Introduction
Doxorubicin (DOX), an anthracycline antibiotic and
antineoplastic agent, was first isolated from Streptomyces
peucetius (1). DOX is a potent
chemotherapeutic agent that is used in the treatment of solid
tumors and malignant hematological diseases (2). DOX exerts its antitumor activity by
inserting into DNA, leading to double-stranded DNA breaks (DSB),
and intercepting DNA topoisomerase II activity (3,4).
However, the clinical use of DOX has been largely restricted due to
its cardiotoxicity, which may lead to the development of
cardiomyopathy and ultimately congestive heart failure (5). The molecular mechanisms underlying
DOX-induced cardiotoxicity include the formation of free radicals,
activation of transcription factor NF-κB, increased lipid
peroxidation and Ca2+ overloading (6–8). The
use of cardioprotective drugs is an alternative approach to reduce
the cardiotoxicity of DOX. Pharmacological and clinical attempts to
reduce the cardiotoxicity of DOX have had little success thus far.
Consequently, it is important to develop a therapy to reduce
DOX-induced cardiotoxicity and increase the antitumor effect of
DOX.
Berberine (BER), a botanical alkaloid, is purified
from the roots and bark of the Berberis species (9). BER reportedly possesses multiple
biological and pharmacological properties, including
anti-diarrheal, anti-fungal, anti-diabetic (10–12),
hepatoprotective and cardioprotective effects. The possible
mechanism of the hepatoprotective effect is that BER inhibits the
activity of CYP 2E1 and CYP 1A2, reduces the production of nitric
oxide and lowers the AST and ALT levels in serum (13,14).
For the cardioprotective property, BER is known to modulate Cdk9
and cyclin T1 protein expression. BER possesses muscarinic
agonist-like properties which may contribute to a reduction in
myocardial damage (15–17). BER also suppresses tumor growth
through the induction of apoptosis and cell cycle arrest in cancer
cells (18–21). Notably, it has been reported that
the acute toxicity of BER was not observed at normal dosage in mice
(22).
Based on these findings, we hypothesized that
combining DOX with BER as a novel strategy for tumor therapy would
not only increase the effect of DOX, but also prevent the
cardiotoxicity induced by DOX. The present study was therefore
performed to test this hypothesis in A549, HepG2 and HeLa cells.
Our observations revealed that BER enhances the antitumor effects
of DOX in A549 and HeLa cells.
Materials and methods
Chemicals
BER was kindly provided by Professor Xue-Gang Li
(Southwest University, Chongqing, China). Dimethyl sulfoxide
(DMSO), trypsin, penicillin, streptomycin,
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-tetrazolium bromide
(MTT) and acridine orange (AO) were purchased from Sigma (St.
Louis, MO, USA). Fetal bovine serum was obtained from Tianhang
Biotechnology Company (Zhejiang, China). DOX was purchased from
Shanxi Powerdone Pharmaceutics Company (Beijing, China).
Cell culture
The human lung carcinoma A549, human cervix
carcinoma HeLa and human hepatoma HepG2 cell lines were purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in Dulbecco’s modified Eagle’s
medium supplemented with 10% fetal bovine serum at 37°C in 5%
CO2. The cells were subcultured at 90% confluence with
0.25% trypsin (w/v) every 2–3 days.
Cell viability assay
The cells were seeded in 96-well plates at different
densities: A549, 7,000 cells/well; HeLa, 6,000 cells/well; and
HepG2, 8,000 cells/well. The stock solutions of DOX and BER [both
dissolved in phosphate-buffered saline (PBS)] were then diluted in
culture medium to obtain the desired concentrations (BER: 0, 1, 10,
100, 200, 400 μM; DOX: 0, 0.1, 1, 10, 100, 200 μM; BER+DOX: 0+0,
1+0.2, 10+2, 50+10, 100+20, 200+60 μM). The MTT assay was used to
detect cell viability. Briefly, 10 μl of MTT (at 5 mg/ml) was added
to each well, at a final concentration of 500 μg/ml. Following 4 h
of incubation under standard conditions, the cell supernatants were
removed. DMSO (100 μl) was then added to dissolve the MTT crystals
(formazan). The absorbance of the sample was read using a Bio-Rad
microplate reader (model 630; Hercules, CA, USA) at 490 nm.
