Introduction
Ascorbic acid (Asc) and its oxidized form,
dehydroascorbic acid, are important in the inhibitory control of
the division and growth of cells in animal tissues (1). Asc has been reported to be a potent
antitumor agent (2), but extremely
high doses are required for carcinostatic effects. To increase the
activity, Asc in combination with supplements (3,4) and
the use of its derivatives generate hydrogen peroxide (5,6). Asc
acylated with palmitic acid on the 6-O-site suppresses cell
growth (7) and DNA synthesis
(8). The 6-O-palmitoyl
derivative of Asc has been demonstrated to exert cytotoxicity to
tumor cells through hydrogen peroxide generation (6). The carcinostatic activity of diverse
Asc derivatives consisting of a palmitoyl moiety and phosphatidyl
moiety has been demonstrated. Their chemical structures are shown
in Table I. In this study, their
activity was compared using human tongue squamous carcinoma cells
(HSC-4). Hyperthermia, is a potent cancer treatment (9), which inhibits the growth of tumor
cells (10–12) and DNA synthesis (13–15),
and is in clinical use for cancer therapy. This study aimed to
examine whether these derivatives of Asc increase tumor cell death
caused by hyperthermia, to further improve cancer treatment.
 | Table IDiverse Asc derivatives examined and
their chemical structures. |
Tumor cells treated with Asc derivatives at 37°C or
42°C were examined. Firstly, differences in the carcinostatic
ability between two types of Asc derivatives, the straight-chain
types with palmitoyl moiety, Asc-2-phosphate-6-O-palmitate
sodium salt (APPS) and 6-O-palmitoyl-Asc (A6-P), as well as
the branched-chain types,
Asc-2-phosphate-6-O-(2′-hexyl)decanoate (APHD) and
Asc-2,3,5,6-O-tetra-(2′-hexyl)decanoate (VCIP), were
examined. Then, differences in the carcinostatic ability between
the isomers, APPS and APHD were assessed. Following that tumor
cells administered with APPS and APHD were observed for
morphological changes. Finally, the side-effects of the Asc
derivatives towards the normal (OUMS-36) cells were examined.
Materials and methods
Cell culture
Human tongue squamous carcinoma (HSC-4) cells were
cultivated in Eagle's minimum essential medium (MEM; Nissui
Pharmaceutical Co., Ltd., Tokyo) supplemented with 18% fetal bovine
serum (FBS; Biological Industries Ltd., Israel) in a humidified
atmosphere of 5% CO2 in air at 37°C.
Examination of carcinostatic effects
The examination of carcinostatic effects was
conducted as previously described (15,16).
Cells were previously cultured for 24 h and suspended in culture
medium at a density of 2×104 cells/ml. The test
solutions of the diverse Asc derivatives were placed into test
tubes. After the solvents were evaporated by jet flow of nitrogen
gas, culture medium was added to the residue and sonicated to
become homogenously emulsified. The cell suspensions and the test
substance were mixed in a glass sample bottle (14 mm i.d. × 40 mm).
The cells were adjusted and diluted to a cell density of
2×104 cells/ml and then, the bottle was tightly covered
with a plastic cap.
Hyperthermic treatment
The suspension was incubated for 60 min at 37°C or
42°C in a water bath (16,17) (BT-23 model, Yamato Scientific Co.,
Ltd., Tokyo) and maintained by sequential culture in a humidified
atmosphere of 5% CO2 in air at 37°C for 24 h.
Cell viability assay
Cell viability was measured using the redox
indicator dye WST-8 (16,18) (Cell Counting kit, Dojin Chemicals,
Kumamoto, Japan). The assay solution became increasingly chromic
according to the mitochondrial dehydrogenase activity. The cultured
cell suspension was transferred into a sampling tube and
centrifuged. After the supernatant was completely removed from the
tube, 110 μl 8% WST-8 was added to the cell precipitate, suspended
and transferred into each well of a 96-well microplate. Following 3
h of incubation at 37°C, the resulting Diformazan solution was
determined by measuring the absorption at 450 nm using a plate
reader (Benchmark, Bio-Rad Laboratories, Hercules, CA, USA).
