Introduction
As a result of poor prognosis, lung cancer is a
major cause of cancer-related mortality. Non-small cell lung cancer
(NSCLC) accounts for approximately 80% of all lung cancer cases and
has a 5-year overall survival rate of less than 15% (1,2).
Approximately 40% of patients diagnosed with NSCLC have
unresectable stage III disease or medically inoperable disease
(3). Although there have been
recent advances and developments in numerous therapeutic
strategies, including surgery, chemotherapy and radiotherapy, poor
survival remains an issue due to the high systemic toxicity and
drug resistance. Therefore, the development of novel approaches for
the diagnosis, treatment and prevention of NSCLC, including
targeted gene treatment as an adjuvant modality or as a
radiosensitizer to treat this lethal disease, is urgently needed to
enhance the survival rate in patients.
One of the targets currently being evaluated for the
treatment of lung cancer is EZH2. EZH2 is a catalytically active
component of the PRC2 complex, which is a human homolog of the
Drosophila protein ‘Enhancer of Zest’. EZH2 is involved in
the transcriptional repression of specific genes by the
trimethylation of lysine 27 and, to a lesser extent, lysine 9 of
histone H3. EZH2 contains a SET domain with an intrinsic histone
lysine methyltransferase activity and directly interacts with and
regulates the activity of the DNA methyltransferases (DNMTs) DNMT1,
DNMT3a and DNMT3b. Various studies have found that the abnormal
expression of EZH2, a potential marker used to distinguish
aggressive from indolent or benign cancers, is involved in the
tumorigenesis of several malignancies, including melanoma,
prostate, breast, bladder and endometrial cancers (4). EZH2 also provides proliferative
advantages to eukaryotic cells through interaction with pathways of
key elements that control cell growth arrest and differentiation
(4–6). As a transcriptional repressor, EZH2 is
involved in controlling cell growth and proliferation by promoting
the S-phase entry and G2/M transition. EZH2 also
promotes the repression of certain genes. This involves histone
deacetylation by histone deacetylase-1 (HDAC-1) with which EZH2
interacts through its PRC2-binding partner EED (7,8).
Recently, the successful use of small interfering
RNA (siRNA) to downregulate gene expression in several model
systems has led to an increasing number of attempts to explore this
methodology in a potentially therapeutic setting. The knockdown of
EZH2 by siRNA inhibits breast cancer cell proliferation, whereas
the pharmacological inhibition of EZH2 results in the apoptosis of
breast cancer cells (9). At
present, few studies concerning NSCLC and its response to
radiotherapy treatment in combination with silencing EZH2 using
siRNAs have been published. Therefore, combining siRNA-EZH2
targeting with irradiation has potential as a therapeutic
option.
In the present study, we aimed to silence EZH2 and
explore the antitumor effect of siRNAs on lung cancer when applied
in combination with radiotherapy. We hypothesized that the
inhibition of the EZH2-mediated signal transduction pathway using
siRNA is an effective strategy to increase the sensitivity of NSCLC
patients to radiotherapy.
Materials and methods
Cell lines and culture
The human lung adenocarcinoma A549 and HTB-56 cell
lines were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA). The cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, USA) and
supplemented with 10% heat-inactivated FBS and 1%
penicillin/streptomycin at 37°C in a humidified incubator with 95%
air and 5% CO2.
siRNA transfection
A549 and HTB-56 cells were plated on six-well plates
at a density of 2×105 cells/well and grown overnight
until 50–80% confluence was achieved to obtain maximum transfection
efficiency. The cells were transfected with validated siRNA for
EZH2 and a negative control vector (Qiagen, Lafayette, CO, USA) at
a concentration of 100 nM using Lipofectamine 2000 transfection
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The medium was replaced with standard
culture medium 6 h post-transfection.
Irradiation treatment
On the days after siRNA transfection, sub-confluent
cell monolayers were treated with γ-ray irradiation from a
60Co source (PLA General Hospital, Beijing, China) at ~2
Gy/min every 3 days for 2 weeks.
