Introduction
Colorectal cancer (CRC), one of the most common
malignant neoplasms in Western countries, has become increasingly
frequent in China, mostly due to the improvement of living
standards and alteration of eating habits. Although overall
survival has climbed to approximately 65% after 5 years, partly due
to the development of systematic therapeutic modalities in past
decades, one-third of advanced patients succumb to progressive
disease (1). Mounting evidence has
demonstrated that several genetic alterations are involved in the
carcinogenetic process of CRC (2).
Identification of multiple cancer-associated molecules may further
our knowledge of the gene regulation throughout the tumorigenetic
process in human CRC.
Peroxisome proliferator-activated receptor γ (PPARγ)
is a member of the steroid receptor superfamily and belongs to the
ligand-activated transcription factors. PPARγ forms a heterodimer
with retinoid X-receptor α in the presence of its ligands, such as
polyunsaturated fatty acids and arachidonic acid derivatives,
following which it regulates the expression of target genes. In
addition to functioning as a significant regulator of adipocyte
differentiation and fatty acid metabolism, PPARγ was found to be
correlated with several types of cancer, including CRC (3,4).
Although aberrations of PPARγ expression have been observed in
several other cancer types, previous studies revealed that PPARγ
was abundant in normal and malignant tissue in the colon (5,6), where
its function is largely unknown. Moreover, the roles of PPARγ in
various stages of CRC are also poorly understood. One recent study
demonstrated that activation of PPARγ inhibited cell growth and
induced cell differentiation in human colorectal cancer through
several pathways involving the induction of PTEN over-expression
(7). PTEN undergoes genetic or
epigenetic inactivation in many malignancies including colorectal
cancer (8,9). The role of the lipid phosphatase
activity of PTEN as a negative regulator of the cytoplasmic
phosphatidylinositol-3-kinase/Akt pathway is well known. However,
certain studies have also revealed that PTEN may relocate to the
nucleus and indicate an additional modulation mechanism (10,11).
Additionally, the question of whether aberrant PTEN is involved in
the initial stages or is a later event in CRC remains
controversial.
To date relatively few studies have focused on the
correlation of expression of PPARγ and PTEN in various tissues. In
this study, we examined the protein expression of PPARγ and PTEN
using immunohistochemical (IHC) staining on tissue microarray (TMA)
in CRC, adenoma and corresponding normal para-cancerous colorectal
tissues and explored their potential clinical significance.
Materials and methods
Materials and patients
Formalin-fixed and paraffin-embedded colorectal
cancer tissues, adenomatous polyps and paraneoplastic normal tissue
specimens were obtained from the archives of the Department of
Gastroenterology, the First Affiliated Hospital of Soochow
University and the National Engineering Center for Biochip in
Shanghai, China. Demographic and clinical data were collected
retrospectively (Table I). A total
of 139 patients comprising 84 males and 55 females (age range
54±28), with CRC (colon cancer, 85; rectal cancer, 54) were
included in this study. None of the patients had received
radiotherapy or chemotherapy prior to surgery. Specimens were
viewed by one pathologist (Dr Jing Fang). CRC tissues were
diagnosed, staged and graded according to the guidelines on the
national diagnosis and treatment of CRC. In these cohort CRC
patients, the median age was 54. The incidence of CRC in China
demonstrates a younger tendency. Therefore, in our study, we chose
54 years as a cut-off value to separate patients into two groups.
In regard to the degree of cancer cell differentiation, 27 cases
were well-differentiated, 81 were moderately differentiated and 31
were poorly differentiated. Lymph node metastases were observed in
50 patients and distant metastases were observed in 23 patients, 13
patients had missing parameters. With the exception of 10 patients
with unclear stage parameters, the ramaining 129 patients were
classified into Dukes’ A (25 cases), B (44 cases), C (37 cases) and
D (23 cases), according to the clinical stages. The 18 adenomatous
polyp specimens were removed by colonoscopy and the 50
para-cancerous normal tissues were resected at least 5 cm away from
the corresponding cancer tissues. Written informed consent was
obtained from each patient according to the institutional
regulations.