Analysis of drug synergism
The combination index (CI) was calculated for the
analysis of the synergistic, antagonistic or additive effects of
the two drugs (23). The CI is
calculated using the formula:
CI=[(D)1/(Dx)1]+[(D)2/(Dx)2],
in which (D)1 is the concentration of the first drug
required to achieve a particular effect in the combination;
(Dx)1 is the concentration of the first drug
that causes an identical effect alone; (D)2 is the
concentration of the second drug which achieves a particular effect
in the combination; (Dx)2 is the
concentration of the second drug that generates the same effect
alone. CI>1 indicates antagonism, CI=1 indicates an additive
effect and CI<1 indicates synergy.
Fluorescent microscopy measurements
To detect apoptosis, A549 cells were stained with
AO. The cells were seeded in 6-well plates at a density of 800,000
cells/well. For the AO procedure, A549 cells were treated with
different concentrations of BER and DOX (BER: 0, 75, 150, 300 μM;
DOX: 0, 1.5, 3, 6 μM; BER+DOX: 0+0, 75+1.5, 150+3, 300+6 μM) for 24
h and then 10 μl of prepared AO working solution (100 μg/ml in PBS)
was added. The cells were immediately examined with a fluorescence
microscope (Olympus U-RFLT50, Tokyo, Japan). Morphologically
apoptotic cells were counted from 10 visual fields of 5 different
areas for each group.
Statistical analysis
Values are presented as the mean ± SEM. One-way
ANOVA and the Student’s t-test were performed. P<0.05 was
considered to indicate a statistically significant result.
Results
BER enhances DOX-mediated cytotoxicity in
solid tumor cells
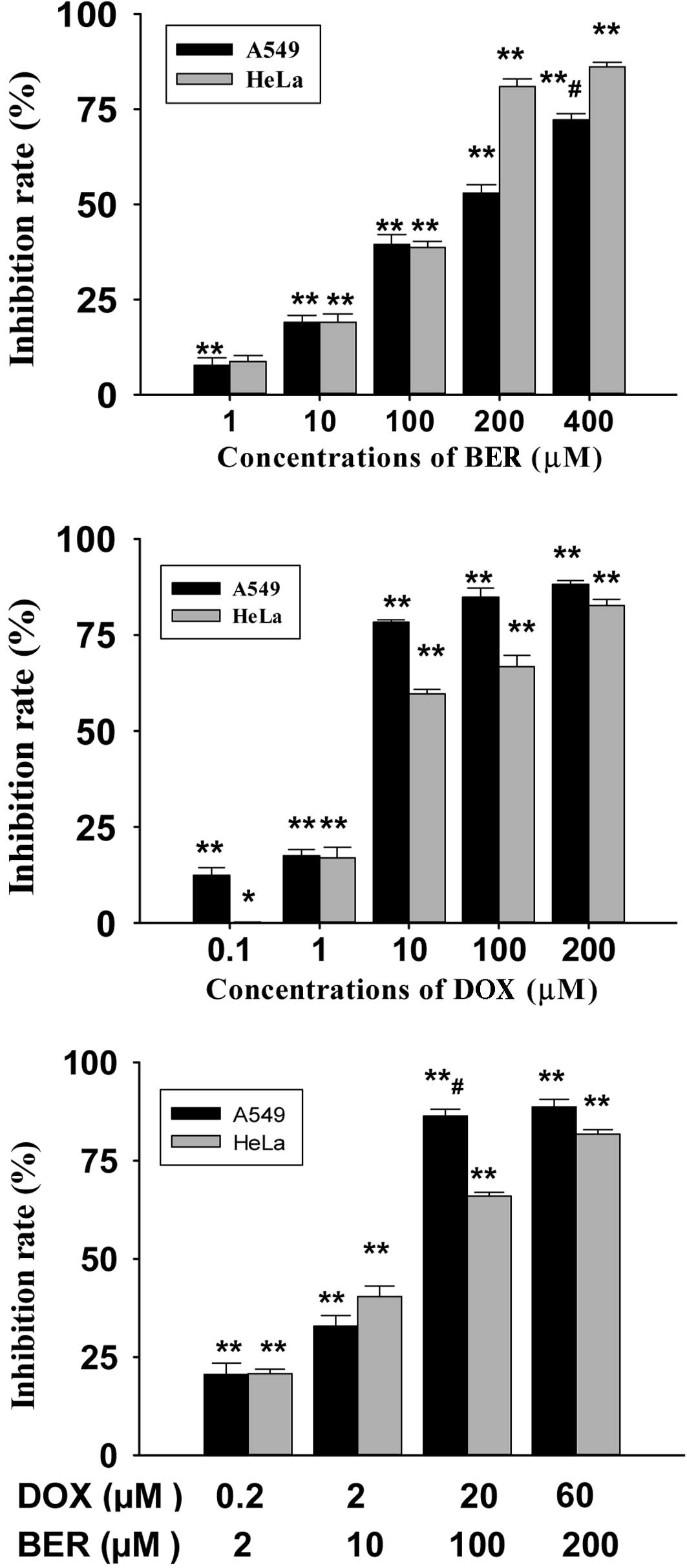
To determine the cell viability following treatment
with different concentrations of DOX and BER in the three cell
lines, the MTT assay was performed. The results indicate that DOX
and BER significantly inhibited cell viability in A549, HeLa and
HepG2 cell lines in a dose-dependent manner (Fig. 1). As shown in Fig. 1, 100 μM BER caused 39.4% inhibition
in A549 cell lines and 200 μM BER had an acute cytotoxic effect in
A549 and HeLa cells. The 50% growth inhibition concentration
(IC50) of BER in A549, HepG2 and HeLa cells following 24
h of incubation was 139.4, 3,587.9 and 159.5 μM, respectively
(Table IA). The IC50 of
DOX and BER in the combination group are shown in Table IB. A549 and HeLa cells were found to
be more sensitive to BER than HepG2 cells (Table I). A549 cells were the most
sensitive to DOX. In the present study, we found that the
IC50 of the combination of BER and DOX was lower than
that of each drug used alone.
