Introduction
Cancer stem cells (CSCs) are defined as the subset
of tumor cells that have an increased ability to self-renew and
generate the diverse cells that comprise the tumor (1,2). CSCs
are considered to be responsible for tumor initiation, metastasis
and resistance to treatments (3).
Cell surface markers which allow the isolation of CSCs are
important in the investigation of the biological behavior of CSCs
and the development of targeted therapies.
CD133, also termed Prominin-1, is a transmembrane
pentaspan protein that was first described as a surface antigen
specific for human hematopoietic stem and progenitor cells
(4,5) and subsequently recognized as a stem
cell marker for other normal tissues. Notably, CD133 has been
reported to be a marker of putative CSCs in brain tumors,
hepatocellular carcinoma, melanoma and prostate, colon, lung,
ovarian and pancreatic cancers (6–16).
This has led to CD133 being referred to as ‘the molecule of the
moment’ (17) in stem cell and
cancer research. However, the significance of CD133 as a marker for
identifying and isolating colon CSCs is controversial (18–21).
CD133 expression, as assessed by antibody staining, appears to be
restricted to a small population of colon cancer cells which form
tumors that recapitulate the morphology of the original tumor in
immunodeficient mice (9–11). By contrast, studies monitoring CD133
expression using a reporter gene or polyclonal antibodies have
shown that CD133 is not restricted to CSCs in primary colon cancer
(18–20). Moreover, CD133neg and
CD133hi metastatic colon cancer cells are able to
initiate tumors (20). Results of a
recent study have shown that CD133 is expressed by CSCs and
differentiated tumor cells, but the AC133 epitope is lost following
CSC differentiation (21). In view
of these contrasting reports, the relevance of CD133 expression to
colorectal tumorigenesis should be considered as a work in
progress.
In this study, we analyzed the expression of CD133
in the SW620 colon cancer cell line to study the correlation
between the levels of CD133 expression and the biology of the
cells. We detected a variation in CD133 expression in different
environments, including culture conditions and nutrient withdrawal.
Contrary to the current paradigm, our data indicate that the
expression of the CD133 antigen is not static, but is regulated by
the microenvironment of the cells; this plasticity may explain the
high expression of CD133 in tumors.
Materials and methods
Cell lines and cell culture
Human SW620 colon cancer cells (22) (ATCC, Manassas, VA, USA) were
routinely passaged in RPMI-1640 medium (Gibco, Carlsbad, CA,
USA)/10% fetal bovine serum (FBS, Gibco) with the following
additives: hydrocortisone (1 μg/ml), thioglycerol (0.01 μg/ml),
insulin (0.025 U/ml), penicillin G (60 mg/l) and streptomycin (100
mg/l). The cells were cultured at 37°C in a humidified atmosphere
containing 10% CO2.
Flow cytometry
Single-cell suspensions were obtained by
trypsinization of the cells in adherent cultures or of the
spheroids grown in hanging drops. Cell preparations were stained
with antibodies against human CD133 (AC133, 1:40; Miltenyi Biotec,
Gladbach, Germany) followed by Alexa488 anti-mouse IgG, or with
APC-conjugated anti-human CD133/1 and CD133/2 antibodies (Miltenyi
Biotec). The cells were analysed on a FACSCalibur flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA). Dead cells, cell
debris, doublets and aggregates were excluded from the analysis
using forward and side scatter and pulse-width gating. The cells
were prepared for sorting by staining with human CD133/1 (1:10, APC
conjugated; Miltenyi Biotec) and 1 μg/ml propidium iodide (PI) to
exclude dead cells during sorting. Cell sorting was performed using
a FACSAria (Becton-Dickinson). Matched isotype primary antibodies
were used as controls.
Spheroid culture in hanging drops
Cells were prepared as single cell suspensions. The
cells were counted and resuspended in medium (RPMI-1640 with 20%
FBS and antibiotics) to a concentration of 1.6×104
cells/ml in a sterile basin. An 8-channel pipette was used to make
rows of 30 μl drops (500 cells/drop) on the up-turned inner surface
of the lid of a tissue culture dish. The lid was then inverted and
placed on top of a culture dish containing 10 ml of PBS. The drops
were incubated at 37°C and 10% CO2 for 6 days. The
resulting spheroids were photographed in phase contrast using a
Nikon microscope with a ×10 lens.
