Introduction
Liposarcomas are the most common class of soft
tissue sarcoma, and are separated into distinct clinicopathological
entities with a characteristic morphological spectrum and exclusive
genetic changes (1,2). Myxoid liposarcoma (MLS) represents one
such entity with the second most common prevalence after
well-differentiated liposarcoma. A significant proportion of MLS
contains a cytogenetic hallmark, t(12;16)(q13;p11), which leads to
the fusion of the CCAAT/enhancer binding protein (C/EBP)
homologous protein (CHOP) and human translocation
liposarcoma (TLS) gene, generating TLS-CHOP fusion
transcript (3–10). In a minor population of MLS, a
variant chromosomal translocation, t(12;22)(q13;q12), has been
documented, resulting in the Ewing sarcoma (EWS)-CHOP
fusion gene (5,6,8–12). Our
recent fusion gene analysis of 172 cases of adipocytic tumors,
comprising 98 cases of lipoma and 74 cases of liposarcoma,
established that TLS-CHOP and EWS-CHOP were specific
to liposarcoma (10). However, of
note, among the distinct entities of liposarcomas, the fusion genes
were detectable in four cases whose histopathological diagnosis was
other than MLS. The present study aimed to re-examine the
clinicopathological features of these four ‘unusual’ cases, and the
results indicated the histopathological variation in MLS.
Materials and methods
Case selection
The patients included in this study were 2 males and
2 females, ranging in age from 32 to 74 years (mean 59), who
presented with a mass lesion ranging from 2.5 to 14 cm in size.
After written informed consent was obtained, tissues from 74
liposarcomas obtained at the time of surgery, and stored at −80°C,
were analyzed by reverse transcription-polymerase chain reaction
(RT-PCR) and DNA sequencing for possible detection of the
TLS-CHOP or EWS-CHOP transcripts (10). Histological subtypes of
liposarcomas, determined by pathologists, consisted of 12
well-differentiated, 41 MLS, 4 de-differentiated, and 17
unclassified. Out of the 74 liposarcomas, 22 (30%) were associated
with the TLS-CHOP fusion transcript, whereas 3 (4%) were
associated with the EWS-CHOP fusion transcript. Histological
subtypes of TLS-CHOP detection consisted of 1
well-differentiated (8% of the subtype), 19 MLS (46% of the
subtype), 1 de-differentiated (25% of the subtype) and 1
unclassified (6% of the subtype). Histological subtypes of
EWS-CHOP detection in liposarcoma included 2 MLS (2% of the
subtype) and 1 de-differentiated (25% of the subtype). Based on the
above, four cases of liposarcoma with detection of either
TLS-CHOP or EWS-CHOP whose postoperative diagnosis
was other than MLS were selected for the current study, consisting
of re-examination of medical records, imaging data and
histopathology. The procurement of frozen tissues and retrospective
data collection were approved by the Review Boards of Osaka
University Hospital and Osaka Medical Center for Cancer and
Cardiovascular Diseases.
Results
Clinically, the patients were 2 male and 2 female,
ranging in age from 32 to 74 (mean 59) years, and presenting with a
mass lesion ranging from 2.5 to 14 cm in size (Table I). Postoperative diagnoses were
well-differentiated liposarcoma (case 1), de-differentiated
liposarcoma (cases 2 and 3), and unclassified (case 4). The
patients underwent wide-resection with or without adjuvant therapy.
Follow-up was available for all 4 patients and ranged from 36 to
129 months (mean 69) after surgery.
 | Table IClinicopathological characteristics of
four cases with detectable TLS-CHOP or EWS-CHOP
transcripts whose diagnosis was other than myxoid liposarcoma. |
Table I
Clinicopathological characteristics of
four cases with detectable TLS-CHOP or EWS-CHOP
transcripts whose diagnosis was other than myxoid liposarcoma.
