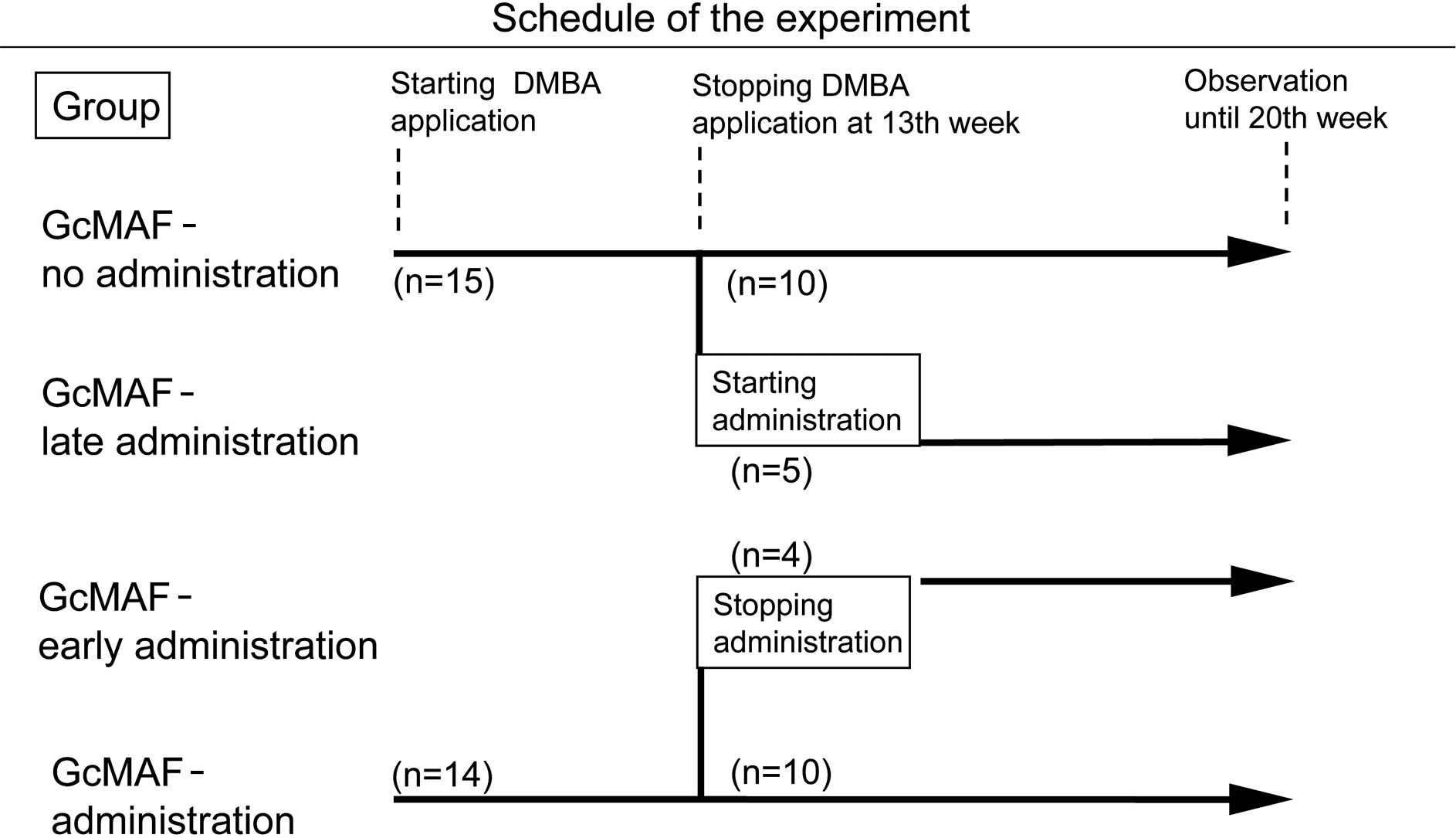
A total of 29 male golden Syrian hamsters, at 5
weeks of age, were purchased from Nihon Animal Inc. (Osaka, Japan).