Immunofluorescence and confocal
microscopy
The sorted cells were seeded on glass cover slips,
fixed in 4% paraformaldehyde and blocked in PBS with 0.2% BSA for
30 min at room temperature prior to incubation with mouse
anti-human CD133/1 antibody (1:40; Miltenyi Biotec) followed by
goat anti-mouse Alexa488 (Invitrogen, Carlsbad, CA, USA). The
nuclei of the cells were stained by incubating with DAPI (1 μg/ml)
for 5 min. The coverslips were mounted on microscope slides.
Confocal microscopy was performed using a Nikon digital eclipse
C1si confocal microscope with EN-C1 software.
Immunohistochemistry
The spheroids were collected and frozen individually
in OCT compound (ProSciTech, Thuringowa, Queensland, Australia).
Frozen sections of the spheroids were fixed in acetone (−20°C, 10
min) and rehydrated in PBS. Endogenous peroxidases were inactivated
by immersing the sections in 0.3% hydrogen peroxide for 20 min. The
sections were incubated overnight at 4°C in a humidified chamber
with mouse anti-human monoclonal CD133/2 antibody (1:40; Miltenyi
Biotec) followed by biotinylated secondary antibody (VECTASTAIN ABC
kit, Vector Laboratories, Burlingame, CA, USA) for 30 min at room
temperature. Each section was further incubated in VECTASTAIN ABC
reagent for 30 min at room temperature. The sections were developed
using DAB (Vector Laboratories) as the substrate and then
counterstained with hematoxylin. Negative controls were stained in
parallel substituting an isotype control with the primary
antibody.
Western blot analysis
Whole protein lysates of cultured cells and
spheroids were used in these experiments. A total of 50 μg of
protein was used per lane. The primary antibodies were
anti-β-tubulin mouse mAb (1:1000) and anti-CD133 (1:200
mouse-anti-CD133/1 at W6B3C1; Miltenyi Biotec). The secondary
antibody was Odyssey anti-mouse 800 (1:10000). Reactive bands were
visualized using an Odyssey infrared photometer (Li-Cor, Lincoln,
NE, USA) according to the manufacturer’s instructions.
Quantitative real time-PCR (qRT-PCR)
Total RNA was prepared from the cultured cells and
spheroids using the RNeasy extraction kit (GE Healthcare,
Piscataway, NJ, USA) and reverse transcribed using high-capacity
cDNA reverse transcription kits (Applied Biosystems, Mulgrave,
Victoria, Australia) according to the manufacturer’s instructions.
qRT-PCR was performed using a 7300 Fast Real-Time PCR system
(Applied Biosystems) using SYBR-Green PCR Master mix (Applied
Biosystems). The human-specific intron-spanning primer pairs for
CD133 were provided by Qiagen (catalog number: QT00075586; Hilden,
Germany). The following primer pair was used for GAPDH: forward,
CAATGACCCCTTCATTGACC; reverse, TGATGACAAGCTTCCCGTTC. The cycle
conditions were as follows: 1 cycle at 50°C for 2 min, followed by
1 cycle at 95°C for 10 min, 40 cycles at 95°C for 15 sec and 60°C
for 1 min. The specificity of the PCR products was tested using
dissociation curves. The relative values of the transcripts were
calculated using the 2-ΔΔCt method, where ΔCt is equal
to the difference in threshold cycles of the target and
reference.
Induction of CD133 by nutrient
withdrawal
CD133neg and CD133hi cells
were plated in complete medium at 1×106 cells/well.
After one day, the medium was changed to DMEM, 5% FBS (control),
DMEM with no serum or glucose-free DMEM with 5% FBS. The cells were
harvested 4 days later and CD133 expression was monitored by
fluorescence-activated cell sorting (FACS). The median and peak
fluorescence channels were determined using the Stat program in
CellQuest.
Statistical analysis
Results are presented as the mean ± SD for three
repeated individual experiments for each group. Statistical
analyses were performed using SPSS software (version 10.0; SPSS,
Inc., Chicago, IL, USA). Correlations between the sample groups and
molecular variables were calculated using the paired t-test.
P<0.05 was considered to indicate a statistically significant
result.
Results
CD133 expression in the SW620 colon
cancer cell line
SW620 cells express two distinct levels of CD133 as
assessed by FACS. CD133neg cells accounted for 70% and
CD133hi cells for 30% of the SW620 cells (Fig. 1). The two subpopulations were always
present, irrespective of the batch of cells or passage number;
however, the relative percentage of each subpopulation varied
occasionally between cultures.