Western blot analysis
To assess EZH2 expression, 30 μg of total cell
protein extracts were obtained from A549 and HTB-56 cells following
centrifugation at 12,000 × g for 30 min at 4°C. The cells were
lysed in buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1
mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1
mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml
leupeptin supplemented with proteinase (Roche Diagnostics,
Mannheim, Germany) and phosphatase inhibitor cocktails (Sigma, St.
Louis, MO, USA). The protein concentrations of cell lysates were
determined using a BCA protein assay kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). The cell lysates were separated by
SDS-PAGE and analyzed according to standard western blotting
procedures. After transferring to PVDF membranes and probing with
1:1,000-diluted anti-EZH2 primary antibodies (Cell Signaling
Technology, Beverly, MA, USA) and β-actin, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
and visualized with ECL reagents (Pierce Biotechnology, Inc.).
Cell proliferation
Cell proliferation was assayed using a cell
proliferation MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide] kit
(Roche Diagnostics) according to the manufacturer’s instructions.
A549 and HTB-56 cells were seeded in triplicate onto 96-well tissue
culture plates at a density of 5×103 cells/well. The
cells were transfected with siRNA-EGFR and treated with irradiation
at ~2 Gy/min. Cell proliferation was determined by assessing the
mitochondrial reduction of MTT. Cell growth was analyzed on a plate
reader at a wavelength of 570 nm using a Universal Microplate
Spectrophotometer (BioTek Instruments, Winooski, VT, USA).
Experiments were performed in triplicate.
Cell cycle analysis by flow
cytometry
For the cell cycle analysis, A549 and HTB-56 cells
were plated at 1×105 cells/well on six-well plates.
Following treatment with siRNA and irradiation, cells from each
group were harvested with 0.125% trypsin, washed twice with
ice-cold PBS and fixed with 70% (v/v) ethanol overnight at 4°C. The
cells were resuspended to a concentration of 1×106
cells/ml in PBS and incubated with 100 μg/ml RNase A and 50 mg/ml
propidium iodide (PI) at room temperature for 30 min. The
distribution of the cells throughout the cell cycle was determined
by flow cytometry (Becton Dickinson, San Jose, CA, USA) and
analyzed with CellQuest software version 3.3 (Becton Dickinson).
Each test was performed a minimum of three times.
Cell apoptosis analysis by flow
cytometry
A549 and HTB-56 cells were seeded at a density of
1×105 cells/well and treated with irradiation in the
presence or absence of siRNA-EZH2. To determine the apoptotic rate,
the cells were recollected with PBS and stained with PI and Annexin
V-FITC. Following staining, cell apoptosis was analyzed by flow
cytometry.
In vivo tumor xenograft studies
A549 cells (1×105 cells/animal) were
subcutaneously injected into the left dorsal flank of 6- to
8-week-old female nude mice. After 2 weeks, the tumors reached 4–5
mm in diameter and the mice were randomly separated into three
treatment groups (n=8 animals per group). Radiotherapy (2 Gy/dose
every 3 days for 2 weeks) was administered to the animals in the
presence or absence of siRNA-EZH2 (60 μg/dose). Control animals
were injected with only PBS and all intratumoral injections were
performed under anesthesia. Subcutaneous tumor growth was measured
every third day and the mean tumor volume was calculated as
width2 × length × 0.52. The research protocol was
approved by the Animal Ethics Committee of PLA General Hospital.
Measurements were performed in a coded and blinded fashion. The
therapeutic effect was determined by statistical analysis using the
Student’s t-test.