The animals were divided into two groups: 14 hamsters with GcMAF
administration by intramuscular injection with 100 pg/hamster to
the thigh twice a week from the beginning of DMBA application,
while 15 hamsters without GcMAF administration served as controls
(Fig. 1). The GcMAF dose of 100
pg/hamster was employed according to the study reported previously
in mice bearing Ehlich ascites tumor (10). These hamsters were treated under
ether anesthesia by painting a cheek pouch three times a week with
1% solution of DMBA (Wako Pure Chemical Industries, Ltd., Osaka,
Japan) dissolved in acetone, as previously described (18,19).
DMBA application was continued until the 13th week. The diameter of
tumors formed was measured using calipers and the body weight of
the hamsters was simultaneously measured once a week.
From the 13th week, when tumors had formed on the
cheek pouches of all of the hamsters, GcMAF administration was
started by intramuscular injection with 100 pg/hamster twice a week
in 5 out of the 15 hamsters in the control group (late
administration of GcMAF) and stopped in 4 out of the 14 hamsters in
the GcMAF-treated group (early administration of GcMAF).
Animal experiments were performed in compliance with
the Guidelines for Animal Experiments of the Hyogo College of
Medicine.
Superoxide generation assay
Peritoneal cells were collected by peritoneal lavage
with cold PBS, washed three times in cold PBS and plated in a 16-mm
multiwell plate in DMEM (Gibco-BRL, Grand Island, NY, USA)
supplemented with 1% fetal bovine serum (FBS; Hyclone Laboratories,
South Logan, UT, USA). The cells were incubated at 37˚C for 30 min
to facilitate the adherence of macrophages to plastic substrate and
then washed with PBS gently to remove non-adherent cells. Various
concentrations of GcMAF were added and the cells were incubated at
37˚C for 3 h. To measure the activation of macrophages, the culture
medium was replaced with 1 ml of PBS containing 20 μg of
cytochrome C (Sigma-Aldrich Co., St. Louis, MO, USA) and incubated
for 10 min. Approximately 30 min after the addition of 10 μl
PBS containing 0.5 μg of phorbol 12-myristyl acetate (PMA;
Sigma-Aldrich Co.), the superoxide-generating activity of
macrophages was determined by measuring the absorbance at 550
nm.
For the in vivo activation assay of
macrophages, GcMAF (100 pg/hamster) was injected into the thigh of
hamsters intramuscularly. The peritoneal cells were harvested 48 to
96 h after injection and assayed for superoxide generation as
described above.
Culture of hamster HCPC-1 cells and
treatment with GcMAF-activated macrophages
HCPC-1 cells were isolated and established from the
7,12-dimethylbenz(α)anthracene-induced epidermoid carcinoma of
golden Syrian hamster cheek pouch. The cells were kindly provided
by Dr G. Shklar (Harvard School of Dental Medicine, Boston, MA,
USA) (20). To examine the
cytocidal effect of GcMAF-activated macrophages on HCPC-1 cells,
the cells were plated at a density of 105 cells/well in
DMEM supplemented with 10% FBS in a multiwell plate and incubated
at 37˚C for 24 h. Non-activated macrophages or macrophages
activated with GcMAF in vitro or in vivo were then
added at the effector to target ratio (E:T ratio) of 5:1 and
further incubated at 37˚C for 48 h. Peritoneal macrophages were
activated either by intramuscular injections with 100 pg GcMAF
twice (1 and 4 days prior to collection) in vivo or by the
addition of 100 pg GcMAF to the medium and incubation at 37°C for 1
h in vitro. Viable HCPC-1 cells were counted at constant
intervals by the nigrosin exclusion test in a hemocytometer.