 | Table ISensitivity of A549, HepG2 and HeLa
cells to the treatment with BER and DOX alone and in
combination. |
Table I
Sensitivity of A549, HepG2 and HeLa
cells to the treatment with BER and DOX alone and in
combination.
| A, IC50 of
BER and DOX alone (μM). |
|---|
|
|---|
| | A549 cells | HepG2 cells | HeLa cells |
|---|
| IC50 | BER | 139.4 | 3,587.8 | 159.5 |
| DOX | 3.1 | 9.2 | 16.7 |
|
| B, IC50 of
combined DOX and BER (μM). |
|
| | A549 cells | HepG2 cells | HeLa cells |
|
| IC50 | BER | 8.6 | - | 98.9 |
| DOX | 1.7 | - | 1.9 |
Synergistic action of BER and DOX
Isobolograms were used to evaluate whether combining
BER and DOX generates a synergistic effect (24). As shown in Fig. 2, the Y-axis shows the
IC50 of BER and the X-axis shows the IC50 of
DOX. The straight line (additivity line) connects the
IC50 values of DOX and BER when the drugs are used
alone. In the present study, we found that the IC50 of
combined DOX and BER was below the straight line, indicating that a
combination of the two drugs may generate a synergistic antitumor
effect in A549 and HeLa cells (Fig.
2). The CI was used to analyze the synergistic effect. The
IC50 of DOX and BER was used to calculate CI. The CI was
(1.7/3.1)+(8.6/139.4)=0.61 in the A549 cells, indicating that
combined DOX and BER generates synergistic effect. In the HeLa
cells, the CI was (1.9/16.7)+(98.9/159.5)=0.73. These results
suggest the synergistic action of BER and DOX in cancer
therapy.
Combined treatment with BER and DOX
causes solid tumor cell apoptosis
To assess whether the decrease in viability was
mediated by inducing apoptosis, cells that had been treated with
the two drugs were stained with AO. Results showed that the single
and combined treatment with DOX and BER induced apoptosis (Fig. 3). The number of apoptotic cells was
increased in the combination group compared with the single
treatment group (Fig. 3D),
suggesting that the combination of DOX and BER synergistically
induces the apoptosis of A549 cells.
 | Figure 3A549 cell apoptosis was assessed by AO
staining. (A) A549 cells were preincubated with BER (75, 150, 300
μM) for 24 h. (B) A549 cells were preincubated with DOX (1.5, 3, 6
μM) for 24 h. (C) A549 cells were preincubated with DOX+BER
(1.5+75, 3+150, 6+300 μM) for 24 h. (D) Apoptosis induced in BER−,
DOX− or BER+DOX-treated cells. In the combination group, the
apoptosis rates were increased compared with the single treatment
groups. **P<0.01, compared with the control group
that was treated with no drugs. N=10. BER berberine; DOX,
doxorubicin; AO, acridine orange. |
Discussion
The findings of the present study indicate that BER,
a botanical alkaloid, is able to enhance the anticancer effect of
DOX in A549 and HeLa cells. Our results have shown that DOX and BER
significantly reduced the viability of A549 and HeLa cells. From
the IC50 of DOX and BER, we found that the
IC50 of the combination of DOX and BER was lower than
the IC50 of the drugs used singly. Notably, the results
of this study demonstrate that combining DOX with BER generates a
synergistic anticancer effect in A549 and HeLa cells.
