Introduction
Multiple myeloma (MM) is a type of malignancy
characterized by clonal plasmocyte hyperplasia that exhibits
multi-drug resistance, tolerance to chemotherapy and a poor
therapeutic outcome (1–3). Recombinant immunotoxins (RITs) with
increased antitumor specificity and reduced cellular resistance
have been designed to target several groups of tumor cell surface
molecules. These surface molecules include cell surface receptors,
cluster designation molecules, oncofetal proteins, angiogenesis
pathway proteins and tumor stromal cells (4). Attempts have been made to kill tumor
cells through specific surface molecules (5). It has been found that a large variety
of monoclonal antibodies (mAb) bind to antigens on cancer cells and
kill cancer cells through apoptosis. However, very few antibodies
are able to kill sufficient numbers of cells to cause tumor
regression in experimental animals and even fewer are useful in the
treatment of malignancy in humans. Therefore it is often necessary
to arm the mAb with a cytotoxic ligand (6). Certain single-chain variable fragments
(scFvs) have produced RITs, but the low affinity of scFvs often
limits the cytotoxicity of RITs and prevents the killing of cancer
cells. DAB389-IL2 and LHRH-PE40 cause complete tumor regression in
some patients (7–10), and the mild side effects of these
anticancer drugs are acceptable. However, a high expression of
specific antigens on the tumor cell surface is extremely rare. For
these reasons we re-evaluate cytokine-mediated RITs in this
study.
MM cells often express over 10,000 interleukin-6
receptor (IL6R) sites/cell (11,12).
Overexpression of IL6R has been found not only in myeloma cells,
but also on the surface of many human tumor cell lines, including
hepatomas (PLC/PRF, Hep3B and HEPG2), leukemias (HL60 and U937),
prostatic carcinomas (LNCaP, DU145 and PC3), gastrointestinal
cancers (BGC-823 and Caco2), and virus-transformed CESS cells
(13,14). However, IL6R is either absent or
expressed at very low levels in normal cells (15,16).
IL6-PE4E and IL6-PE40 have been constructed to evaluate
their therapeutic efficacy against a variety of IL6R-overexpressing
malignancies. The two forms of RIT demonstrated significant
antitumoral effects with mild hepatotoxicity for human hepatoma and
acute myelocytic leukemia in mice (17,18).
These results indicated that, despite IL6R being expressed in
several normal cell types and sIL6R being expressed in the serum,
both forms of RIT have significant antitumor effects to target the
IL6R-overexpressing tumor cells.
Mature human IL6 has a stable central structure that
binds to IL6R, and the deletion of the N-terminal 28 amino acids in
IL6 does not affect its receptor-binding activity (19–21).
The most common mutant of Pseudomonas exotoxin (PE) is
PE38KDEL, in which the domain Ia (amino acids 1–252) and a portion
of the domain Ib (amino acids 365–380) have been deleted and in
which the C-terminal amino acids 609–613 (REDLK) of PE were
replaced to improve cytotoxicity by the KDEL sequence. The KDEL
sequence improves the cytotoxicity of PE38 by increasing the
affinity of the receptor which transports the toxin from the
transreticular Golgi apparatus to the endoplasmic reticulum (ER),
where it is translocated to the cytosol and kills cells by
inactivating elongation factor 2 (EF2) (22,23).
Based on the above theories and facts, we generated
a new structure of IL6-mediated immunotoxin to kill
IL6R-overexpressing tumors. This new immunotoxin was evaluated in
three aspects: the specific cytotoxicity to IL6R-overexpressing MM
cells in vitro, antitumor effects and side effects in
vivo using immunocompetent mice and murine MM tumor models. The
recombinant immunotoxin IL6(T23)-PE38KDEL was found to be capable
of killing IL6R-overexpressing cancer cells and causing significant
tumor regression.
Materials and methods
Animals and cell lines
For the cytotoxicity and antitumor assays, 6- to
8-week-old female BALB/c mice weighing 16–18 g were purchased and
bred under pathogen-free conditions in the animal center of Jilin
University, Changchun, China. Animal care and use were in
compliance with institutional guidelines. U266 (human myeloma),
SP2/0 (mouse myeloma), and CEM (T lymphoid leukemia) cells were
purchased from the Tumor Cell Bank at the Chinese Academy of
Medical Sciences.
Design and synthesis of a recombinant
toxin gene
The target toxin IL6(T23)-PE38KDEL is a fusion
protein that was constructed by connecting human IL6 missing
N-terminal 23 amino acids to PE38KDEL. The IL6(T23)-PE38KDEL gene
sequence was encoded using E. coli preferred codons by Jcat
software (24). The complete gene
sequence with restriction enzyme sites NcoI and XhoI
was synthesized, cloned into the pUC57 vector and then transformed
into the E. coli DH5α strains by the Shanghai Sangon
Biotechnology Company.