The effect of the addition of heat-inactivated DMBA-
induced tumor-bearing hamster serum on macrophage-directed
cytotoxicity with GcMAF in HCPC-1 cells was also studied, since it
was reported that the tumoricidal activity of activated macrophages
was markedly enhanced by the addition of tumor-bearing patient
serum in the human retinoblastoma cell line W24 (6).
The tumor-bearing hamster serum, ~20 weeks after
DMBA application, was collected from 5 hamsters and mixed together
just before the experiment. The tumor-bearing or normal hamster
serum was added to the culture medium at the final concentration of
5% after heat-inactivation at 56˚C for 30 min.
Statistical analysis
Statistical analysis of the data was per-formed by
using the Student's t-test. P<0.05 was considered to be
statistically significant.
Results
Suppression of carcinogenesis and tumor
growth by GcMAF administration
SCC was produced from the 9th to the 11th week after
DMBA application in all 15 hamsters of the control group without
GcMAF administration, and all died of tumor burden within 20 weeks.
Out of the 14 hamsters, 2 animals with GcMAF administration did not
develop tumors, while the remaining 12 hamsters showed a
significant delay of tumor development for ~3.5 weeks in addition
to suppressed tumor growth. These 12 hamsters survived until the
20th week of the experimental period (Table I and Fig. 2). The body weight loss associated
with tumor burden was significantly higher in the control group
than that in the GcMAF-treated group (Fig. 3).
 | Table IEffect of GcMAF administration on
DMBA-induced hamster cheek pouch carcinogenesis. |
Table I
Effect of GcMAF administration on
DMBA-induced hamster cheek pouch carcinogenesis.
| Treatment | Tumor prevalence
(%) | Onset of tumor
formation (weeks) | Tumor diameter
(mm) | Tumor death (%) | Mean survival time
(weeks) |
|---|
| | |
| | |
|---|
| | | 13th week | 16th week | Ratio | | |
|---|
| GcMAF-no
administration (n=10) | 10/10 (100) | 9.9±0.9 | 5.3±3.9 | 17.9±5.0 | 3.38 | 10/10 (100) | 15.0±2.1 |
| GcMAF-late
administration (n=5) | 5/5 (100) | | 3.0±1.5 | 8.0±2.9 | 2.67 | 5/5 (100) | 17.4±0.5b |
| GcMAF-early
administration (n=4) | 4/4 (100) | 13.4±0.8 | 2.3±1.8 | 7.7±2.7 | 3.35 | 0/4 (0) | >20c |
|
GcMAF-administration (n=10) | 8/10 (80) | | 1.5±1.2 | 2.8±2.5 | 1.87a | 0/8 (0) | >20c |
When GcMAF administration was started from the 13th
to the 20th week in 5 tumor-bearing hamsters in the control group,
tumor growth was slightly suppressed and all of the hamsters died
of tumor burden; however, body weight loss was significantly
inhibited and the mean survival time was extended. In contrast,
when GcMAF administration was stopped in the 13th week in 4
tumor-bearing hamsters in the GcMAF-treated group, tumor growth and
body weight loss were promoted, but none of the hamsters died
within the 20-week period (Table I,
Figs. 2 and 3).
Macrophage activation with GcMAF assayed
by superoxide generation
When hamster peritoneal macrophages were treated
with various concentrations of GcMAF in vitro, superoxide
generation was increased 9-fold in a dose-dependent manner,
indicating that the macrophage activation needed to cause efficient
tumoricidal effect (Table II).
