Introduction
Bladder cancer is an increasingly significant
international public health problem. In the USA, bladder cancer is
the second most common genitourinary malignant disease, with 69,250
new cases and 14,990 mortalities estimated in 2011 (1). The incidence of bladder cancer
increases with age, peaking between 50 and 70 years, and the
disease is approximately three times more common in males than
females (2). The established risk
factors for bladder cancer include tobacco smoke, exposure to
industry-related aromatic amines and the uptake of drugs such as
phenacetin, chlornaphazine and cyclophosphamide (3). Exposure to these chemical carcinogens
may lead to direct and indirect DNA damage, genome instability and
carcinogenesis (4). However, the
precise mechanism of bladder cancer development remains
unclear.
The cell cycle and cell proliferation are controlled
by cyclins, cyclin-dependent protein kinases (Cdks) and Cdk
inhibitors (5). The alteration of
various components of the cell cycle regulatory mechanism that
controls the progression of cells from a quiescent to a growing
state contributes to the development of numerous types of human
cancer (6). Cdk6, in cooperation
with cyclin D, drives cell cycle progression from G1 to S phase
through the phosphorylation and subsequent inactivation of the
retinoblastoma 1 protein (7).
Aberrant Cdk6 expression has been reported in pancreatic cancer
(8), T-cell lymphoma (9), malignant glioma (10) and medulloblastoma (11), suggesting the involvement of Cdk6 in
cancer. However, the expression of Cdk6 in bladder cancer has not
been reported, although the roles of Cdk6 partner proteins such as
p16 in bladder cancer development have been investigated previously
(12,13). To investigate the role of Cdk6 in
bladder cancer development, we examined the Cdk6 expression in
cases of bladder cancer and their adjacent tissues using an
immunohistochemistry (IHC) assay and evaluated the correlation
between Cdk6 expression and bladder cancer progression.
Materials and methods
Tissue microarray and ethics
statement
The tissue microarray (TMA) for the bladder cancer
was obtained from Shanghai Outdo Biotech Company (Shanghai, China).
The use of human bladder transitional cell carcinomas (TCCs) and
their adjacent tissues in this study was approved by the Clinical
Research Ethics Board of the First Hangzhou People Hospital
(Hangzhou, China). The study was conducted in accordance with the
Declaration of Helsinki guidelines.
IHC
The IHC assay was carried out as previously
described (14). The monoclonal
mouse anti-Cdk6 antibody (1:50 dilution; Millipore, Billerica, MA,
USA) was used for primary antibody incubation at 4°C overnight. A
slide incubated without the primary antibody was used as a negative
control.
Evaluation of immunostaining
The Cdk6 staining was blindly and independently
examined by two pathologists. In certain cases where discrepancy
between the two observers occurred, the immunostained slides were
reviewed in a double viewing microscope to resolve the discrepancy.
Cdk6 staining intensity was scored as 0, 1+, 2+ or 3+. The
percentage of Cdk6-positive cells was scored as: 1 (0–25%), 2
(26–50%), 3 (51–75%) and 4 (76–100%). The level of Cdk6 staining
was evaluated by the immunoreactive score (IRS) (15), which is calculated by multiplying
the scores of the staining intensity and the percentage of positive
cells.
Statistical analysis
The paired Student's t-test was applied to evaluate
the differences in Cdk6 expression levels between the bladder
cancer cases and the adjacent tissues. The independent t-test was
used to evaluate the differences in Cdk6 expression levels between
different stages of bladder cancer. The differences in clinical
characteristics and the expression levels of Cdk6 were evaluated
using the χ2 test between patient subgroups. P<0.05
was considered statistically significant and all tests were
two-sided. SPSS version 11.5 (SPSS Inc., Chicago, IL, USA) software
was used for all analyses.
Results
Clinicopathological features of TMAs
A total of 62 bladder tissues (31 pairs of bladder
cancer cases and adjacent tissues) were used for TMA construction.
The distributions of selected demographic characteristics of
bladder cancer patients are listed in Table I. Of the 31 bladder cancer patients,
26 were male and 5 were female. Patient age ranged between 48 and
83 years (median, 67). Due to the loss of biopsy cores or
insufficient tumor cells being present in the cores, only 29 pairs
of bladder cancer cases (29 adjacent tissues and 31 bladder cancer
cases) could be evaluated for Cdk6 staining. The 31 bladder cancer
tissues included seven cases of non-invasive, low-grade papillary
lesions (excluding carcinomas in situ) and 24 cases of
invasive bladder cancer.
 | Table IClinical characteristics of bladder
cancer patients. |
Table I
Clinical characteristics of bladder
cancer patients.