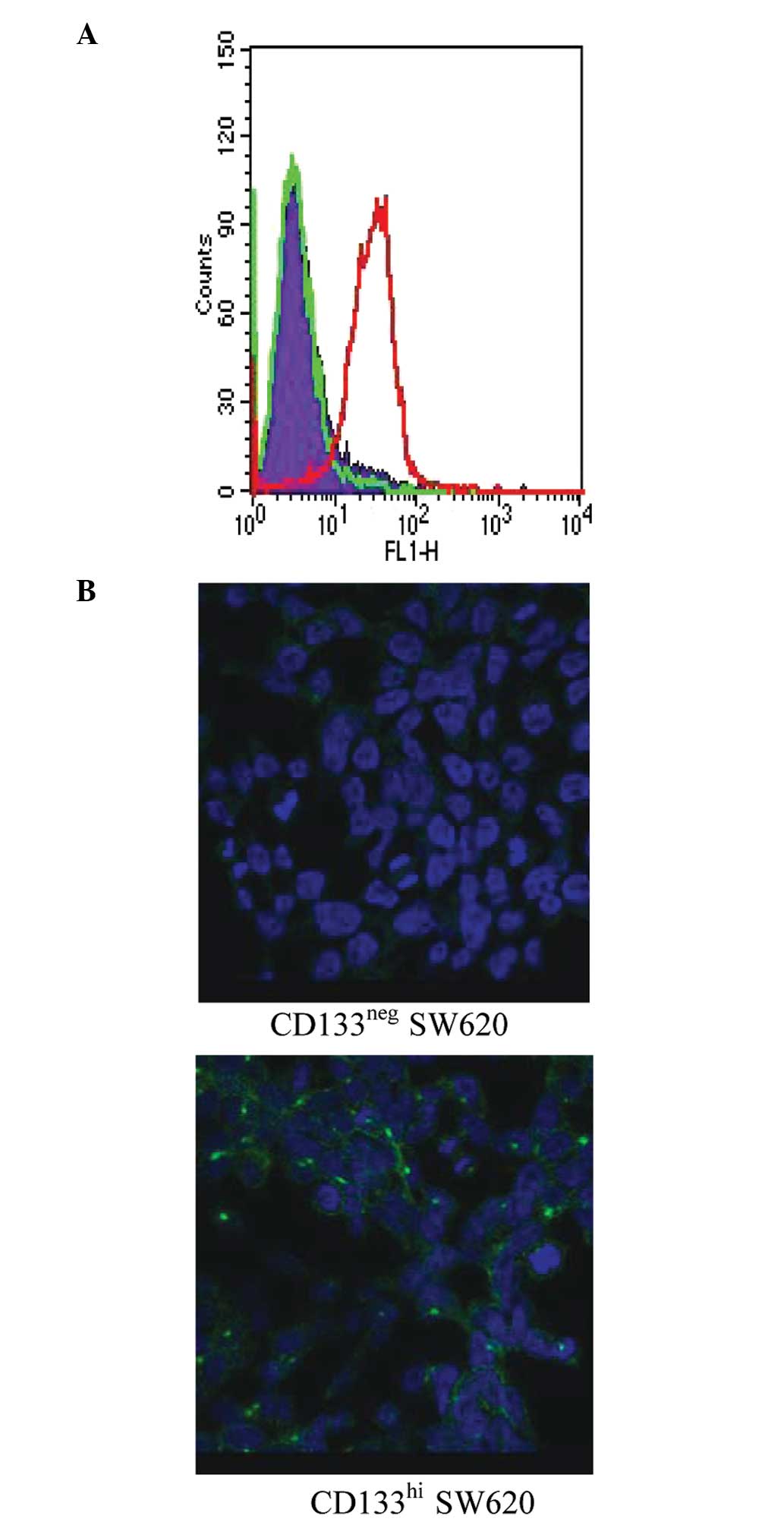
CD133neg and
CD133hi expression remains stable following sorting
To investigate the stability of CD133 expression in
the negative and positive subsets, SW620 cells were sorted into
separate CD133neg and CD133hi pools. The two
subpopulations were grown separately and analysed for CD133
expression 8 days after sorting by flow cytometry (Fig. 2A) and immunofluorecence microscopy
(Fig. 2B). The two methods showed
that CD133 expression in CD133neg and CD133hi
SW620 cells is stable during normal passage in culture.