Intramuscular administration of GcMAF to hamsters also showed an
~3-fold increase of superoxide generation in macrophages as
compared to the control at 48 and 96 h post injection.
 | Table IISuperoxide generation in macrophages
with GcMAF treatment in vitro and in vivo. |
Table II
Superoxide generation in macrophages
with GcMAF treatment in vitro and in vivo.
| GcMAF
treatment | Generation of
superoxide (nmol/min/106 cells) | Ratio |
|---|
| In vitro
experiment (treatment with GcMAF at 37˚C for 3 h) |
| GcMAF no
administration | 0.094±0.02 | 1.0 |
| GcMAF (0.1
pg/ml) | 0.209±0.04 | 2.2 |
| GcMAF (1
pg/ml) | 0.335±0.04 | 3.6 |
| GcMAF (10
pg/ml) | 0.586±0.05 | 6.2 |
| GcMAF (100
pg/ml) | 0.848±0.06 | 9.0 |
| In vivo
experiment (intramuscular injection of 100 pg of
GcMAF/hamster) |
| GcMAF no
administration | 0.094±0.02 | 1.0 |
| 48 h post
injection | 0.293±0.03 | 3.1 |
| 96 h post
injection | 0.324±0.05 | 3.4 |
Cytocidal effect of GcMAF-activated
macrophages on HCPC-1 cells
The cytocidal effect of GcMAF-activated macrophages
was examined by treatment with an E:T ratio of 5:1 at 37˚C for 48 h
on HCPC-1 cells. As shown in Table
III, the number of viable cells was decreased by 69% even with
the addition of non-activated macrophages, but decreased to ~55% in
the case of macrophages activated with 100 and 200 pg of GcMAF. A
similar or stronger effect was obtained by treatment with
macrophages activated in vivo as well as in vitro
(Table IV). To investigate the
effect of tumor-bearing hamster serum on macrophage-directed
cytotoxicity in HCPC-1 cells, tumor-bearing hamster serum was added
at a final concentration of 5% to the medium. As shown in Table V, the tumor-bearing hamster serum
was more cytotoxic than normal serum, and the number of viable
cells was decreased by 60%. This was a similar level to that
obtained through the addition of non-activated macrophages.
However, treatment with GcMAF-activated macrophages reduced the
viable cells by 46% and the combined treatment with GcMAF-activated
macrophages and tumor-bearing hamster serum markedly reduced the
viable cells by 11%. HCPC-1 cells attacked by macrophages became
round and were eradicated (Fig.
4).
 | Table IIICytocidal effect of peritoneal
macrophages activated with GcMAF in vitro on HCPC-1
cells. |
Table III
Cytocidal effect of peritoneal
macrophages activated with GcMAF in vitro on HCPC-1
cells.
| Macrophages (Mφ)
treated | No. of viable
HCPC-1 cells (×104) | Ratio |
|---|
| Control | 100.3±10.0 | 1.00 |
| Non-activated
Mφ | 69.0±4.5 | 0.69a |
| Activated Mφ with 1
pg of GcMAF | 66.5±3.4 | 0.66a |
| Activated Mφ with
10 pg of GcMAF | 62.3±4.6 | 0.62a |
| Activated Mφ with
100 pg of GcMAF | 54.8±5.6 | 0.55a |
| Activated Mφ with
200 pg of GcMAF | 54.3±2.6 | 0.54a |
 | Table IVComparison of cytocidal effect of
peritoneal macrophages activated with GcMAF in vitro and
in vivo on HCPC-1 cells. |
Table IV
Comparison of cytocidal effect of
peritoneal macrophages activated with GcMAF in vitro and
in vivo on HCPC-1 cells.
| Macrophages (Mφ)
treated | No. of viable
HCPC-1 cells (×104) | Ratio |
|---|
| Control | 93.6±9.0 | 1.00 |
| GcMAF | 83.6±11.0 | 0.90 |
| Non-activated
Mφ | 71.6±1.8 | 0.76 |
| In vitro
activated Mφ | 61.9±3.0 | 0.66a |
| In vivo
activated Mφ | 39.5±4.2 | 0.42b |
 | Table VElevated cytocidal effect of
peritoneal macrophages activated with GcMAF in vivo by the
addition of tumor-bearing hamster serum on HCPC-1 cells. |
Table V
Elevated cytocidal effect of
peritoneal macrophages activated with GcMAF in vivo by the
addition of tumor-bearing hamster serum on HCPC-1 cells.