DOX has been found to have anticancer activities
against a range of solid tumors. However, the therapeutic use of
DOX has been limited by its serious dose-related cardiotoxicity
(25). BER has been reported to be
safe and beneficial in the treatment of patients with chronic
congestive heart failure (16).
Therefore, combining DOX with BER is a novel strategy for the
treatment of cancer and reduction of the cardiotoxicity induced by
DOX.
BER is a naturally occurring botanical alkaloid that
is found in the roots and bark of the Berberis species. In
clinical use, BER possesses anti-inflammatory, anti-diarrheal and
anti-fungal effects. BER has also been reported to possess
anticancer properties and anti-metastatic effects in non-small cell
lung cancer A549 cells (26). The
mechanism of its antitumor effect is that BER induces apoptosis and
cell cycle arrest in cancer cells (27,28).
However, the anticancer effect of BER is associated with the cell
type; the IC50 of BER in the HepG2 cell line is 3,587.8
μM, which is extremely high for an antitumor drug, but the
IC50 of BER is lower in A549 and HeLa cells. Our results
have shown that BER induces apoptosis in A549 cells. Notably, the
combination of DOX and BER also synergistically induced the
apoptosis of A549 cells (Fig. 3).
These data suggest that the induction of apoptosis is the mechanism
by which the combination of DOX and BER inhibits the growth of A549
cells. However, more investigations are required to demonstrate the
efficacy of the combination of DOX and BER in treating cancer
patients.
The induction of apoptosis is one of the antitumor
mechanisms of DOX and BER. This is in accordance with the theory of
‘independent similar action’ (29).
Therefore, combining DOX with BER may achieve a synergistic
antitumor effect. In the present study, we used isobologram
illustrations to detect the synergism. A combination of the two
drugs generated synergistic antitumor effects in A549 (CI=0.61) and
HeLa (CI=0.73) cells (Fig. 3).
Thus, more studies should be conducted to detect the mechanism of
the synergistic anticancer action of DOX and BER.
In conclusion, we confirmed that the combination of
DOX and BER synergistically generates anticancer effects in A549
and HeLa cells in vitro, possibly mediated by inducing
apoptosis. With regard to HepG2 cells, the IC50 of BER
is extremely high for an antitumor agent. The combination of DOX
with BER is a novel strategy that has potential in the treatment of
cancer patients.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81001454), the Research Fund for the
Doctoral Program of Southwest University, China (SWU109036), the
Fundamental Research Funds for the Central Universities
(XDJK2010C061) and the Fund for Construction of Scientific and
Technical Innovation of Chongqing (CSTC, 2009CB1010).
Abbreviations:
|
AO
|
acridine orange
|
|
BER
|
berberine
|
|
CI
|
combination index
|
|
DOX
|
doxorubicin
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-tetrazolium bromide
|
|
IC50
|
the 50% growth inhibition
concentration
|
References
|
1
|
Henderson IC and Frei TE III: Adriamycin
and the heart. N Engl J Med. 300:310–312. 1979. View Article : Google Scholar
|
|
2
|
Christiansen S and Autschbach R:
Doxorubicin in experimental and clinical heart failure. Eur J
Cardiothorac Surg. 30:611–616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Binaschi M, Capranico G, Dal Bo L and
Zunino F: Relationship between lethal effects and topoisomerase II
mediated double-strand DNA breaks produced by anthracyclines with
different sequence specificity. Mol Pharmacol. 51:1053–1059.
1997.
|
|
4
|
Hennig UG, Rudd NL and Hoar DI:
Kinetochore immunofluorescence in micronuclei: a rapid method for
the in situ detection of aneuploidy and chromosome breakage in
human fibroblasts. Mutat Res. 203:405–414. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boucek RJ Jr, Dodd DA, Atkinson JB, Oquist
N and Olson RD: Contractile failure in chronic doxorubicin-induced
cardiomyopathy. J Mol Cell Cardiol. 29:2631–2640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doroshow JH: Effect of anthracyline
antibiotics on oxygen radical formation in rat heart. Cancer Res.