CD133 expression in SW620 cells is
induced by anchorage-independent growth or nutrient withdrawal
The expression of CD133 was stable in selected
subpopulations under standard culture conditions (Fig. 2). To test whether this observation
was also true under anchorage-independent conditions, we collected
SW620 spheroids and monitored the CD133 expression by FACS
analysis. Spheroids (30 per cell line) were collected, pooled and
trypsinized to yield single cell suspensions. The cells were then
stained with CD133 antibody for FACS analysis. There was
significant CD133 re-expression in CD133neg cells grown
as spheroids, with >50% of the cells converting to
CD133hi; CD133 expression in the CD133hi
spheroids remained stable (Fig.
3A). Control cells grown as monolayers and tested in parallel
remained true to the original phenotype (data not shown),
demonstrating that the re-expression of CD133 was induced by
anchorage-independent growth. The re-expression of CD133 in the
spheroids was confirmed by immunohistochemical staining and western
blotting. A strong, patchy expression of the CD133 antigen was
detected in the CD133neg cell spheroids (Fig. 3B), consistent with the distribution
detected in primary colon tumors (9); the marked increase in CD133 protein
expression was confirmed by immunoblotting (Fig. 3C). At the mRNA level, the level of
the CD133 gene expression in the spheroids of CD133neg
cells was significantly increased compared with that of cells from
the monolayer cultures; a smaller but consistent increase in CD133
gene expression was also detected in CD133hi and
parental SW620 cells grown as spheroids (Fig. 3D).
 | Figure 3CD133 expression in
CD133neg and CD133hi SW620 cell spheroids.
(A) Within each cell line, spheroids were pooled and disrupted into
single cell suspensions. CD133 reactivity was assessed by FACS.
Solid purple, CD133; green overlay, negative control. (B)
Immunohistochemical staining of CD133 in spheroids. Brown, CD133;
blue, nuclear stain; magnification ×200. (C) CD133 protein
expression in spheroids compared with cell monolayers by western
blot analysis. Upper panel, CD133 reactivity; lower panel,
β-tubulin reactivity. Lane 1, CD133neg spheroids; lane
2, CD133neg cell monolayers; lane 3, CD133hi
spheroids; lane 4, CD133hi monolayers. (D) Expression of
CD133 by qRT-PCR, standardized to CD133 expression of
CD133neg monolayer culture cells. *P<0.05
compared with CD133neg monolayer culture cells. SP,
spheroid; FACS, fluorescence-activated cell sorting; qRT-PCR,
quantitative real time-PCR. |
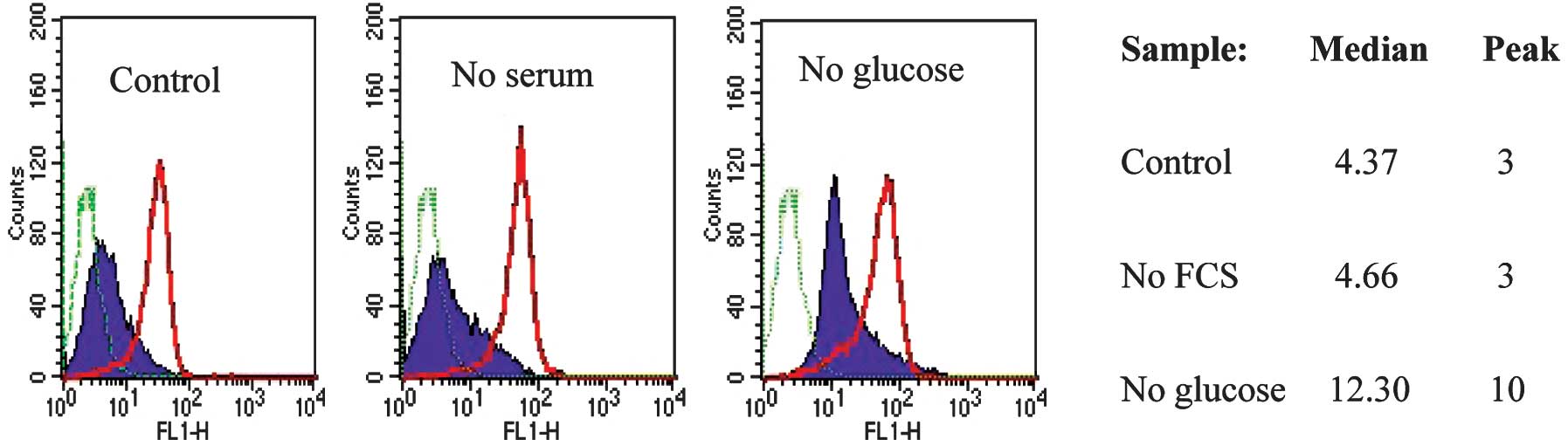
The re-expression of CD133 in spheroids, where cells
are packed tightly in a small volume of medium, raises the
possibility that nutrient or oxygen deficiency regulates CD133
antigen expression. To test this hypothesis, monolayer cultures of
CD133neg cells were exposed to low oxygen, low serum,
low glucose or low glucose plus low serum. After 5 days, the CD133
expression of the cells under each culture condition was monitored
by FACS analysis. Cells cultured without FBS and without glucose
had low viability following 5 days in culture and were not analysed
further, but all the other samples had high viability. A reduction
in oxygen level to 2% did not affect CD133 antigen expression (data
not shown). CD133neg cells grown in medium without
glucose showed a marked shift in CD133 expression, whereas FBS
withdrawal did not have a significant effect (Fig. 4). Neither glucose nor serum
withdrawal had an effect on CD133 expression in CD133hi
SW620 cells (Fig. 4).