| Macrophages (Mφ)
and serum | No. of viable
HCPC-1 cells (×104) | Ratio |
|---|
| Control | 116.4±15.7 | 1.00 |
| + Normal serum | 103.4±6.7 | 0.89 |
| + Tumor-bearing
serum | 68.6±14.1 | 0.59a |
| + Non-activated
Mφ | 72.0±4.5 | 0.62b |
| + Non-activated Mφ
+ normal serum | 68.0±3.8 | 0.58c |
| + Non-activated Mφ
+ tumor-bearing serum | 52.0±9.6 | 0.45c |
| + In vivo
activated Mφ | 53.6±4.3 | 0.46c |
| + In vivo
activated Mφ + normal serum | 50.6±6.9 | 0.43c |
| + In vivo
activated Mφ + tumor-bearing serum | 13.0±2.6 | 0.11c |
Discussion
Inflammation of cancer tissues induced by the
intratumoral administration of Bacillus Calmette-Guerin
(BCG) or other bacterial cells has been widely established to
result in the regression of local as well as metastasized tumors,
suggesting the development of specific immunity against the tumor
(21,22). Since cancer tissues comprise
alkylphospholipids, inflamed cancer tissues release
lysoalkylphospholipids and alkylglycerols (23–26).
Lysoalkylphospholipids and alkylglycerols are both potent
macrophage-activating agents, and inflammation-derived macrophage
activation is the principal macrophage activation process requiring
serum vitamin D3-binding protein (Gc Protein) (16,27–31).
Hydrolysis of the Gc protein with the inducible membrane
β-galactosidase of inflammation-primed B cells as well as the
membranous Neu-1 sialidase of T cells generates GcMAF. GcMAF can be
generated enzymatically in vitro by the stepwise treatment
of highly purified Gc protein with immobilized β-galactosidase and
sialidase (10,17,28).
Pilot studies have reported GcMAF antitumor effects on a variety of
cancers, such as colorectal, breast and prostate cancer (11–13).
Moreover, 83% of patients with oral SCC were found to have
decreased precursor activity of serum Gc protein and, by contrast,
increased Nagalase activity, which efficiently deglycosylated Gc
protein (5). Therefore, exogenous
administration of GcMAF is considered to inhibit oral
carcinogenesis and tumor growth. This study aimed to investigate
the antitumor effect of GcMAF on oral cancer by using a
well-established DMBA-induced hamster cheek pouch carcinogenesis
model and its derived carcinoma cell line.
Consequently, administration of GcMAF from the
beginning of DMBA application to cheek pouches resulted in
decreased tumor prevalence and a significant delay in tumor
formation. Tumor growth was retarded significantly and no death due
to tumor burden was noted until the 20th week of observation.
On the other hand, all hamsters without GcMAF
administration developed tumors by the 11th week and died by the
20th week. This antitumor effect of GcMAF was further evidenced by
starting or stopping GcMAF administration halfway through the
experiment. On the other hand, tumor growth was slightly suppressed
when GcMAF administration was commenced from the halfway point, but
the mean survival time was prolonged significantly. On the other
hand, stopping GcMAF administration at the halfway point promoted
tumor growth, but maintained no tumor death and the mean survival
time was extended (Fig. 2 and
Table I). In this case, since the
mean tumor diameter between two subgroups of 10 hamsters without
GcMAF administration and 5 hamsters with late administration of
GcMAF differed by more than 2-fold, the results in tumor growth and
mean survival time may be biased. However, when the two subgroups
of early and late administration of GcMAF were compared, early
administration of GcMAF was found to be more effective in the
elongation of life span than late administration. In addition,
GcMAF administration has no observed side effects and has prevented
body weight loss in tumor-bearing animals (Fig. 3). To evaluate the macrophage
activation with GcMAF, the generation of superoxide from peritoneal
macrophages was measured. Superoxide was efficiently generated in a
dose-dependent manner by in vitro treatment with GcMAF.