 | Table ICharacteristics of study
population. |
Table I
Characteristics of study
population.
| Age at enrolment
(mean ± SD) | Gender | Location |
|---|
|
|
|---|
| Male | Female | Colon | Rectum |
|---|
| Cancer (n=139) | 54±28 | 84 | 55 | 85 | 54 |
| Adenoma (n=18) | 48±12 | 11 | 7 | 12 | 6 |
| Normal (n=50) | 55±27 | 35 | 15 | 32 | 18 |
Reagents
Primary rabbit polyclonal antibodies against human
PPARγ and PTEN were obtained from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). The EnVision+™ goat-anti rabbit kit was a product
of GenTech company.
Construction and sectioning of tissue
microarray
The TMAs were assembled using a manual tissue
arrayer. In brief, the CRCs, adenomas and normal tissues were
embedded in paraffin blocks and 5-μm sections stained with
hematoxylin-eosin (HE) were obtained to select representative areas
for biopsies. The selected donor cores (diameter 1.0 or 1.5 mm)
were extracted from areas of individual donor paraffin blocks and
precisely arrayed into a new recipient block with a custom-built
instrument (Beecher Instruments, Silver Spring, MD, USA). Tissue
specimens from 139 CRCs, 18 adenomatous polyps and 50
para-cancerous benign mucosas were arranged in 4 recipient paraffin
blocks. A total of 3 core tissue biopsies were obtained from each
specimen. After construction, 4-μm slides were cut from these
recipient blocks and used in series for IHC staining.
IHC staining to TMA sections
Microarray sections (4-μm) were soaked in xylene
overnight to remove any adhesive from the tape transfer system.
Slides were deparaffinized and rehydrated and antigens were then
retrieved by autoclave. Following incubation in 10% normal goat
serum for 20 min at room temperature, the sections were stained
with the primary antibodies at 1:100 dilution overnight at 4°C. The
antibodies used were monoclonal (rabbit antihuman) with PPARγ and
PTEN purchased from Santa Cruz Biotechnology Inc. The primary
antibodies were further examined using a biotin-free, horseradish
peroxidase enzyme-labeled polymer conjugated to anti-rabbit
secondary antibodies (GeneTech).
Each of the IHC-stained sections was scored
according to the sum of the extension area and intensity (12). Nuclear staining with yellow and/or
brown was scored for PPARγ, whereas complete cytoplasm staining was
assessed for PTEN. The intensities were scored as 0 (negative), +
(faint/), ++ (moderate), and +++ (strong). The immunoreactive areas
were categorized as 0 (<5%), + (5–25%), ++ (26–50%), +++
(51–75%) and ++++ (>75%). Combined scores ≥ ++ were considered
positive and staining scores ≥ +++ were regarded as strong
positive.
Statistical analysis
Statistical analysis of PPARγ and PTEN stainings and
their correlations with clinicopathological variables were carried
out using the SPSS program, version 13.0 (SPSS, Inc.). Pearson’s
χ2 test or Fisher’s exact test were applied to compare
the staining index and clinicopathological parameters of the two
molecules. The significance level was defined as P<0.05.
This study was approved by the Ethics Committee of
Soochow University.