Discussion
CD133 expression characterizes immature cells in the
lower part of the intestinal crypt and is suggested to be a
hallmark of colorectal cancer stem cells (CRCS) in primary tumors
(9–11). In the present study, we investigated
the role of CD133 in a colon cancer cell line, SW620, in which
CD133neg and CD133hi cells are present.
CD133neg and CD133hi subpopulations were
obtained by FACS sorting and used for functional studies. Following
serial passages, the expression of CD133 (detected with the AC133
epitope antibody) was stable in the two subsets. Notably, the
difference in the CD133 mRNA level between the CD133hi
and CD133neg cells was confirmed at the RNA level by
qRT-PCR (Fig. 3D). Doubts have been
shed on the reliability of AC133 as an accurate measure of CD133
expression, with reported AC133 epitope loss upon CSC
differentiation (21) and cell
cycle-dependent variation of the CD133 epitope (23). Our results have shown that CD133
antibody staining of the AC133 epitope is correlated with CD133
mRNA expression in SW620 cells under different growth conditions
and that CD133 expression is stable during the monolayer culture of
SW620.
As a model for CD133 expression during the
tumorigenesis of colon cancer cells, CD133neg and
CD133hi cells were grown in spheroid cultures using the
hanging drop method. This method is based on the ability of cells
to aggregate and form homogeneous spheroids without the need for
polymer scaffolds such as matrigel, polyglycolic acid or
microporous supports (24). The
spheroids are in vitro 3D tissue structures that mimic the
in vivo tissue organization and microenvironment of tumors
(25,26). We detected the significant
re-expression of CD133 in CD133neg cells at the protein
and mRNA level during spheroid formation. CD133 re-expression in
CD133neg cells only occurred when the cells were grown
as spheroid and not when passaged as adherent cells. There are two
possible explanations for this phenomenon: a switch from
cell/matrix to cell/cell interactions or reduced nutrient
availability resulting from high local cell densities. Since there
are relative nutrient and oxygen supply insufficiencies in the
microenvironment of primary human tumors and xenograft cells
(27), and considering that CD133
is a marker of bioenergetic stress in human glioma (28), we postulated that the CD133
re-expression observed in our study was correlated with the energy
metabolism of the cells. Nutrient withdrawal markedly induced CD133
expression in CD133neg SW620 monolayers cultured without
glucose. Our findings suggest that CD133 re-expression in the
tumorigenesis of CD133neg SW620 cells may be mediated by
the microenvironment of the tumor cells.
CD133 appears to have no obvious functional role in
driving tumorigenesis, invasion and metastasis (29,30);
however, CD133 is expressed in tumors, particularly at the invasive
front or in conditions that mimic invasion (31). Our present findings support these
results and complement them by showing the upregulation of CD133 in
SW620 cells under the conditions of glucose deprivation.
In conclusion, our data indicate a plasticity of
CD133 antigen expression, which makes it unsuitable as a marker of
either colon stem cells or colon CSCs. It is likely that CD133
expression is maintained or induced during tumor formation and
serves as a marker of cells undergoing metabolic stress responses.
The potential role of CD133 in protecting cells from bioenergetic
stress, and its possible link to the maintenance of an immature
phenotype, requires further investigation.
Acknowledgements
We thank Francesca Walker, Huihua Zhang and Tony
Burgess (Ludwig Institute, Melbourne) for assistance with all
experiments.