Results
Effective inhibition of cell
proliferation following combination treatment
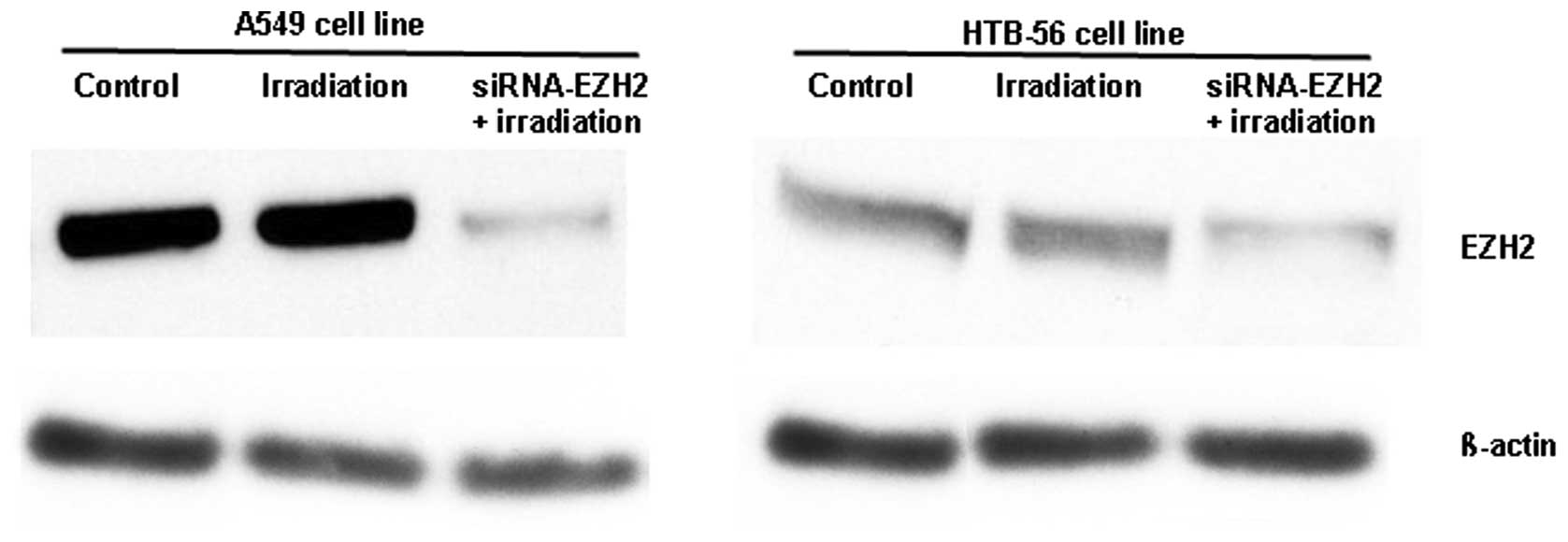
Using western blotting, we found that the
transfection of siRNA-EZH2 into A549 and HTB-56 cells downregulated
EZH2 expression by ~87 and ~75%, respectively (Fig. 1). In addition, the expression of
EZH2 was markedly higher in A549 than in HTB-56 cells. In the
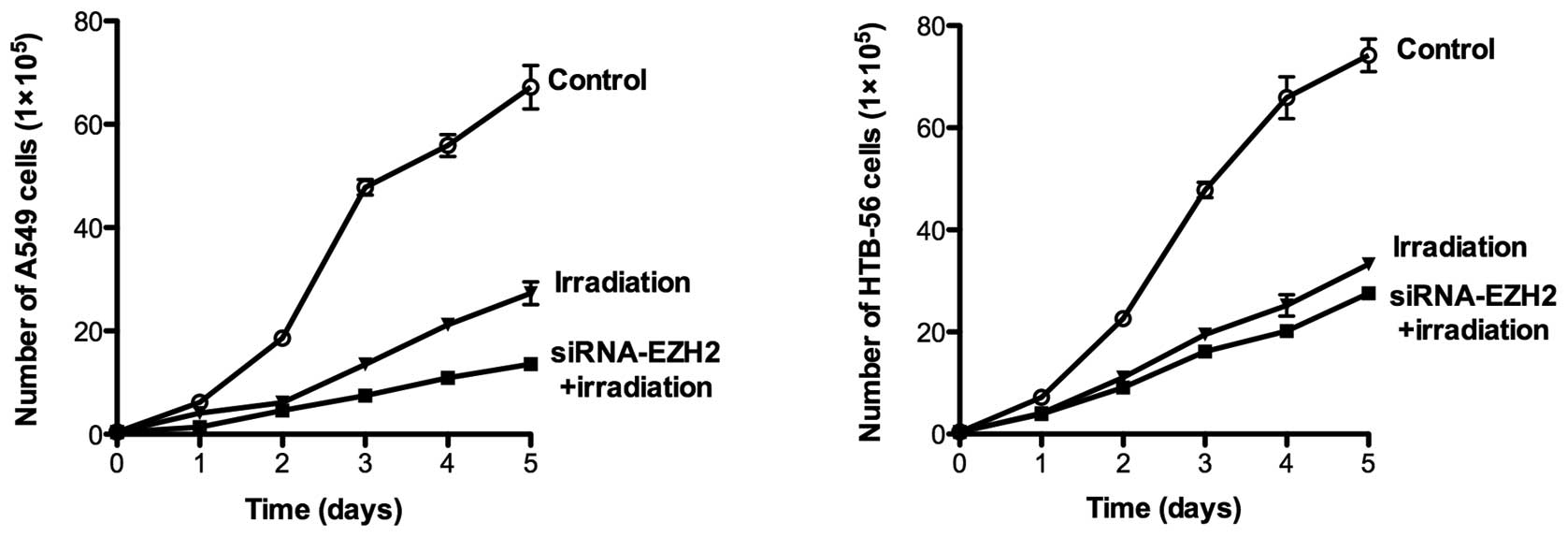
subsequent experiments, we tested the hypothesis that cooperative
anti-proliferative effects occur by combining siRNA-EZH2 treatment
with ionizing radiation in A549 and HTB-56 cells. The two cell
lines were irradiated with 2 Gy γ-rays at different time points in
the presence or absence of siRNA-EZH2. Following treatment, daily
analysis of cell viability on days 1–5 demonstrated that the
combined treatment significantly inhibited cell proliferation
(P<0.001) in A549 and HTB-56 cells. The number of A549 and
HTB-56 cells was reduced by 79.7 and 62.8%, respectively, following
irradiation in the presence of siRNA-EZH2. In cells that received
only radiotherapy, the number of A549 and HTB-56 cells was reduced
by 59.3 and 55.1%, respectively (Fig.
2).
Effects of irradiation on the cell cycle
in the presence of siRNA-EZH2
The cell cycle distribution of A549 and HTB-56 cells
was investigated via FACS in the radiotherapy only and
combination-treated groups. Following irradiation, the S-phase
fraction of A549 and HTB-56 cells was altered in the presence and
absence of siRNA-EZH2 (Fig. 3). The
downregulation of EZH2 in A549 and HTB-56 cells for 48 h produced a
17 and 11% increase, respectively, in the number of cells in the
G2/M phase following irradiation compared with the cells
that received only radiotherapy. This result indicates that
siRNA-EZH2 arrested the cells in the G2/M phase,
delaying cell cycle progression. Furthermore, the change caused by
the siRNA differed between the A549 and HTB-56 cells, although they
received the same treatment.