| No. | Age/Gender | Location | Size (cm) | Postoperative
diagnosis | Differential
diagnosis and dominant cells | Myxomatous
component | Adjuvant therapy | Follow-up after wide
resection | Fusion gene |
|---|
| 1 | 70/M | Thigh | >10 | WDLS | ND | ND | No | 72 mo ANED | TLS-CHOP |
| 2 | 74/F | Thigh | 2.5 | DDLS | Pleomorphic sarcoma,
spindle cells | <10% | No | 129 mo ANED | TLS-CHOP |
| 3 | 58/M | Retro-peritoneum | ND | DDLS | Pleomorphic MFH,
spindle cells | <10% | Chemo, Radio | 22 mo rec; 40 mo
SD | EWS-CHOP |
| 4 | 32/F | Back | 14 | Unclassified | Synovial sarcoma,
spindle cells | <5% | Chemo | 36 mo ANED, DID | TLS-CHOP |
Re-examination of the clinical records identified
that three of the four cases (cases 2, 3 and 4 in Table I) experienced considerable
difficulty in making definitive diagnosis. Histopathologically, two
cases postoperatively diagnosed as de-differentiated liposarcoma
(cases 2 and 3 in Table I)
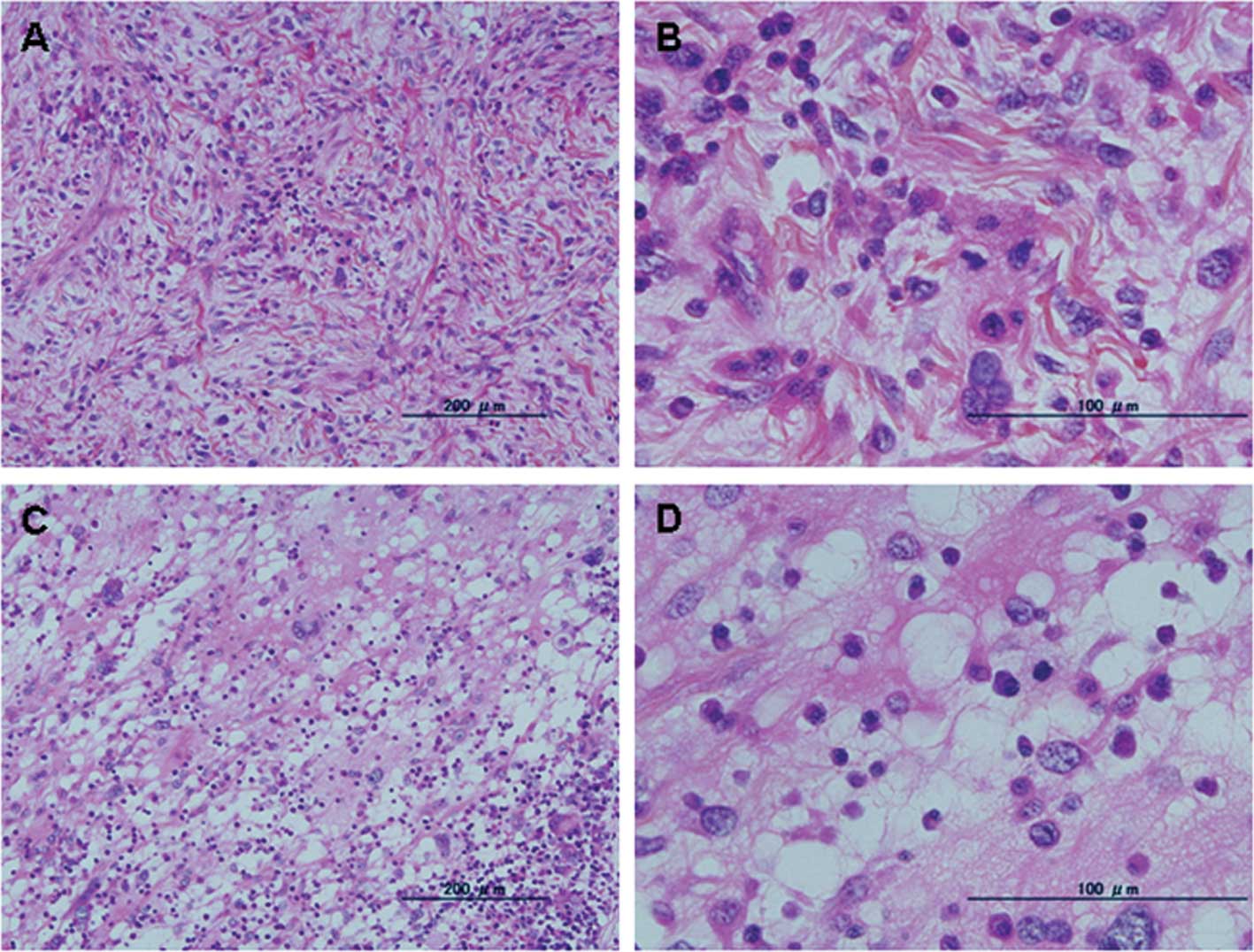
exhibited variable cellularity and cytological pleomorphism, and
contained large bizarre cells with foamy cytoplasm (Fig. 1A and 1B). In one case
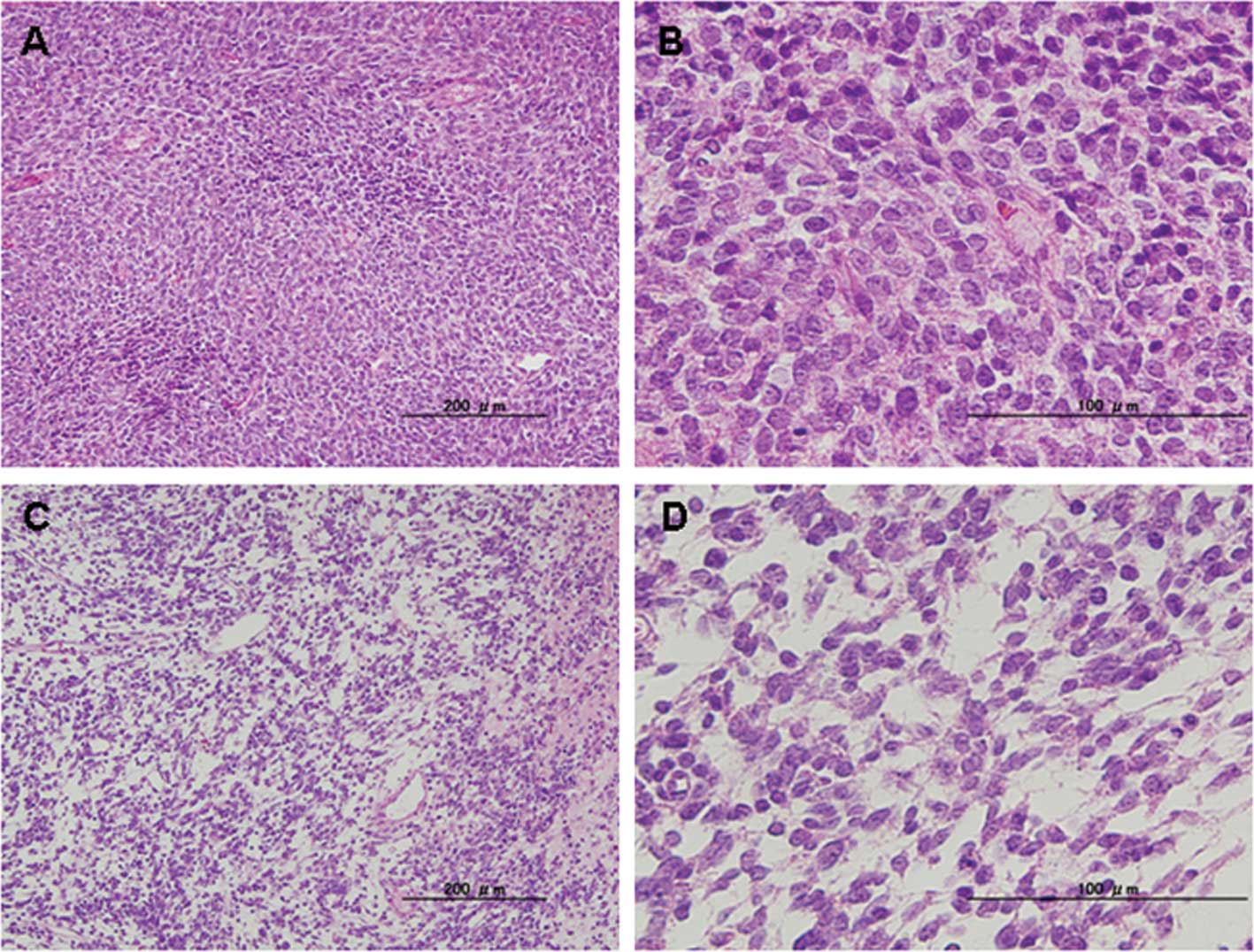
postoperatively diagnosed as unclassified (case 4 in Table I), spindle cells were densely
proliferated (Fig. 2A and B);
however, the tentative diagnosis of monophasic synovial sarcoma was
postulated by the subsequent RT-PCR analysis, which did not detect
any synovial sarcoma, translocated to X chromosome
(SYT)-sarcoma, synovial, X breakpoint (SSX) fusion
transcripts for the definitive diagnosis (13). Re-examination of the histopathology
identified that these three cases contained areas of myxomatous
change as a minor component (<10%) (Figs. 1C and D and 2C and D).