Crystal violet staining
The carcinostatic activities were evaluated using a
crystal violet stain assay followed by cell morphological
observation (18,19). The cell suspensions and the test
tube substance were mixed in a 24-well culture plate (Becton,
Dickinson and Co., Franklin Lakes, NJ, USA). The stain was then
removed and the wells were rinsed thoroughly with running water
until no additional dye leached from the wells. Cell morphology was
observed under a phase-contrast microscope (Olympus IX-70).
Statistical analysis
Student's t-test was used for statistical analysis,
with p (probability) values <0.05 considered as indicative of
statistical significance.
Results
Carcinostatic effects of diverse Asc
derivatives and hyperthermia
The Asc derivatives were added to the HSC-4 cells.
The culture samples were then heated in a water bath for 60 min at
37°C or 42°C, and were maintained by sequential culture for 24 h at
37°C. The carcinostatic effects were measured using a redox
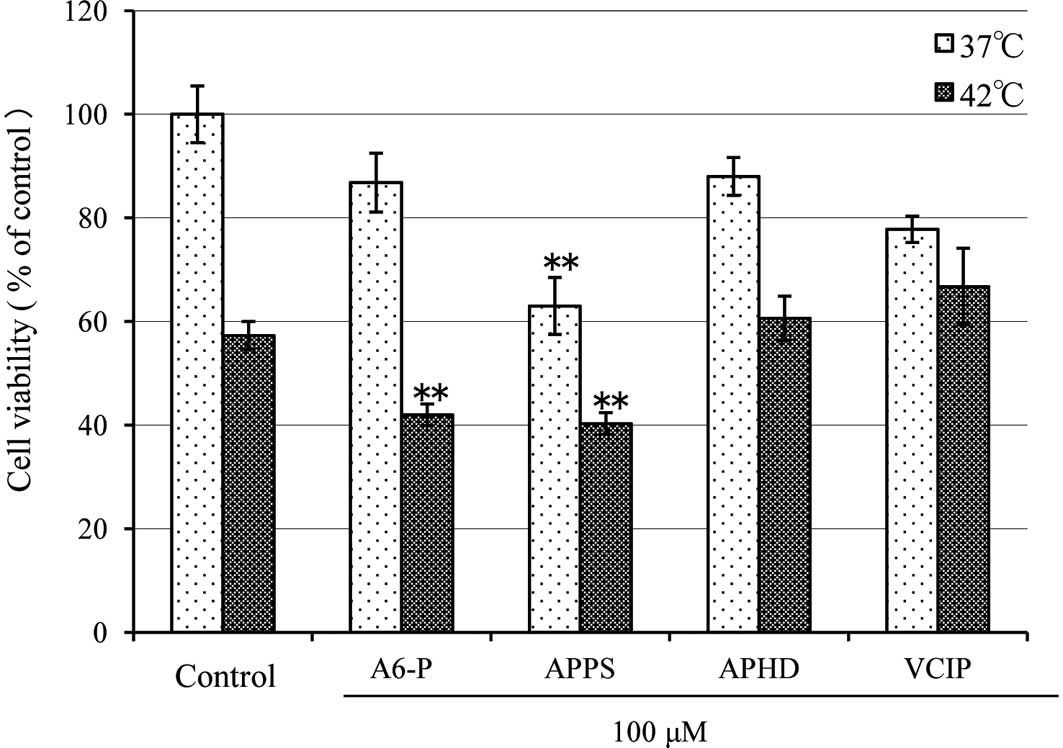
reaction-based WST-8 assay (Fig.
1). The cell viability of the control at 37°C was considered to
be 100%. At 37°C, A6-P, APPS, APHD and VCIP yielded cell survival
rates of 86.8±5.7, 63.0±5.5 (P<0.0001), 88.01±3.70 and
77.8±2.55%, respectively. The cell viability for the control was
reduced to 57.3±2.7% at 42°C (P<0.0001). At 42°C, A6-P, APPS,
APHD and VCIP decreased cell viability to 42.0±2.1 (P<0.0001),
40.3±2.1 (P<0.0001), 60.6±4.3 and 66.8±7.4%, respectively. The
carcinostatic activities of A6-P and APPS were markedly increased
with hyperthermia.