Effects on cell apoptosis following
combined treatment
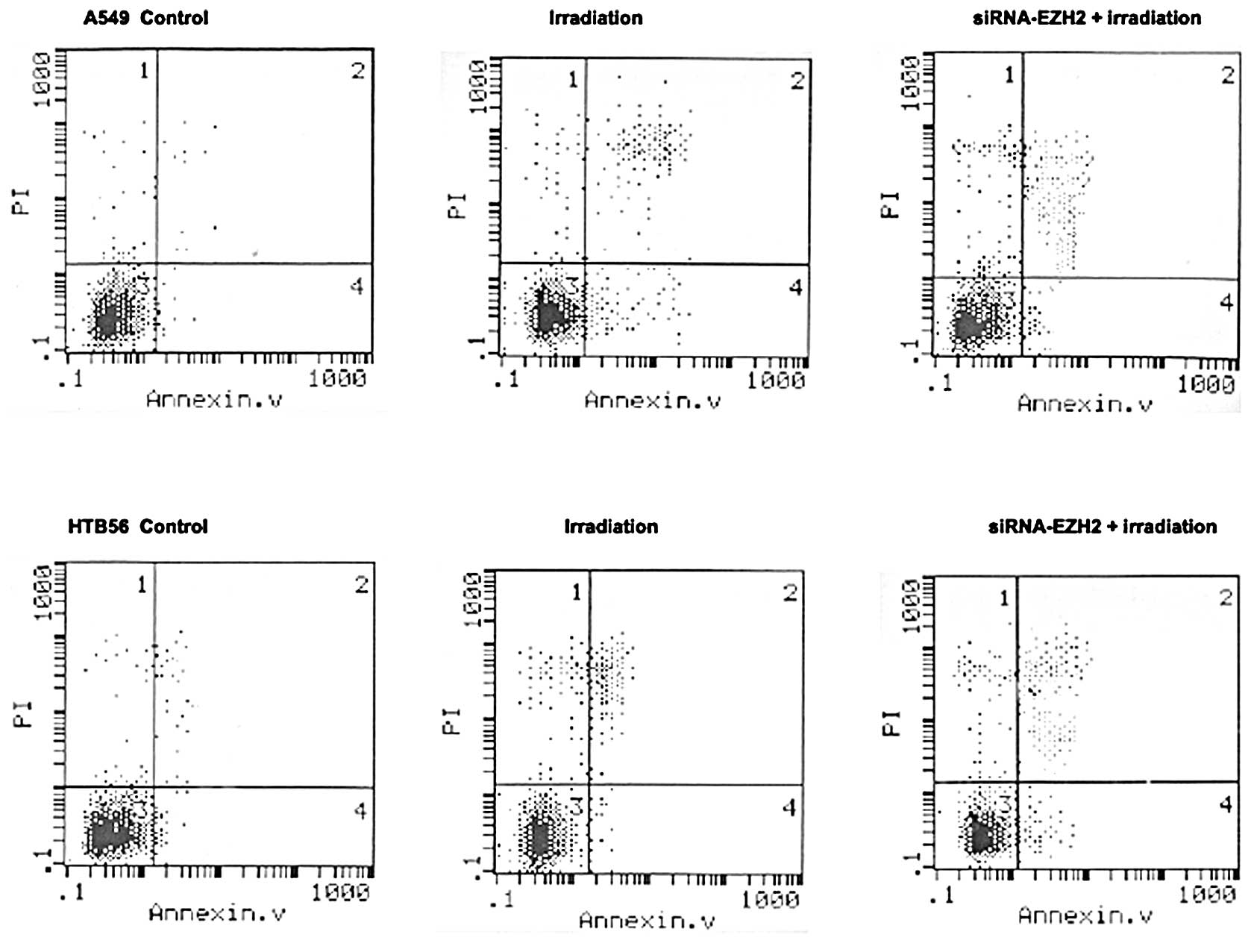
The results of the flow cytometry showed that
irradiation treatment combined with siRNA-EZH2 induced changes in
the percentage of apoptotic cells in A549 and HTB-56 cells compared
with cells that received irradiation alone (Fig. 4). Some divergence in apoptosis was
observed between the A549 and HTB-56 cells. The percentage of cells
undergoing apoptosis in the A549 and HTB-56 cells was 14.35±1.05
and 12.23±2.18%, respectively, following irradiation with 2 Gy
γ-rays, and 23.41±2.35 and 15.35±3.14%, respectively, following
treatment with irradiation coupled with siRNA-EZH2. A significant
difference was found between the irradiation-only and combination
therapy groups (P<0.01).