References
|
1
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells - perspectives on current status and future
directions: AACR workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar
|
|
3
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin AH, Miraglia S, Zanjani ED, et al:
AC133, a novel marker for human hematopoietic stem and progenitor
cells. Blood. 90:5002–5012. 1997.PubMed/NCBI
|
|
5
|
Miraglia S, Godfrey W, Yin AH, et al: A
novel five-transmembrane hematopoietic stem cell antigen:
isolation, characterization, and molecular cloning. Blood.
90:5013–5021. 1997.PubMed/NCBI
|
|
6
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taylor MD, Poppleton H, Fuller C, et al:
Radial glia cells are candidate stem cells of ependymoma. Cancer
Cell. 8:323–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
10
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Todaro M, Alea MP, Di Stefano AB, et al:
Colon cancer stem cells dictate tumor growth and resist cell death
by production of interleukin-4. Cell Stem Cell. 1:389–402. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eramo A, Lotti F, Sette G, et al:
Identification and expansion of the tumorigenic lung cancer stem
cell population. Cell Death Differ. 15:504–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma S, Chan KW, Hu L, et al: Identification
and characterization of tumorigenic liver cancer stem/progenitor
cells. Gastroenterology. 132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Monzani E, Facchetti F, Galmozzi E, et al:
Melanoma contains CD133 and ABCG2 positive cells with enhanced
tumourigenic potential. Eur J Cancer. 43:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Curley MD, Therrien VA, Cummings CL, et
al: CD133 expression defines a tumor initiating cell population in
primary human ovarian cancer. Stem Cells. 27:2875–2883.
2009.PubMed/NCBI
|
|
16
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizrak D, Brittan M and Alison MR: CD133:
molecule of the moment. J Pathol. 214:3–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shmelkov SV, Butler JM, Hooper AT, et al:
CD133 expression is not restricted to stem cells, and both
CD133+ and CD133- metastatic colon cancer
cells initiate tumors. J Clin Invest. 118:2111–2120.
2008.PubMed/NCBI
|
|
19
|
Horst D, Kriegl L, Engel J, Kirchner T and
Jung A: CD133 expression is an independent prognostic marker for
low survival in colorectal cancer. Br J Cancer. 99:1285–1289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kojima M, Ishii G, Atsumi N, Fujii S,
Saito N and Ochiai A: Immunohistochemical detection of CD133
expression in colorectal cancer: A clinicopathological study.
Cancer Sci. 99:1578–1583. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kemper K, Sprick MR, de Bree M, et al: The
AC133 epitope, but not the CD133 protein, is lost upon cancer stem
cell differentiation. Cancer Res. 70:719–729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leibovitz A, Stinson JC, McCombs WB III,
McCoy CE, Mazur KC and Mabry ND: Classification of human colorectal
adenocarcinoma cell lines. Cancer Res. 36:4562–4569.
1976.PubMed/NCBI
|
|
23
|
Jaksch M, Múnera J, Bajpai R, Terskikh A
and Oshima RG: Cell cycle-dependent variation of a CD133 epitope in
human embryonic stem cell, colon cancer, and melanoma cell lines.
Cancer Res. 68:7882–7886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kelm JM, Timmins NE, Brown CJ, Fussenegger
M and Nielsen LK: Method for generation of homogeneous
multicellular tumor spheroids applicable to a wide variety of cell
types. Biotech Bioeng. 83:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dertinger H and Hulser DF: Intercellular
communication in spheroids. Recent Results Cancer Res. 95:67–83.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sutherland RM: Cell and environment
interactions in tumor microregions: the multicell spheroid model.
Science. 240:177–184. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: a review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
28
|
Griguer CE, Oliva CR, Gobin E, Marcorelles
P, Benos DJ, Lancaster JR and Gillespie GY: CD133 is a marker of
bioenergetic stress in human glioma. PLoS One. 3:e36552008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du L, Wang H, He L, Zhang J, Ni B, Wang X,
Jin H, et al: CD44 is of functional importance for colorectal
cancer stem cells. Clin Cancer Res. 14:6751–6760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horst D, Scheel SK, Liebmann S, Neumann J,
Maatz S, Kirchner T and Jung A: The cancer stem cell marker CD133
has high prognostic impact but unknown functional relevance for the
metastasis of human colon cancer. J Pathol. 219:427–434. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kirkland SC: Type I collagen inhibits
differentiation and promotes a stem cell-like phenotype in human
colorectal carcinoma cells. Br J Cancer. 101:320–326. 2009.
View Article : Google Scholar : PubMed/NCBI
|