| Clinical
characteristics | N (%) |
|---|
| Total | 31 (100) |
| Age (years) |
| ≤67 | 16 (51.6) |
| >67 | 15 (48.4) |
| Gender |
| Female | 5 (16.1) |
| Male | 26 (83.9) |
| Stage of bladder
cancer |
| Superficial | 7 (22.6) |
| Invasive | 24 (77.4) |
Cdk6 expression is increased in bladder
cancer
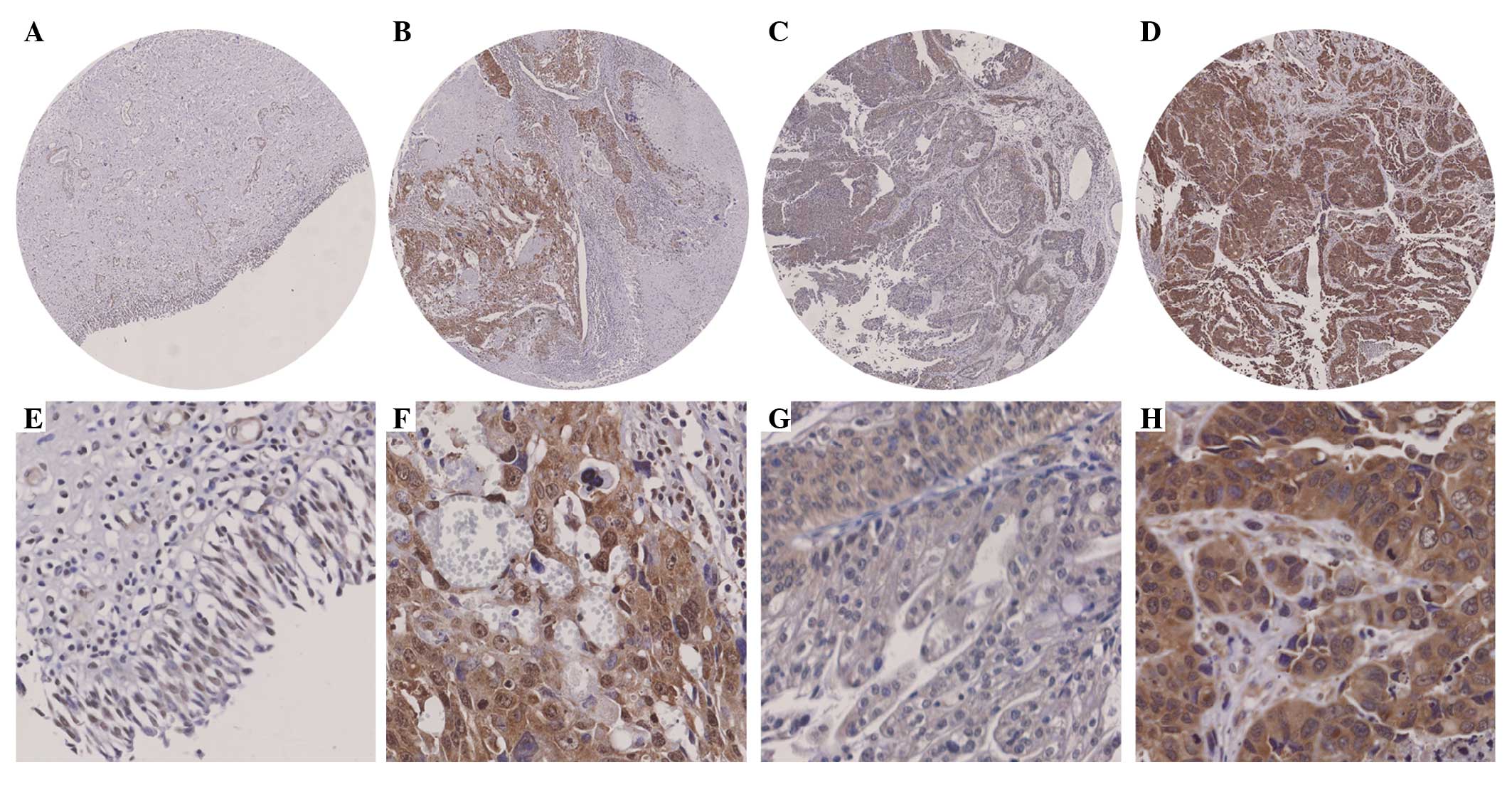
The Cdk6 staining was detected in the cytoplasm and
nuclei (Fig. 1A-H). Cytoplasmic and
nuclear Cdk6 staining was increased in 38% (11/29) and 65% (19/29)
of all bladder cancer cases compared with their adjacent tissues
(Fig. 1A and B) and the differences
were significant (P=0.005 and P<0.001 for cytoplasmic and
nuclear staining, respectively; paired-samples t-test; Table II). No significant difference was
found in either cytoplasmic or nuclear Cdk6 staining between the
superficial bladder cancer cases and their adjacent tissues
(P=0.086 and P=0.172, respectively; paired-samples t-test; Table II). However, significant
differences in cytoplasmic and nuclear Cdk6 staining were observed
between the invasive bladder cancer cases and their adjacent
tissues (P=0.005 and P<0.001, respectively; paired-samples
t-test; Fig. 1A and B; Table II).