Results
Expression of PPARγ and PTEN in different
tissues
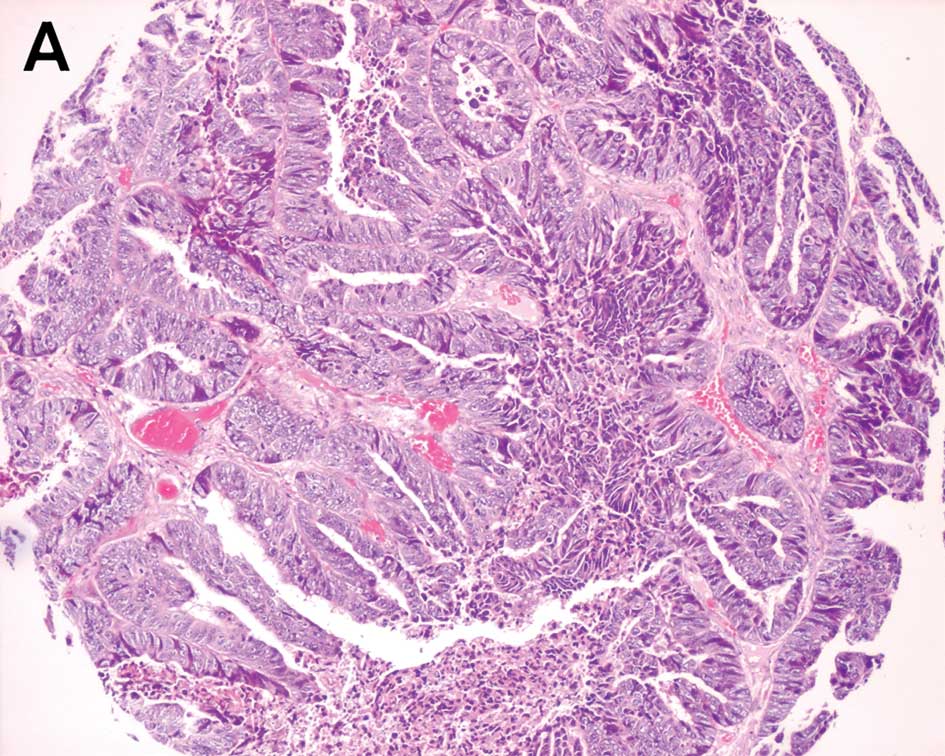
The expression of PPARγ protein was mainly located
in the cell nucleus, with a minority in the cytoplasm. Positive
expression was detected in 125 specimens in 139 CRC tissues, 15
specimens in 18 adenomas and 41 in 50 corresponding normal
colorectal tissues, whereas strong positive rates were 25%
(35/139), 17% (3/18) and 34% (17/50), respectively (Fig. 1). No significant differences were
found among the CRC, adenoma and normal colorectal mucosa regarding
IHC staining in our study (P=0.055). (Table II). Furthermore, there was also no
signifcantly different expression observed between the cancer
tissues and their normal paracancerous counterparts following the
exclusion of the adenoma data (P=0.112).
 | Table IIExpression of PPARγ and PTEN in
different colorectal tissues. |
Table II
Expression of PPARγ and PTEN in
different colorectal tissues.
| Groups | Numbers | PPARγ (%) | P-valuea | PTEN (%) | P-valuea |
|---|
|
|
|---|
| − | 1+ | 2+ | 3+ | − | 1+ | 2+ | 3+ |
|---|
| Cancer | 139 | 13 (9.4) | 1 (0.7) | 90 (64.7) | 35 (25.2) | 0.055 | 6 (4.3) | 4 (2.9) | 74 (53.2) | 55 (39.6) | 0.100 |
| Adenoma | 18 | 3 (16.7) | 0 (0.0) | 12 (66.6) | 3 (16.7) | | 1 (5.6) | 2 (11.1) | 12 (66.7) | 3 (16.6) | |
| Normal | 50 | 7 (14.0) | 2 (4.0) | 24 (48.0) | 17 (34.0) | | 1 (2.0) | 1 (2.0) | 29 (58.0) | 19 (38.0) | |
The expression of PTEN protein was commonly observed
in the cytoplasm in CRC, adenoma and normal colorectal mucosa.
Positive immunostainings were observed in 129 tissues of 139 CRC
specimens, 15 of 18 adenomas and 48 in normal colorectal mucosa
tissues (Fig. 1). No statistically
significant difference was found between these tissue types.
(P=0.100) (Table II). Similarly,
when the cancerous and para-cancerous tissues were isolated, a
significantly different expression of PTEN was not observed
(P=0.846).
We further investigated the relationship between the
PPARγ and PTEN, according to our originally conservative plan.
Notably, the expression of PPARγ was found to be positively
correlated with that of PTEN in this cohort tissue in our study
(r=0.375, P=0.000).