Morphological changes in tumor cells
observed by crystal violet stain assay
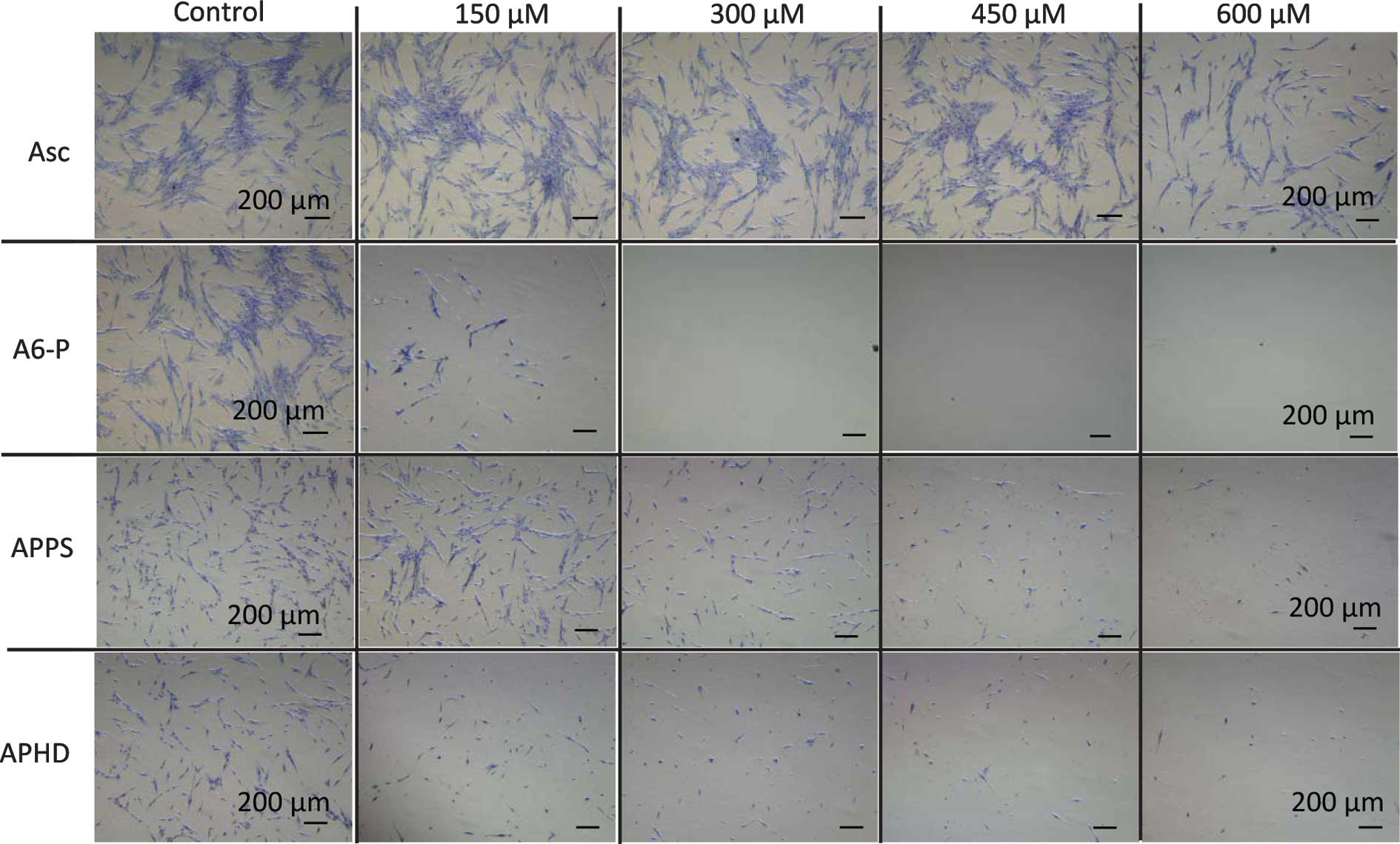
Morphological observations in the tumor cells was
performed by crystal violet staining, while the carcinostatic
effects were assayed. The morphological changes occurred in the
HSC-4 cells treated with APPS or APHD, which exhibited the greatest
carcinostatic activities (Fig. 2).