Clinical report (case 4)
A 32-year-old female presented with a large mass
measuring 14 cm located on her back (Fig. 3A), which had occasional pain from
one month prior to presentation. Computed tomography demonstrated
that the mass lesion was predominantly located under the fascia of
the back muscle, but partly penetrated into the thorax (Fig. 3B). Magnetic resonance imaging
revealed non-homogeneous enhancement of the lesion following
gadolinium diethylenetriaminepentaacetic acid injection. Open
biopsy clearly supported the malignant nature of the lesion
(Fig. 2A and B), and wide-resection
was achieved with adjuvant pre- and post-operative chemotherapy.
There were no signs of recurrence or metastasis prior to the
patient's death due to suicide at 36 months after surgery.
Discussion
According to the World Health Organization (WHO)
classification, MLS is defined as ‘a malignant tumor composed of
uniform round to oval-shaped primitive non-lipogenic mesenchymal
cells and a variable number of lipoblasts in a prominent myxoid
stroma with a characteristic branching vascular pattern’ (2). In this retrospective analysis of four
cases, their postoperative diagnoses, divided into various classes,
were revised to MLS following the detection of the TLS-CHOP
or EWS-CHOP fusion transcripts. With the exception of one
case (case 1) whose histopathological material was not available,
re-examination proved that these cases shared a common
characteristic, i.e., a myxomatous area as a minor component
(<10%), indicating that there are unusual MLS cases whose myxoid
stroma is not prominent.
Dominant components (>90%) of the current cases
resembled pleomorphic sarcoma (case 2), pleomorphic malignant
fibrous histiocytoma (case 3) and monophasic synovial sarcoma (case
4). In cases 2 and 3, the dominant component had been regarded as a
de-differentiated feature of the de-differentiated liposarcoma. As
shown in mixed-type liposarcomas consisting of combined patterns of
well-differentiated liposarcoma and MLS, these cases (and possibly
also case 1) may represent an extreme variant of the morphological
spectrum within MLS (14). On the
other hand, in case 4, the contradiction between histopathological
appearance and the negative detection of SYT-SSX fusion
transcripts resulted in a delay in classification, and this case
was considered to comprise exclusively round-cell type MLS.
In conclusion, the present study indicates the
histopathological variation in MLS and the significance of fusion
gene detection for definitive diagnosis. In case of i) difficulty
in providing definitive diagnosis of soft tissue sarcoma by
standard histopathological examination; ii) negative detection of
the histopathological candidate fusion gene (as in case 4 in this
study); and iii) recognition of myxoid stroma even as a minor
component, we propose that analysis of TLS-CHOP and
EWS-CHOP fusion genes may aid diagnosis of unusual MLS.
Further studies on the correlation between fusion genes and
clinicopathological characteristics of MLS are required to
establish the specific identity of this class.
Acknowledgements
This study was supported in part by the Japan
Society for the Promotion of Science (Grant no. 22591683), the
Nakatomi Foundation and the Osaka Medical Research Foundation for
Incurable Diseases.
References
|
1
|
Weiss SW and Goldblum JR: Liposarcoma.
Enzinger and Weiss's Soft Tissue Tumors. 4th edition. Mosby; St.