Correlations between the expression of
PPARγ/PTEN and the clinicopathological parameters
The association between the IHC pattern of PPARγ and
clinicopathological features including age, gender, histological
grade, lymph node and distant metastasis were not statistically
significant (P>0.05). Similarly, there were no significant
associations between the expression of PTEN and age, gender,
clinical stage and lymph node metastasis. However, PTEN protein
expression was found to be correlated with CRC histological grade
(P=0.006) and distant metastasis (P=0.015). PPARγ expression was
also shown to correlate with clinical stage in this cohort of CRC
patients (P=0.004). (Table
III).
 | Table IIICorrelations between the expression of
PPARγ or PTEN and the clinicopathological data in the CRC
patients. |
Table III
Correlations between the expression of
PPARγ or PTEN and the clinicopathological data in the CRC
patients.
| Clinicopathological
data | Number | PPARγ | P-valuea | PTEN | P-valuea |
|---|
|
|
|---|
| − | 1+ | 2+ | 3+ | − | 1+ | 2+ | 3+ |
|---|
| Age |
| <54 | 45 | 7 | 0 | 26 | 12 | 0.274 | 3 | 2 | 24 | 16 | 0.576 |
| ≥54 | 94 | 6 | 1 | 64 | 23 | | 3 | 2 | 50 | 39 | |
| Gender |
| Male | 84 | 9 | 1 | 51 | 23 | 0.647 | 5 | 4 | 45 | 30 | 0.234 |
| Female | 55 | 4 | 0 | 39 | 12 | | 1 | 0 | 29 | 25 | |
| Histological
grade |
| Low | 31 | 6 | 0 | 17 | 8 | 0.165 | 5 | 2 | 18 | 6 | 0.006 |
| Moderate | 81 | 6 | 0 | 53 | 22 | | 1 | 2 | 43 | 35 | |
| High | 27 | 1 | 1 | 20 | 5 | | 0 | 0 | 13 | 14 | |
| Lymph-node
metastasis |
| Negative | 89 | 6 | 0 | 59 | 24 | 0.259 | 3 | 2 | 42 | 42 | 0.069 |
| Positive | 50 | 7 | 1 | 31 | 11 | | 3 | 2 | 32 | 13 | |
| Distant
metastasis |
| Negative | 103 | 7 | 1 | 65 | 30 | 0.175 | 2 | 3 | 58 | 40 | 0.015 |
| Positive | 23 | 4 | 0 | 16 | 3 | | 4 | 1 | 8 | 10 | |
| Dukes’ stage |
| A | 25 | 2 | 0 | 21 | 2 | 0.004 | 0 | 1 | 12 | 12 | 0.095 |
| B | 44 | 1 | 0 | 23 | 20 | | 1 | 1 | 23 | 19 | |
| C | 37 | 5 | 1 | 22 | 9 | | 1 | 1 | 25 | 10 | |
| D | 23 | 4 | 0 | 16 | 3 | | 4 | 1 | 8 | 10 | |
Discussion
Although it has been identified in other cancer
cells, including prostate, gastric, pancreatic and lung cancer, but
found to be absent from their corresponding benign tissues
(13–16), PPARγ was found to be expressed
ubiquitously in colorectal epithelial cells and cancer cells by
certain authors (17–19). The first problem to be addressed in
the study is therefore the exact expression status of PPARγ in
various colorectal specimens.
In their study, Mansen et al (17) found that expression levels of PPARγ
and PPARβ/δ in colorectal tissues of mice were higher than those in
the small intestine, and that the expression of PPARγ was gradually
increased from crypt to lumen. Similarly, Fujisawa et al
(18) found that PPARγ was
expressed highly in human normal colon epithelium and cancer cells.