Similarly, in vivo treatment with GcMAF generated superoxide
to the same extent at 48 and 96 h post injection (Table II).
Highly activated macrophages with GcMAF have already
been reported to have a strong tumoricidal activity (6–9). In
order to investigate the antitumor effect of GcMAF on DMBA-induced
hamster cheek pouch carcinogenesis, tumoricidal activity of
GcMAF-activated macrophages was examined using HCPC-1, which is a
squamous carcinoma cell line derived from DMBA-induced cheek pouch
carcinoma. Consequently, macrophages activated in vitro and
in vivo with GcMAF demonstrated a significant tumoricidal
activity on HCPC-1 cells, as compared to non-activated macrophages.
This activity was dose-dependent with a plateau in 100 pg
administration of GcMAF (Tables
III and IV). Since it was
reported that the tumoricidal activity of macrophages
photodynamically activated with hematoporphyrin derivative was
markedly enhanced on human retinoblastoma cells by the addition of
retinoblastoma patient serum in culture (6), the effect of the addition of
tumor-bearing hamster serum was tested on tumoricidal activity for
HCPC-1 cells. As expected, a marked tumoricidal activity for HCPC-1
cells was observed and 89% of the cells were killed in contrast to
57% of cells killed by activated macrophages and normal hamster
serum (Table V). This finding
suggests that tumor-bearing hamsters carry IgG-antibodies against
antigens, such as tumor-associated antigen (32–35)
for cheek pouch carcinoma. Additionally, Fc-receptors of activated
macrophages promote the ingestion of tumor cells. Since
GcMAF-treated macrophages develop a large number of Fc-receptors
(4,17,36),
this enhanced cell killing is considered to be due to an
Fc-receptor-mediated process.
Although GcMAF therapy has been found to show
curative effects on a variety of cancers (10–13,37,38),
Yamamoto et al stated that the efficacy of GcMAF therapy for
a variety of cancer types depends on the degree of cell membrane
abnormality (12,13). Undifferentiated tumor cells are
killed more efficiently than differentiated tumor cells.
Adenocarcinoma, such as breast and prostate cancer, is
undifferentiated and killed rapidly by the activated macrophages,
whereas well-differentiated tumor cells, such as SCC cells, are
slowly killed by the activated macrophages. SCC occupies
approximately 90% of oral cancer, the majority of which is
well-differentiated SCC. DMBA-induced cheek pouch carcinoma is also
well-differentiated SCC. These facts suggest that GcMAF therapy is
not as efficacious in oral cancer as compared to other types of
cancer. In the present study, however, GcMAF exhibited a marked
retardation in tumor development and growth was demonstrated by,
along with increased survival time, without any noteworthy side
effects. GcMAF treatment may therefore have therapeutic potential
for oral cancer, as supported by this animal model.
Acknowledgements
This study was supported by a Grant-in-Aid for
Scientific Research from the Ministry of Education, Science and
Culture of Japan (no. 09672086 to M.U. and no. 11771304 to H.K.),
and a Grant-in-Aid for Researchers, Hyogo College of Medicine, to
S.H.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Von Dongen GA and Snow GB: Prospectives
for future studies in head and neck cancer. Eur J Surg Oncol.
23:485–491. 1997.
|
|
3
|
Gross L: Immunological defect in aged
population and its relationship to cancer. Cancer Res. 18:201–204.
1965.PubMed/NCBI
|
|
4
|
Ngwenya BZ and Yamamoto N: Activation of
peritoneal macrophages by lysophosphatidylcholine. Biochim Biophys
Acta. 839:9–15. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto N, Naraparaju VR and Urade M:
Prognostic utility of serum α-N-acetylgalactosaminidase and
immunosuppression resulted from deglycosylation of serum Gc protein
in oral cancer patients. Cancer Res. 57:295–299. 1997.
|
|
6
|
Yamamoto N and Hoober JK: Tumoricidal
capacities of macrophages photodynamically activated with
hematoporphyrin derivative. Photochem Photobiol. 56:245–250. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fidler JJ and Schroit AJ: Recognition and
destruction of neoplastic cells by activated macrophages:
discrimination of altered self. Biochim Biophys Acta. 948:151–173.