 | Table IICdk6 staining in bladder lesions. |
Table II
Cdk6 staining in bladder lesions.
| Stages | Subcellular | Tissue | Cdk6 staining (mean ±
SD) | P-valuea |
|---|
| Superficial
(n=7) | Cytoplasmic | Adjacent | 6.29±2.43 | 0.086 |
| | Bladder cancer | 8.29±2.93 | |
| Nuclear | Adjacent | 3.57±1.99 | 0.172 |
| | Bladder cancer | 5.57±3.31 | |
| Invasive (n=22) | Cytoplasmic | Adjacent | 8.82±2.81 | 0.005 |
| | Bladder cancer | 10.64±2.08 | |
| Nuclear | Adjacent | 6.73±3.14 | <0.001 |
| | Bladder cancer | 9.36±3.14 | |
Correlation between Cdk6 expression and
clinicopathological parameters
In the bladder cancer patients, we did not find any
significant correlations between either cytoplasmic or nuclear Cdk6
expression and the clinicopathological characteristics, including
age and gender (data not shown). However, cytoplasmic and nuclear
Cdk6 staining were increased in invasive bladder cancer cases
compared with that in the superficial bladder cancer cases (P=0.026
and P=0.006, respectively; independent-samples t-test; Fig. 1C and D; Table III), suggesting that cytoplasmic
and nuclear Cdk6 expression correlates with bladder cancer
progression.
 | Table IIICdk6 staining in bladder lesions. |
Table III
Cdk6 staining in bladder lesions.
| Subcellular | Stages | Cdk6 staining (mean ±
SD) | P-valuea |
|---|
| Cytoplasmic | Superficial
(n=7) | 8.29±2.93 | 0.026 |
| Invasive (n=24) | 10.58±2.08 | |
| Nuclear | Superficial
(n=7) | 5.57±3.31 | 0.006 |
| Invasive (n=24) | 9.56±3.09 | |
Discussion
In the present study, we examined Cdk6 expression in
cases of bladder cancer and their adjacent tissues and evaluated
the correlation between Cdk6 expression and the clinical
characteristics of bladder cancer patients. Similar to other types
of cancer, Cdk6 expression was increased in bladder cancer. DNA
amplification is a common mechanism found in numerous types of
human tumors and may result in the overexpression of genes whose
products are involved in cell proliferation. However, Cdk6
overexpression was not restricted to cases with gene amplification
and previous studies have reported that the aberrant
post-transcriptional regulation of Cdk6 resulted in Cdk6
overexpression. For example, miR-9 was found to be methylated in
acute lymphoblastic leukemia patients and the methylation of miR-9
was associated with its downregulation (16). The epigenetic downregulation of
miR-9 induced the upregulation of its target, Cdk6 (16). The elucidation of the mechanism of
increased Cdk6 expression in bladder cancer requires further
investigation.
At the initiation of cell cycle progression, cyclin
D enhances the cell transition through the G1 phase of the cell
cycle by binding to and activating Cdk6 (7). The specific binding of p16 to Cdk6
inhibits the catalytic activity of the cyclin D-Cdk6 complex and
consequently arrests the cell cycle at the G1 phase (17). Alteration of this pathway results in
the onset of cancer cell cycle progression and tumor development.
The p16 gene deletion is reportedly an early event in bladder
cancer (18,19). Our data have shown that Cdk6
expression was increased in the cases of invasive bladder cancer,
suggesting that the overexpression of Cdk6 is a subsequent effect
of the dysfunction of p16 contributing to bladder cancer
development.
Identifying biomarkers for bladder cancer in
conjunction with traditional cancer stages may improve early
diagnosis and patient care. However, reliable biomarkers,
particularly for advanced bladder cancer, are lacking. As Cdk6
expression may be determined by means of IHC on formalin-fixed
paraffin-embedded sections, this marker is well suited for the
routine diagnostic setting as well as evaluation in controlled
clinical studies.