Effects of combination therapy on
subcutaneous tumor growth
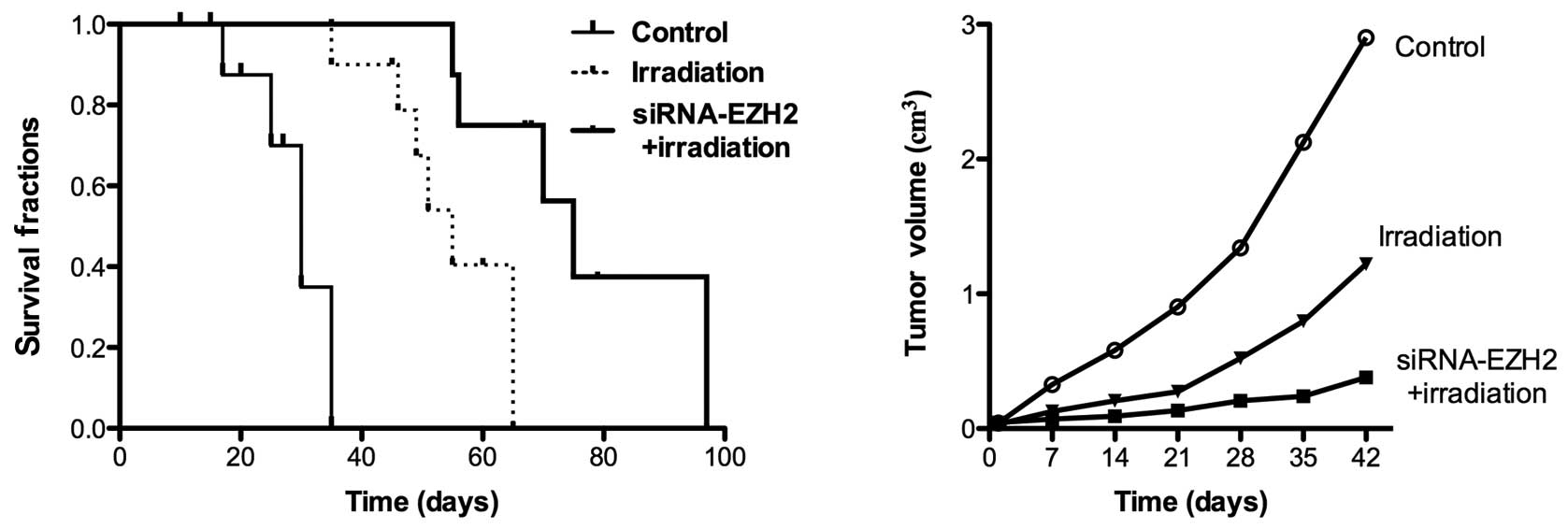
When tumors were established 2 weeks after A549 cell
injection, the nude mice were divided into three groups: a control
group without any treatment and two groups treated with irradiation
for 2 weeks in the presence and absence of siRNA-EZH2. The tumor
growth and survival rates were evaluated following the application
of the different therapeutic methods. The tumor size was measured 6
weeks later and was 2.1±0.03 cm3 in control animals,
0.7±0.04 cm3 following radiotherapy alone and 0.23±0.01
cm3 following combination therapy. Treatment with
irradiation for 2 weeks induced the inhibition of tumor growth in
all the mice (Fig. 5). Although
complete tumor regression was not observed in the combination
therapy and irradiation-only groups, treatment with siRNA-EZH2 and
radiotherapy caused an increased inhibition of A549 tumor growth
and increased the survival rate of tumor-bearing mice, thus,
demonstrating the enhanced effect of the combined treatment in
vivo.