The morphological observations in the HCS-4 cells treated with
APPS, were decreased cell numbers, cell shrinkage and pycnosis
(nuclear condensation) indicative of apoptosis and cell
deformation. At 42°C, the morphological changes of the cells were
increased and fragmentation of the cells was also observed.
Cytotoxicities of diverse Asc derivatives
on OUMS-36 cells
The diverse Asc derivatives were added to OUMS-36
cells and the culture samples were maintained by sequential culture
for 24 h at 37°C. The cytotoxicities were measured by a WST-8 assay
(Figs. 3 and 4). The cell viability of the controls in
the absence of the Asc derivative was considered to be 100%. The
order of magnitude of cytotoxicity at a dose of 600 μM was as
follows: A6-P>APHD>Asc>APPS. APPS did not injure the
OUMS-36 cells, even at 600 μM (Fig.
3). This tendency was also exhibited in the morphological
observation (Fig. 5)
Discussion
Among the studied diverse ascorbic acid (Asc)
derivatives, the straight chain type palmitic acid-phosphorus acid
derivative, APPS, demonstrated the greatest antitumor effect,
whereas, the branched chain type, APHD, did not demonstrate any
antitumor effects. This finding may be due to the fact that the
straight chain type is considered to be more permeable through the
cell membrane than the branched type. Asc radicals, which are
produced by the enzyme-catalyzed esterolysis of APPS, are absorbed
into the cells where they injure DNA leading to cell death. By
contrast, the branched types, APHD and VCIP, are less permeable or
not permeable through the cell membranes, and therefore not
effective for carcinostasis. From the morphological observations of
the HCS-4 cells treated with APPS, a decrease in cell number, cell
shrinkage and pycnosis (nuclear condensation) indicative of
apoptosis and cell deformation were observed. These observed
morphological changes were increased upon combination with
hyperthermia treatment where further fragmentation of the cells was
also observed. Whether the Asc derivatives were cytotoxic to the
normal cells was then examined. A6-P was found to be cytotoxic to
the tumor cells and normal (OUMS-36) cells, APHD was cytotoxic to
the normal cells, but scarcely to the tumor cells, and APPS was
cytotoxic to the tumor cells but not the normal cells at a dose of
600 μM. The results demonstrate that APPS has a marked
carcinostatic advantage over A6-P. This benefit may be due to the
addition of the of 2-O-phosphatidyl moiety, which adjusts
the molecular LHB (lipophilicity-hydrophilicity balance) to be more
hydrophilic. By contrast, the branched chain type APHD, an isomer
of APPS, was almost ineffective, even at 100 μM. This finding may
be due to the difference in molecular structure, which is related
to surface activity and cell membrane permeability.
In conclusion, APPS exhibited a marked carcinostatic
effect, and therefore may be developed as a potent antitumor agent
with limited side-effects towards normal cells, and as a promoter
by combination with hyperthermia. APPS has also been demonstrated
to inhibit invasion of human fibrosarcoma cells (HT-1080) through
the constituted basement membrane Matrigel and metastasis of mouse
melanoma cells (B16-BL6)transplanted from the tail vein in mice
(20).
Acknowledgements
The authors thank Dr Shinya Kato for his technical
assistance.
References
|
1
|
Edgar JA: Dehydroascorbic acid and cell
division. Nature. 2273:24–26. 1970. View
Article : Google Scholar
|
|
2
|
Cameron E, Pauling L and Leibovitz B:
Ascorbic acid and cancer: a review. Cancer Res. 39:663–681.
1779.
|
|
3
|
Poydock ME, Reikert D and Rice J:
Influence of vitamins C and B12 on the survival rate of mice
bearing ascites tumor. Exp Cell Biol. 50:88–91. 1982.PubMed/NCBI
|
|
4
|
Pierson HF, Fisher JM and Rabinovitz M:
Depletion of extracellular cysteine with hydroxocobalamin and
ascorbate in experimental murine cancer chemotherapy. Cancer Res.