A major limitation of the present study was the
relatively small number of cases of bladder cancer tissues for IHC
study. Nevertheless, the potential of Cdk6 as a biomarker for
bladder cancer identified in this investigation may be useful for
distinguishing between non-invasive superficial and invasive cases
of bladder cancer. To confirm this hypothesis, more studies should
be performed with an independent large cohort of patients. To the
best of our knowledge, this is the first study to demonstrate the
involvement of Cdk6 in bladder cancer development.
In conclusion, findings of our study showed that
Cdk6 expression was increased in cases of invasive bladder cancer
and that an increased Cdk6 expression may contribute to bladder
cancer development and serve as a biomarker for bladder cancer.
Acknowledgements
We thank Dr Mingjuan Jin from the Department of
Epidemiology & Health Statistics, Zhejiang University School of
Medicine, for assistance with statistical analyses. This study was
supported by the Hangzhou Science-Technology Development Program,
Zhejiang, China (No. 20090833B04) (to Dr Gang Wang).
Abbreviations:
|
Cdk6
|
cyclin-dependent kinase 6
|
|
IHC
|
immunohistochemistry
|
|
TMA
|
tissue microarray
|
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pryor WA: Cigarette smoke radicals and the
role of free radicals in chemical carcinogenicity. Environ Health
Perspect. 105(Suppl 4): 875–882. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franekova M, Halasova E, Bukovska E, et
al: Gene polymorphisms in bladder cancer. Urol Oncol. 26:1–8. 2008.
View Article : Google Scholar
|
|
5
|
Murray AW: Recycling the cell cycle:
cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santamaria D and Ortega S: Cyclins and
CDKS in development and cancer: lessons from genetically modified
mice. Front Biosci. 11:1164–1188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyerson M and Harlow E: Identification of
G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell
Biol. 14:2077–2086. 1994.PubMed/NCBI
|
|
8
|
Lee KH, Lotterman C, Karikari C, et al:
Epigenetic silencing of MicroRNA miR-107 regulates cyclin-dependent
kinase 6 expression in pancreatic cancer. Pancreatology. 9:293–301.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chilosi M, Doglioni C, Yan Z, et al:
Differential expression of cyclin-dependent kinase 6 in cortical
thymocytes and T-cell lymphoblastic lymphoma/leukemia. Am J Pathol.
152:209–217. 1998.PubMed/NCBI
|
|
10
|
Costello JF, Plass C, Arap W, et al:
Cyclin-dependent kinase 6 (CDK6) amplification in human gliomas
identified using two-dimensional separation of genomic DNA. Cancer
Res. 57:1250–1254. 1997.PubMed/NCBI
|
|
11
|
Mendrzyk F, Radlwimmer B, Joos S, et al:
Genomic and protein expression profiling identifies CDK6 as novel
independent prognostic marker in medulloblastoma. J Clin Oncol.
23:8853–8862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raspollini MR, Nesi G, Baroni G, et al:
p16(INK4a) expression in urinary bladder carcinoma. Arch Ital Urol
Androl. 78:97–100. 2006.PubMed/NCBI
|
|
13
|
Shariat SF, Tokunaga H, Zhou J, et al:
p53, p21, pRB, and p16 expression predict clinical outcome in
cystectomy with bladder cancer. J Clin Oncol. 22:1014–1024. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen G, Cheng Y, Zhang Z, et al:
Prognostic significance of cytoplasmic p27 expression in human
melanoma. Cancer Epidemiol Biomarkers Prev. 20:2212–2221. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German).
|
|
16
|
Rodriguez-Otero P, Roman-Gomez J,
Vilas-Zornoza A, et al: Deregulation of FGFR1 and CDK6 oncogenic
pathways in acute lymphoblastic leukaemia harbouring epigenetic
modifications of the MIR9 family. Br J Haematol. 155:73–83. 2011.
View Article : Google Scholar
|
|
17
|
Russo AA, Tong L, Lee JO, et al:
Structural basis for inhibition of the cyclin-dependent kinase Cdk6
by the tumour suppressor p16INK4a. Nature. 395:237–243. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eissa S, Ahmed MI, Said H, et al: Cell
cycle regulators in bladder cancer: relationship to
schistosomiasis. IUBMB Life. 56:557–564. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Osman HG, Abadeer NY, Mohamed SE and Fahmy
AK: Expression of p16, p15 and Cyclin D1 in bladder cancer and
correlation with cancer progression. Internet J Urol. 4:http://www.ispub.com/journal/the-internet-journal-of-urology/volume-4-number-2/expression-of-p16-p15-and-cyclin-d1-in-bladder-cancer-and-correlates-with-cancer-progression-and-clinical-out-come.html.
|