1988.PubMed/NCBI
|
|
8
|
Keller R: The macrophage response to
infectious agents: mechanisms of macrophage activation and tumor
cell killing. Res Immunol. 144:271–273. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klostergard J: Macrophage tumoricidal
mechanisms. Res Immunol. 144:274–276. 1993. View Article : Google Scholar
|
|
10
|
Yamamoto N and Naraparaju VR:
Immunotherapy of BALB/c mice bearing Ehrlich ascites tumor with
vitamin D-binding protein-derived macrophage activating factor.
Cancer Res. 57:2187–2192. 1997.PubMed/NCBI
|
|
11
|
Yamamoto N, Suyama H, Nakazato H, Yamamoto
NY and Koga Y: Immunotherapy of metastatic colorectal cancer with
vitamin D-binding protein-derived macrophage activating factor,
GcMAF. Cancer Immunol Immunother. 57:1007–1016. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto N, Suyama H, Yamamoto NY and
Ushijima N: Immunotherapy of metastatic breast cancer patients with
vitamin D-binding protein-derived macrophage activating factor
(GcMAF). Int J Cancer. 122:461–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto N, Suyama H and Yamamoto N:
Immunotherapy for prostate cancer with Gc protein-derived
macrophage-activating factor, GcMAF. Transl Oncol. 1:65–72. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto N, Willett NP and Lindsay DD:
Participation of serum proteins in the inflammation-primed
activation of macrophages. Inflammation. 18:311–322. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Link RP, Perlman KL, Pierce EA, Schnoes HK
and DeLuca HF: Purification of human serum vitamin D-binding
protein by 25-hydroxyvitamin D3-Sepharose chromatography. Anal
Biochem. 157:262–269. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto N and Kumashiro R: Conversion of
vitamin D3 binding protein (group-specific component) to a
macrophage activating factor by the stepwise action of
beta-galactosidase of B cells and sialidase of T cells. J Immunol.
151:2794–2802. 1993.
|
|
17
|
Yamamoto N: Structural definition of a
potent macrophage activating factor derived from vitamin D3-binding
protein with adjuvant activity for antibody production. Mol
Immunol. 33:1157–1164. 1996. View Article : Google Scholar
|
|
18
|
Nishimura N, Urade M, Hashitani S, et al:
Increased expression of cyclooxygenase (COX)-2 protein in
DMBA-induced hamster cheek pouch carcinogenesis and chemopreventive
effect of a selective COX-2 inhibitor celecoxib. J Oral Pathol Med.
33:614–621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Segawa E, Hashitani S, Toyohara Y, et al:
Inhibitory effect of sulindac on DMBA-induced hamster cheek pouch
carcinogenesis and its derived cell line. Oncol Rep. 21:869–874.
2009.PubMed/NCBI
|
|
20
|
Odukoya O, Schwartz J, Weichselbaum R and
Shklar G: An epidermoid carcinoma cell line derived from hamster
7,12-dimethylbenz(α)anthracene-induced buccal pouch tumors. J Natl
Cancer Inst. 71:1253–1264. 1983.
|
|
21
|
Zbar B and Tanaka T: Immunotherapy of
cancer: regression of tumors after intralesional injection of
living Mycobacterium bovis. Science. 172:271–273. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morton D, Eibler FR, Malmgren RA and Wood
WC: Immunological factors which influence response to immunotherapy
in malignant melanoma. Surgery. 68:158–164. 1970.PubMed/NCBI
|
|
23
|
Yamamoto N and Ngwenya BZ: Activation of
mouse peritoneal macrophages by lysophospholipids and ether
derivatives of neutral lipids and phospholipids. Cancer Res.