43:460–472. 1983.PubMed/NCBI
|
|
7
|
Ichihara S, Yamada Y, Kawai Y, Osawa T,
Furuhashi K, Duan Z and Ichihara G: Roles of oxidative stress and
Akt signaling in doxorubicin cardiotoxicity. Biochem Biophys Res
Commun. 359:27–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Temma K, Chugun A, Akera T, Hara Y, Sasaki
T and Kondo H: Ca2+ overloading causes the negative
inotropic effect of doxorubicin in myocytes isolated from
guinea-pig hearts. Eur J Pharmacol. 322:235–242. 1997.
|
|
9
|
Timothy CB and Gregory SK: Berberine:
therapeutic potential of an alkaloid found in several medicinal
plants. Altern Med Rev. 2:94–102. 1997.
|
|
10
|
Takase H, Yamamoto K, Ito K and Yumioka E:
Pharmacological studies on antidiarrheal effects of berberine and
geranii herb. Nihon Yakurigaku Zasshi. 102:101–112. 1993.(In
Japanese).
|
|
11
|
Iwazaki RS, Endo EH, Ueda-Nakamura T,
Nakamura CV, Garcia LB and Filho BP: In vitro antifungal activity
of the berberine and its synergism with fluconazole. Antonie Van
Leeuwenhoek. 97:201–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Wei J, Xue R, et al: Berberine
lowers blood glucose in type 2 diabetes mellitus patients through
increasing insulin receptor expression. Metabolism. 59:285–292.
2010. View Article : Google Scholar
|
|
13
|
Zhao X, Zhang JJ, Wang X, Bu XY, Lou YQ
and Zhang GL: Effect of berberine on hepatocyte proliferation,
inducible nitric oxide synthase expression, cytochrome P450 2E1 and
1A2 activities in diethylnitrosamine- and phenobarbital- treated
rats. Biomed Pharmacother. 62:567–572. 2008. View Article : Google Scholar
|
|
14
|
Janbaz KH and Gilani AH: Studies on
preventive and curative effects of berberine on chemical-induced
hepatotoxicity in rodents. Fitoterapia. 71:25–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou JY, Zhou SW, Tang JL, Xu Y and Ying
Y: Effect of berberine on Cdk9 and cyclin T1 expressions in
myocardium of diabetic rats. J Med Coll PLA. 23:45–51. 2008.
View Article : Google Scholar
|
|
16
|
Zeng XH, Zeng XJ and Li YY: Efficacy and
safety of berberine for congestive heart failure secondary to
ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol.
92:173–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salehi S and Filtz TM: Berberine possesses
muscarinic agonist-like properties in cultured rodent
cardiomyocytes. Pharmacol Res. 63:335–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patil JB, Kim J and Jayaprakasha GK:
Berberine induces apoptosis in breast cancer cells (MCF-7) through
mitochondrial-dependent pathway. Eur J Pharmacol. 645:70–78. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuo CL, Chou CC and Yung BY: Berberine
complexes with DNA in the berberine-induced apoptosis in human
leukemic HL-60 cells. Cancer Lett. 93:193–200. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsang CM, Lau EP, Di K, et al: Berberine
inhibits Rho GTPases and cell migration at low doses but induces G2
arrest and apoptosis at high doses in human cancer cells. Int J Mol
Med. 24:131–138. 2009.PubMed/NCBI
|
|
21
|
Kim JB, Yu JH, Ko E, et al: The alkaloid
berberine inhibits the growth of Anoikis-resistant MCF-7 and
MDA-MB-231 breast cancer cell lines by inducing cell cycle arrest.
Phytomedicine. 17:436–440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kheir MM, Wang YG, Hua L, Hu J, Li LL, Lei
F and Du L: Acute toxicity of berberine and its correlation with
the blood concentration in mice. Food Chem Toxicol. 48:1105–1110.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tallarida RJ: Drug synergism: Its
detection and applications. J Pharmacol Exp Ther. 298:865–872.
2001.PubMed/NCBI
|
|
25
|
Yoshida M, Shiojima I, Ikeda H and Komuro
l: Chronic doxorubicin cardiotoxicity is mediated by oxidative DNA
damage-ATM-p53-apoptosis pathway and attenuated by pitavastatin
through the inhibition of Rac1 activity. J Mol Cell Cardiol.
47:698–705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng PL, Hsieh YS, Wang CJ, Hsu JL and
Chou FP: Inhibitory effect of berberine on the invasion of human
lung cancer cells via decreased productions of
urokinase-plasminogen activator and matrix metalloproteinase-2.
Toxicol Appl Pharmacol. 214:8–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan K, Zhang C, Feng J, et al: Induction
of G1 cell cycle arrest and apoptosis by berberine in bladder
cancer cells. Eur J Pharmacol. 661:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meeran SM, Katiyar S and Katiyar SK:
Berberine-induced apoptosis in human prostate cancer cells is
initiated by reactive oxygen species generation. Toxicol Appl
Pharmacol. 229:33–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bliss CI: The toxicity of poisons applied
jointly. Ann Appl Biol. 26:585–615. 1939. View Article : Google Scholar
|