In the present study, our results revealed that PPARγ was highly
expressed in three tissues and that no statistically significant
difference was observed, consistent with the study by Eun et
al (19). Therefore, in
combination with the results of previous studies and our
investigations, we propose that the colorectal basal cells
decreased in fissionability and developed differentiated potential
followed by the increased expression of PPARγ during the
differentiation process. These results demonstrate that, although a
variety of gene aberrations such as deletion of APC and P53 and
activation of RAS occur during the progression of CRC, the protein
expression of PPARγ may exhibit no marked change, indicating that
the variation of PPARγ protein expression may not be involved in
the progression of CRC. However, whether other patterns of PPARγ
aberrations such as mutation, rearrangement or post-translational
modification are involved in the pathogenesis of CRC is poorly
understood. Therefore, together with other studies, we propose that
PPARγ expression may not be considered as an early diagnostic and
progression index in CRC. Nevertheless, the decreasing expression
tendency of PPARγ found in advanced colorectal cancers in this
study may indicate the prognostic potential of PPARγ. Furthermore,
activation of PPARγ demonstrating an anti-oncogene role in CRC may
indicate that although PPARγ was expressed equally in benign
tissues and cancer cells of the colorectum, PPARγ activity may
decrease in malignant tissues through several pathways such as
mutation, post-translational modification, competitive inhibition
of PPARβ/δ and decreasing of the endogenic ligands (20,21).
Therefore, the specific role of PPARγ in the development and
progression of CRC requires additional studies.
As a tumor suppressor gene, PTEN on chromosome
10q23.3 encodes a dual-specificity phosphatase that negatively
regulates the phosphoinositol-3-kinase/Akt pathway and mediates
cell-cycle arrest and apoptosis. Several studies have revealed that
PTEN aberration is involved in the development of a number of types
of cancer (22,23). One study also found that PTEN is
involved in CRC (24). However,
Zhou et al (25) revealed
that distinct PTEN aberrations may occur in variant genetic
backgrounds. Furthermore, it was found that the mutation in the ATP
motif of PTEN may affect its subcelluar localization and tumor
suppressive function (11,26). In our study, PTEN protein was mostly
expressed in the cytoplasm, but not in the nucleus, and no
significant difference was observed between CRC, adenoma and normal
colorectal mucosa, which was consistent with previous studies
(27,28). However, whether the unmatched paired
design and different genetic backgrounds were responsible for the
results requires additional studies. Notably, the correlations
between PTEN expression and histological grade or distant
metastasis were also noted in CRC tissues, which demonstrated that
deletion or low expression of PTEN may not play a role in the
primary stage of malignant transformation but may be involved
elsewhere in the progression of cancer, such as aggravation of
malignant differentiation and metastasis. Based on our present
results, PTEN expression may not be a marker for early diagnosis of
CRC but may have some potential to predict malignant aggravation
and distant metastasis and may also be a potential prognostic
marker in CRC, which requires verification by future predictive or
prognostic statistical analyses. Moreover, the positive correlation
between the expression of PPARγ and PTEN observed in our study may
indicate the modulation relationship of these two proteins during
colorectal carcinogenesis, which provides additional evidence to
support the hypothesis that PTEN may be a downstream regulatory
factor of PPARγ.
In this study, we elevated efficiency and reduced
cost and labor in studies of target gene expression through the use
of TMA, a new molecular biotechnology, when compared with
immunostaining of conventional specimens. The cell morphology and
protein expressions were studied in parallel and the variance in
results under various experimental conditions currently experienced
with conventional technology may be avoided. Therefore, it is
feasible that CRC may be studied using TMA in combination with
other molecular biotechnology in the near future. TMA technology,
which is fast, convenient and economical, may have a potentially
dominant position in macro-scale examination of tissue
specimens.
Acknowledgements
This work is supported by the 135 Medical Important
Talent Foundation of Jiangsu Province, China (No.37RC2002037) and
the National 863 Project about Functional Genomic and Biochip (No.
2002AA2Z2021).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer Statistics 2009. CA Cancer J Clin. 59:1–25. 2009.