Discussion
For most NSCLC patients, radiation therapy is
crucial in the treatment of the disease. However, long-term
survival of more than 5 years remains poor. Additionally, the
resistance to radiation from different lung cancer types limits the
therapeutic efficacy of current treatments (10). Therefore, according to the rules for
the development of more effective and less toxic treatments, there
is a search for new modalities via molecular, biological, genetic
and immunological methods with the aim of enhancing the survival
and quality of life of NSCLC patients. This is an urgent challenge
in current clinical cancer therapies. It has been demonstrated that
EZH2 is overexpressed in a number of malignancies with aggressive
forms and that the cell growth of breast cancer is significantly
influenced following the knockdown of EZH2 with siRNA (9,11). The
purpose of the present study was to test the hypothesis that
therapeutic efficiency increases following irradiation therapy in
combination with siRNA-EZH2 downregulation in human A549 and HTB-56
cells in vitro and in vivo.
In the present study, following the transfection of
siRNA-EZH2 into A549 and HTB-56 cells, the inhibition of cell
proliferation caused by irradiation was enhanced compared with
radiotherapy alone. These results markedly suggest that the reduced
expression of EZH2 by siRNA treatment is closely associated with
the therapeutic efficiency of irradiation on cancer cells.
Furthermore, these findings demonstrate that the inhibitory
efficiency of the combination treatment on cell proliferation was
markedly higher in A549 cells than that in HTB-56 cells due to the
different expression levels of EZH2. This observation indicates a
possible reason for the divergence of the clinical irradiation
therapy on NSCLC patients. Additionally, we evaluated the effect of
siRNA-EZH2 on cellular sensitivity to irradiation in terms of
changes in the cell cycle and cell apoptosis. Different mechanisms,
including several related signaling pathways that delay or inhibit
molecular transitions in cancer cells, have been investigated and
found to influence cell cycle control. These control pathways are
critical for genomic integrity and for the repair and survival of
cells exposed to DNA-damaging agents (6,12,13).
We found that EZH2 silencing arrested the cells in S and
G2/M phases, delayed the cell cycle progression and
increased cell apoptosis when combined with irradiation therapy.
The possible mechanism of these phenomena is that the process of
DNA repair was blocked with a corresponding effect on cell cycle
arrest and apoptosis. This hypothesis is consistent with a previous
study, which reported that EZH2 silencing with siRNA in breast
cancer and ovarian cancer cells resulted in cell cycle arrest in
the G0/G1 phase.
Based on these results, we found that the expression
of EZH2 is different in A549 and HTB-56 cells. This may explain the
divergent activity of radiation therapy on these cells and th
reason for the treatment with siRNA inducing greater therapeutic
efficiency in A549 cells compared to HTB-56 cells in terms of cell
proliferation, cell cycle progression and apoptosis. This finding
suggests that a higher sensitivity to irradiation therapy was
induced in A549 cells after the knockdown of EZH2. These data also
serve as reminders that a biological treatment, including specific
or new biomarkers of lung cancer, should be personalized in the
clinical therapy of NSCLC patients. Additionally, we established
subcutaneous lung cancer xenografts with A549 cells in
immunodeficient mice and evaluated the inhibition of tumor growth
induced by combined ionizing radiation and siRNA-EZH2 treatment. We
found that the combined therapy with intratumoral injections of
siRNA-EZH2 into A549 tumor xenografts significantly increased the
survival rate and inhibition of tumor growth (P<0.01) compared
with radiotherapy alone.
Combination therapies using radiotherapy and
biological agents to target cancer have been investigated for
several years. Research on cancer cell growth signaling pathways
provides potential benefits in addition to the possibility of
enhancing and improving therapeutic outcomes. Such combination
therapies generate more specific tumor responses and decreased
toxicity to normal tissues. Additionally, partially effective
therapeutic modalities may be combined without having to
significantly reduce their doses to avoid treatment-related
toxicities. In the present study, by defining the proposed role of
EZH2 in the process of lung cancer treatment following irradiation,
a new biological marker and therapeutic target were identified to
demonstrate that the application of gene silencing methods with
siRNA are potentially useful as a highly specific tool in the
modulation of tumor-promoting effects.