45:4727–4731. 1985.PubMed/NCBI
|
|
5
|
Hacker MP, Khokhar AR, Brown DB, McCormack
JJ and Krakoff IH: Ascorbato(1,2-diaminocyclohexane): platinum (II)
complexes, a new series of water-soluble antitumor drugs. Cancer
Res. 45:4748–4753. 1985.PubMed/NCBI
|
|
6
|
Miwa N, Yamazaki H, Nagaoka Y, Kageyama K,
Onoyama Y, Matsui-Yuasa I, Otani S and Morisawa S: Altered
production of the active oxygen species is involved in enhanced
cytotoxic action of acylated derivatives of ascorbate to tumor
cells. Biochim Biophys Acta. 18:144–151. 1988.PubMed/NCBI
|
|
7
|
Miwa N and Yamazaki H: Potentiated
susceptibility of ascites tumor to acyl derivatives of ascorbate
caused by balanced hydrophobicity in the molecule. Exp Cell Biol.
54:245–249. 1986.PubMed/NCBI
|
|
8
|
Kageyama K, Onoyama Y, Kimura M, Yamazaki
H and Miwa N: Enhanced inhibition of DNA synthesis and release of
membrane phospholipids in tumour cells treated with a combination
of acylated ascorbate and hyperthermia. Int J Hyperthermia.
7:85–91. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pajonk F, Ophoven A and McBride WH:
Hyperthermia-induced proteasome inhibition and loss of androgen
receptor expression in human prostate cancer cells. Cancer Res.
65:4836–4843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harris M: Criteria of viability in
heat-treated cells. Exp Cell Res. 44:658–661. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palzer RJ and Heidelberger C: Studies on
the quantitative biology of hyperthermic killing of HeLa cells.
Cancer Res. 33:415–421. 1973.PubMed/NCBI
|
|
12
|
Gerner EW, Boone R, Connor WG, Hicks JA
and Boone ML: A transient thermotolerant survival response produced
by single thermal doses in HeLa cells. Cancer Res. 36:1035–1040.
1976.PubMed/NCBI
|
|
13
|
Mondovì B, Finazzi Agrò A, Rotilio G,
Strom R, Moricca G and Rossi Fanelli A: The biochemical mechanism
of selective heat sensitivity of cancer cells. II Studies on
nucleic acids and protein synthesis. Eur J Cancer. 5:137–146.
1969.PubMed/NCBI
|
|
14
|
Henle KJ and Leeper DB: Effects of
hyperthermia (45 degrees) on macromolecular synthesis in Chinese
hamster ovary cells. Cancer Res. 39:2665–2674. 1979.PubMed/NCBI
|
|
15
|
Kageyama K, Onoyama Y, Nakanishi M and Ito
K: New culture tube with an inside wall devised for studies of
short-term hyperthermia. Int J Hyperthermia. 4:567–570. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka H, Kageyama K, Kusumoto K, Asada R
and Miwa N: Antitumor and antiinvasive effects of diverse new
macrocyclic lactones, alkylolides and alkenylolides, and their
enhancement by hyperthermia. Oncol Rep. 18:1257–1262.
2007.PubMed/NCBI
|
|
17
|
Asada R, Kageyama K, Tanaka H, Mimura H
and Miwa N: The antitumor activities of the structurally-similar
two-species aromatics Tonalide and Pearlide and the enhancement of
their effects by hyperthermia. Mol Med Rep. 2:33–37.
2009.PubMed/NCBI
|
|
18
|
Asada R, Kageyama K, Tanaka H, Matsui H,
Kimura M, Saitoh Y and Miwa N: Antitumor effects of nano-bubble
hydrogen-dissolved water are enhanced by coexistent platinum
colloid and the combined hyperthermia with apoptosis-like cell
death. Oncol Rep. 24:1463–1470. 2010.
|
|
19
|
Saito K, Oku T, Ata N, Miyasiro H and
Saiki I: A modified and convenient method for assessing tumor cell
invasion and migration and its application to screening for
inhibitors. Biol Pharm Bull. 20:345–348. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JW, Kayasuga A, Nagao N,
Masatsuji-Kato E, Tuzuki T and Miwa N: Repressions of actin
assembly and RhoA localization are involved in inhibition of tumor
cell motility by lipophilic ascorbyl phosphate. Int J Oncol.
23:1561–1567. 2003.PubMed/NCBI
|