View Article : Google Scholar
|
|
2
|
Hung KE and Chung DC: New insights into
the molecular pathogenesis of colorectal cancer. Drug Discov Today
Dis Mech. 3:439–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glass CK and Rosenfeld MG: The coregulator
exchange in transcriptional functions of nuclear receptors. Genes
Dev. 14:121–141. 2000.PubMed/NCBI
|
|
4
|
Kliewer SA, Sundseth SS, Jones SA, Brown
PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard
JM and Lehmann JM: Fatty acids and eicosanoids regulate gene
expression through direct interactions with peroxisome
proliferator-activated receptors alpha and gamma. PNAS.
94:4318–4323. 1997. View Article : Google Scholar
|
|
5
|
Matthiessen MW, Pedersen G, Albrektsen T,
Adamsen S, Fleckner J and Brynskov J: Peroxisome
proliferator-activated receptor expression and activation in normal
human colonic epithelial cells and tubular adenomas. Scand J
Gastroenterol. 40:198–205. 2005. View Article : Google Scholar
|
|
6
|
Su WD, Bush CR, Necela BM, Calcagno SR,
Murray NR, Fields AP and Thompson EA: Differential expression,
distribution, and function of PPARγ in the proximal and distal
colon. Physiol Genomics. 30:342–353. 2007.
|
|
7
|
Schwab M, Reynders V, Loitsch S, Shastri
YM, Steinhilber D, Schröder O and Stein J: PPARγ is involved in
mesalazine-mediated induction of apoptosis and inhibition of cell
growth in colon cancer cells. Carcinogenesis. 29:1407–1414.
2008.
|
|
8
|
Eng C: PTEN: one gene, many syndromes. Hum
Mutat. 22:183–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nassif NT, Lobo GP, Wu X, Henderson CJ,
Morrison CD, Eng C, Jalaludin B and Segelov E: PTEN mutations are
common in sporadic microsatellite stable colorectal cancer.
Oncogene. 23:617–628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song MS, Salmena L, Carracedo A, Egia A,
Lo-Coco F, Teruya-Feldstein J and Pandolfi PP: The
deubiquitinylation and localization of PTEN are regulated by a
HAUSP-PML network. Nature. 455:813–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lobo GP, Waite KA, Planchon SM, Romigh T,
Nassif NT and Eng C: Germline and somatic cancer-associated
mutations in the ATP-binding motifs of PTEN influence its
subcellular localization and tumor suppressive function. Hum Mol
Genet. 18:2851–2862. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rahman MA, Dhar DK, Yamagicgi E, Maruyama
S, Sato T, Hayashi H, Ono T, Yamanoi A, Kohno H and Nagasue N:
Coexpression of inducible nitric oxide synthase and COX-2 in
hepatocellular carcinoma and surrounding liver: possible
involvement of COX-2 in the angiogenesis of hepatitis C
virus-positive cases. Clin Cancer Res. 7:1325–1332. 2001.
|
|
13
|
Nagata D, Yoshihiro H, Nakanishi M,
Naruyama H, Okada S, Ando R, Tozawa K and Kohri K: Peroxisome
proliferator-activated receptor-gamma and growth inhibition by its
ligands in prostate cancer. Cancer Detect Prev. 32:259–66. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato H, Ishihara S, Kawashima K, Moriyama
N, Suetsugu H, Kazumori H, Okuyama T, Rumi MA, Fukuda R, Nagasue N
and Kinoshita Y: Expression of peroxisome proliferator-activated
receptor (PPAR) γ in gastric cancer and inhibitory effects of PPARγ
agonists. Br J Cancer. 83:1394–1400. 2000.
|
|
15
|
Sun WH, Chen GS, Ou XL, Yang Y, Luo C,
Zhang Y, Shao Y, Xu HC, Xiao B, Xue YP, et al: Inhibition of COX-2
and activation of peroxisome proliferator-activated receptor gamma
synergistically inhibits proliferation and induces apoptosis of
human pancreatic carcinoma cells. Cancer Lett. 275:247–255. 2009.
View Article : Google Scholar
|
|
16
|
Chen D, Jin GF, Wang Y, Wang H, Liu H, Liu
Y, Fan W, Ma H, Miao R, Hu Z, et al: Genetic variants in peroxisome
proliferator-activated receptor-γ gene are associated with risk of
lung cancer in a Chinese population. Carcinogenesis. 29:342–350.