47:2008–2013. 1987.PubMed/NCBI
|
|
24
|
Yamamoto N, Ngwenya BZ and Pieringer PA:
Activation of macrophages by ether analogues of lysophospholipids.
Cancer Immunol Immunother. 25:185–192. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto N, St Claire DA Jr, Homma S and
Ngwenya BZ: Activation of mouse macrophages by alkylglycerols,
inflammation products of cancerous tissues. Cancer Res.
48:6044–6049. 1988.PubMed/NCBI
|
|
26
|
Homma S and Yamamoto N: Activation process
of macrophage after in vitro treatment of mouse lymphocytes with
dodecylglycerol. Clin Exp Immunol. 79:307–313. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Homma S, Yamamoto M and Yamamoto N:
Vitamin D binding protein (group-specific component, Gc) is the
sole serum protein required for macrophage activation after
treatment of peritoneal cells with lysophosphatidylcholine. Immunol
Cell Biol. 71:249–257. 1993. View Article : Google Scholar
|
|
28
|
Naraparaju VR and Yamamoto N: Roles of
beta-galactosidase of B lymphocytes and sialidase of T lymphocytes
in inflammation-primed activation of macrophages. Immunol Lett.
43:143–148. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamamoto N and Homma S: Vitamin D3 binding
protein (group-specific component) is a precursor for the
macrophage-activating signal factor from
lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci
USA. 88:8539–8543. 1991. View Article : Google Scholar
|
|
30
|
Yamamoto N, Homma S, Haddad JG and
Kowalski MN: Vitamin D3 binding protein required for in vitro
activation of macrophages after dodecylglycerol treatment of mouse
peritoneal cells. Immunology. 74:420–424. 1991.PubMed/NCBI
|
|
31
|
Yamamoto N, Homma S and Millman I:
Identification of the serum factor required for in vitro activation
of macrophage – role of vitamin D3-binding protein (group specific
component, Gc) in lysophospholipid activation of mouse peritoneal
macrophages. J Immunol. 147:273–280. 1991.PubMed/NCBI
|
|
32
|
Zhang S, Cordon-Cardo C, Zhang HS, et al:
Selection of tumor antigens as targets for immune attack using
immunohistochemistry. I Focus on gangliosides. Int J Cancer.
73:42–49. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang S, Zhang HS, Cordon-Cardo C, et al:
Selection of tumor antigens as targets for immune attack using
immunohistochemistry. II Blood group-related antigens. Int J
Cancer. 73:50–56. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Zhang HS, Cordon-Cardo C,
Ragupathi G and Livingston PO: Selection of tumor antigens as
targets for immune attack using immunohistochemistry. III Protein
antigen. Clin Cancer Res. 4:2669–2676. 1998.PubMed/NCBI
|
|
35
|
Musselli C, Ragupathi G, Gilewski T,
Panageas KS, Spinat Y and Livingston PO: Reevaluation of the
cellular immune response in breast cancer patients vaccinated with
MUC1. Int J Cancer. 97:660–667. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ngwenya BZ and Yamamoto N: Effects of
inflammation products on immune systems: lysophosphatidylcholine
stimulates macrophages. Cancer Immunol Immunother. 21:174–182.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamamoto N, Urade M and Ueda M: Potent
tumoricidal capacity of macrophages activated by Gc protein-derived
macrophage activating factor (GcMAF) and its therapeutic efficacy
for prostate, breast and colorectal cancers. J Immunother.
28:6422005. View Article : Google Scholar
|
|
38
|
Yamamoto N and Ueda M: Therapeutic
efficacy of vitamin D-binding protein (Gc protein)-derived
macrophage activating factor (GcMAF) for prostate and breast
cancers. Immunology. Medmond Ltd; Bologna, Italy: pp. 201–204.
2004
|