Louis: pp. 641–93. 2001
|
|
2
|
Antonescu CR and Ladanyi M: Myxoid
liposarcoma. World Health Organization Classification of Tumours:
Pathology and Genetics of Tumours of Soft Tissue and Bone. Fletcher
CDM, Unni KK and Mertens F: IARC Press; Lyon: pp. 40–43. 2002
|
|
3
|
Antonescu CR, Elahi A, Humphrey M, Lui MY,
Healey JH, Brennan MF, Woodruff JM, Jhanwar SC and Ladanyi M:
Specificity of TLS-CHOP rearrangement for classic myxoid/
round cell liposarcoma: absence in predominantly myxoid
well-differentiated liposarcomas. J Mol Diagn. 2:132–138. 2000.
|
|
4
|
Panagopoulos I, Mertens F, Isaksson M and
Mandahl N: A novel FUS/CHOP chimera in myxoid
liposarcoma. Biochem Biophys Res Commun. 279:838–845.
2000.PubMed/NCBI
|
|
5
|
Antonescu CR, Tschernyavsky SJ, Decuseara
R, Leung DH, Woodruff JM, Brennan MF, Bridge JA, Neff JR, Goldblum
JR and Ladanyi M: Prognostic impact of P53 status, TLS-CHOP
fusion transcript structure, and histological grade in myxoid
liposarcoma: a molecular and clinicopathologic study of 82 cases.
Clin Cancer Res. 7:3977–3987. 2001.PubMed/NCBI
|
|
6
|
Hosaka T, Nakashima Y, Kusuzaki K, Murata
H, Nakayama T, Nakamata T, Aoyama T, Okamoto T, Nishijo K, Araki N,
et al: A novel type of EWS-CHOP fusion gene in two cases of
myxoid liposarcoma. J Mol Diagn. 4:164–171. 2002.
|
|
7
|
Domoto H, Hosaka T, Oikawa K, Ohbayashi T,
Ishida T, Izumi M, Iwaya K, Toguchida J, Kuroda M and Mukai K:
TLS-CHOP target gene DOL54 expression in liposarcomas
and malignant fibrous histiocytomas. Pathol Int. 52:497–500. 2002.
View Article : Google Scholar
|
|
8
|
Bode-Lesniewska B, Frigerio S, Exner U,
Abdou MT, Moch H and Zimmermann DR: Relevance of translocation type
in myxoid liposarcoma and identification of a novel
EWSR1-DDIT3 fusion. Genes Chromosomes Cancer. 46:961–971.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alaggio R, Coffin CM, Weiss SW, Bridge JA,
Issakov J, Oliveira AM and Folpe AL: Liposarcomas in young
patients: a study of 82 cases occurring in patients younger than 22
years of age. Am J Surg Pathol. 33:645–658. 2009.PubMed/NCBI
|
|
10
|
Kubo T, Matsui Y, Naka N, Araki N, Myoui
A, Endo K, Yasui N, Ohtani O, Suzuki K, Kimura T, Yoshikawa H and
Ueda T: Specificity of fusion genes in adipocytic tumors.
Anticancer Res. 30:661–664. 2010.PubMed/NCBI
|
|
11
|
Matsui Y, Ueda T, Kubo T, Hasegawa T,
Tomita Y, Okamoto M, Myoui A, Kakunaga S, Yasui N and Yoshikawa H:
A novel type of EWS-CHOP fusion gene in myxoid liposarcoma.
Biochem Biophys Res Commun. 348:437–440. 2006.PubMed/NCBI
|
|
12
|
Suzuki K, Matsui Y, Endo K, Kubo T,
Hasegawa T, Kimura T, Ohtani O and Yasui N: Myxoid liposarcoma with
EWS-CHOP type 1 fusion gene. Anticancer Res. 30:4679–4684.
2010.
|
|
13
|
Ladanyi M, Antonescu CR, Leung DH,
Woodruff JM, Kawai A, Healey JH, Brennan MF, Bridge JA, Neff JR,
Barr FG, et al: Impact of SYT-SSX fusion type on the
clinical behavior of synovial sarcoma: a multi-institutional
retrospective study of 243 patients. Cancer Res. 62:135–140.
2002.
|
|
14
|
De Vreeze RSA, De Jong D, Koops W,
Nederlof PM, Ariaens A, Haas RL and van Coevorden F: Oncogenesis
and classification of mixed-type liposarcoma: a radiological,
histopathological and molecular biological analysis. Int J Cancer.
128:778–786. 2011.PubMed/NCBI
|