In conclusion, this study has shown that EZH2
silencing with siRNA, enhanced A549 and HTB-56 cell sensitivity to
irradiation in vitro and in vivo and that this effect
was influenced by the EZH2 expression level. The information
obtained from the present study may be used to develop new
treatments for the clinical therapy of NSCLC patients.
References
|
1
|
Fidias P and Novello S: Strategies for
prolonged therapy in patients with advanced non-small-cell lung
cancer. J Clin Oncol. 28:5116–5123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fuld AD, Dragnev KH and Rigas JR:
Pemetrexed in advanced non-small-cell lung cancer. Expert Opin
Pharmacother. 11:1387–1402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whitehurst AW, Bodemann BO, Cardenas J,
Ferguson D, Girard L, Peyton M, Minna JD, Michnoff C, Hao W, Roth
MG, et al: Synthetic lethal screen identification of
chemosensitizer loci in cancer cells. Nature. 446:815–819. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Francis H and Solomon B: The current
status of targeted therapy for non-small cell lung cancer. Intern
Med J. 40:611–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suvà ML, Riggi N, Janiszewska M,
Radovanovic I, Provero P, Stehle JC, Baumer K, Le Bitoux MA, Marino
D, Cironi L, et al: EZH2 is essential for glioblastoma cancer stem
cell maintenance. Cancer Res. 69:9211–9218. 2009.PubMed/NCBI
|
|
6
|
Lennes IT and Lynch TJ: Quality indicators
in cancer care: development and implementation for improved health
outcomes in non-small-cell lung cancer. Clin Lung Cancer.
10:341–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaguchi J, Sasaki M, Sato Y, Itatsu K,
Harada K, Zen Y, Ikeda H, Nimura Y, Nagino M and Nakanuma Y:
Histone deacetylase inhibitor (SAHA) and repression of EZH2
synergistically inhibit proliferation of gallbladder carcinoma.
Cancer Sci. 101:355–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujii S, Ito K, Ito Y and Ochiai A:
Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by
increasing histone H3 methylation. J Biol Chem. 283:17324–17332.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsang DP and Cheng AS: Epigenetic
regulation of signaling pathways in cancer: role of the histone
methyltransferase EZH2. J Gastroenterol Hepatol. 26:19–27. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonzalez ME, Li X, Toy K, DuPrie M,
Ventura AC, Banerjee M, Ljungman M, Merajver SD and Kleer CG:
Downregulation of EZH2 decreases growth of estrogen
receptor-negative invasive breast carcinoma and requires BRCA1.
Oncogene. 28:843–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fiskus W, Wang Y, Sreekumar A, Buckley KM,
Shi H, Jillella A, Ustun C, Rao R, Fernandez P, Chen J, et al:
Combined epigenetic therapy with the histone methyltransferase EZH2
inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor
panobinostat against human AML cells. Blood. 114:2733–2743. 2009.
View Article : Google Scholar
|
|
12
|
Panousis D, Patsouris E, Lagoudianakis E,
Pappas A, Kyriakidou V, Voulgaris Z, Xepapadakis G, Manouras A,
Athanassiadou AM and Athanassiadou P: The value of TOP2A, EZH2 and
paxillin expression as markers of aggressive breast cancer:
relationship with other prognostic factors. Eur J Gynaecol Oncol.
32:156–159. 2011.PubMed/NCBI
|
|
13
|
Tang X, Milyavsky M, Shats I, Erez N,
Goldfinger N and Rotter V: Activated p53 suppresses the histone
methyltransferase EZH2 gene. Oncogene. 23:5759–5769. 2004.
View Article : Google Scholar : PubMed/NCBI
|