2008.
|
|
17
|
Mansen A, Guardiola-Diaz H, Rafter J,
Branting C and Gustafsson JA: Expression of the peroxisome
proliferators-activated receptor (PPAR) in the mouce colonic
mucosa. Biochem Biophys Res Commun. 222:844–851. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujisawa T, Nakajima A, Fujisawa N,
Takahashi H, Ikeda I, Tomimoto A, Yonemitsu K, Nakajima N, Kudo C,
Wada K, et al: Peroxisome proliferator-activated receptor gamma
(PPARgamma) suppresses colonic epithelial cell turnover and colon
carcinogenesis through inhibition of the beta-catenin/T cell factor
(TCF) pathway. J Pharmacol Sci. 106:627–38. 2008. View Article : Google Scholar
|
|
19
|
Eun CS, Han DS, Lee SH, Paik CH, Chung YW,
Lee J and Hahm JS: Attenuation of colonic inflammation by PPARγ in
intestinal epithelial cells: effect on toll-like receptor pathway.
Dig Dis Sci. 51:693–697. 2006.
|
|
20
|
Feilchenfeldt J, Brundler MA, Soravia C,
Totsch M and Meier CA: Peroxisome proliferators-activated receptors
(PPARs) and associated transcription factors in colon cancer:
reduced expression of PPARgamma-coactivator 1 (PGC-1). Cancer Lett.
203:25–33. 2004. View Article : Google Scholar
|
|
21
|
Hisatake J, Ikezoe T, Carey M, Holden S,
Tomoyasu S and Koeffler HP: Down-regulation of prostate-specific
antigen expression by ligands for peroxisome proliferator-activated
receptor gamma (troglitazone) in human prostate cancer. Cancer Res.
19:5494–5498. 2000.
|
|
22
|
Puzio-Kuter AM, Martin MC, Kinkade CW,
Wang X, Shen TH, Matos T, Shen MM, Cordon-Cardo C and Abate-Shen C:
Inactivation of p53 and Pten promotes invasive bladder cancer.
Genes and Dev. 23:675–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwanaga K, Yang YN, Raso MG, Ma L, Hanna
AE, Thilaganathan N, Moghaddam S, Evans CM, Li H, Cai WW, et al:
Pten inactivation accelerates oncogenic K-ras-initiated
tumorigenesis in a mouse model of lung cancer. Cancer Res.
68:1119–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loupakis F, Pollina L, Stasi I, Ruzzo A,
Scartozzi M, Santini D, Masi G, Graziano F, Cremolini C, Rulli E,
et al: PTEN expression and KRAS mutations on primary tumors and
metastases in the prediction of benefit from cetuximab plus
irinotecan for patients with metastatic colorectal cancer. J Clin
Oncol. 27:2622–2629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou XP, Loukola A, Salovaara R,
Nystrom-Lahti M, Peltomaki P, De la Chapelle A, Aaltonen LA and Eng
C: PTEN mutational spectra expression levels and subcellular
localization in microsatellite stable and unstable colorectal
cancers. Am J Pathol. 161:439–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lobo GP, Waite KA, Planchon SM, Romigh T,
Houghton JA and Eng C: ATP modulates PTEN subcellular localization
in multiple cancer cell lines. Hum Mol Genet. 17:2877–2885. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bowen KA, Doan HQ, Zhou BP, Wang Q, Zhu Y,
Rychahou PG and Evers BM: PTEN loss induces epithelial-mesenchymal
transition in human colon cancer cells. Anticancer Res.
29:4439–4449. 2009.PubMed/NCBI
|
|
28
|
Rychahou PG, Kang JH, Gulhati P, Doan HQ,
Chen LA, Xiao SY, Chung DH and Evers BM: Akt2 over expression plays
a critical role in the establishment of colorectal cancer
metastasis. PNAS. 105:20315–20320. 2008. View Article : Google Scholar : PubMed/NCBI
|