Expression and purification of
recombinant protein
The gene fragment encoding IL6(T23)-PE38KDEL was
inserted between the NcoI and XhoI sites of the
expression vector pET28a(+) plasmid (Novagen). The recombinant
plasmid carrying the fusion gene was expressed via
isopropyl-β-D-1-thiogalacto-pyranoside (IPTG) induction in the
E. coli BL21 (λDE3) cells. The expressed product was
purified from the inclusion bodies of the bacterial cells as
previously described (25,26), with slight modifications. In brief,
following sonication and centrifugation of the bacterial cells, the
inclusion bodies were washed extensively with 2.5% Triton X-100 and
TE buffer and then dissolved, denatured and reduced in
guanidine-dithioerythritol solution. Following denaturation, the
inclusion body protein was refolded by dilution in a renaturation
buffer containing arginine and reduced glutathione to facilitate
redox shuffling and then concentrated through a Millipore Amicon
concentrator (30 kDa). The solution containing the refolded protein
was centrifuged, filtered, and applied onto a 20-ml Q HP column
attached to a fast protein liquid chromatography system ÄKTA
Explorer 100, then washed with 20 mM Tris-Cl, pH 7.4, and eluted
with a stepwise gradient of 20 mM Tris-Cl, pH 7.4, containing 1.0 M
NaCl. The fraction containing the peak cytotoxic activity was then
diluted 3-fold with 20 mM Tris-Cl, pH 7.4, and loaded onto an 8-ml
monoQ column, which was then eluted with a linear gradient to
obtain the cytotoxicity protein. After removing endotoxin through a
polymyxin B column, the concentrated monoQ-purified protein was
loaded on a Superdex 200 column and eluted with phosphate-buffered
saline (PBS). The single elution peak was collected and saved. For
the in vitro and in vivo studies, a batch of active
IL6(T23)-PE38KDEL was produced with a low endotoxin content. The
recombinant immunotoxin IL6-PE40 was prepared and identified as
described (11). The protein
concentration of the purified chimeric toxins was determined by the
Bradford assay (27). The endotoxin
concentrations of RIT were determined by a colorimetric Limulus
test (28).
In vitro cytotoxicity assay
The number of IL6Rs in cells was measured by IL6
receptor-binding assays (13). The
specific cytotoxicity of IL6(T23)-PE38KDEL and IL6-PE40 was
assessed in triplicate using two IL6R-positive cell lines, U266 and
SP2/0, and one IL6R-negative cell line, CEM, by MTS colorimetric
assay (29). Briefly, the cells
were washed three times with RPMI-1640 medium to remove autocrine
IL6 and seeded in a 96-well cell culture plate at 1×104
cells/well (200 μl). Following 0.22 μm membrane filtration,
sterilization and dilution in PBS containing 0.2% serum albumin,
various concentrations of IL6(T23)-PE38KDEL (20 μl) were added to
the cell suspension. After adding RIT, the cells were incubated at
37°C for 30 h and 20 μl/well MTS/PMS was added. After 3 h the
plates were read at 490 nm using a microreader.
For competition experiments, various amounts of
recombinant IL6 were added 20 min before IL6(T23)-PE38KDEL (40
ng/ml) was added to U266 and SP2/0 cells. The growth inhibition
ratios of tumor cells were measured by MTS assay. For the
morphological observation of IL6(T23)-PE38KDEL cytotoxicity, U266,
SP2/0 and CEM cells were plated in a 96-well cell culture plate.
After 4 h incubation, 50 ng/ml IL6(T23)-PE38KDEL was added to the
cells for 30 h. The control groups were seeded and cultured in the
same conditions but without IL6(T23)-PE38KDEL.
Toxicity and maximum tolerated dose in
mice
Groups of 10 female BALB/c mice were given single or
multiple (QD for 10 days) injections of increasing doses of
IL6(T23)-PE38KDEL intravenously (i.v.) through the tail vein, and
the animals were observed over 2 weeks. The LD50 was
calculated using the Trimmed Spearman-Karber program. The maximum
cumulative tolerated dose of IL6(T23)-PE38KDEL in mice was
determined. Side effects were determined by autopsy and
histopathology in the animals administered with lethal levels of
RIT.
To determine the side effects in liver and renal
cells, and the effect on the number of peripheral blood cells
following treatment, a group of mice receiving the high dose of
IL6(T23)-PE38KDEL (0.4 mg/kg/day for 10 days) was sacrificed 10
days after the treatment. Whole blood was collected in heparinized
tubes and the differential blood cells were counted from each mouse
in quadruplicate using a hemocytometer. Plasma was collected
following centrifugation and kept frozen at -20°C. Biochemical
parameters were analyzed and measured with a multi-test
analyzer.
In vivo antitumor assay
The antitumor activity of IL6(T23)-PE38KDEL was
evaluated in female BALB/c mice previously injected with MM cells.
SP2/0 cells (1×107) were injected intraperitoneally
(i.p.) into the mice and the development of MM was monitored by
body weight and the serum paraprotein level (30). Five days after the injection (DPI)
of the MM cells, the mice were randomly divided into 4 groups of 10
mice each. Three groups received a daily injection of
IL6(T23)-PE38KDEL in 0.1 ml PBS at doses of 0.1, 0.2 and 0.4
mg/kg/day for 10 days by i.v. through the tail vein. The control
group received the corresponding volume of PBS in the same manner.
Mice that died during the treatment with IL6(T23)-PE38KDEL were
subjected to histopathological examination. Differences in survival
time of the experimental animals were evaluated with a log-rank
test and Kaplan-Meier survival curves. P<0.05 was considered to
indicate a statistically significant difference. To study the
effect of the method of IL6(T23)-PE38KDEL administration, one group
of MM-treated mice (10 mice) received a daily i.p. injection of
IL6(T23)-PE38KDEL at a dose of 0.4 mg/kg/day for 10 days. The
control group received the same volume of PBS in the same manner.
The mice were sacrificed on day 15 and dissected to determine the
effect of interventional therapy. Whole blood was collected, and
the main physiological and biochemical parameters of the blood were
measured.
Results
Expression and purification of
IL6(T23)-PE38KDEL
For the preparation of IL6(T23)-PE38KDEL, the
transformed E. coli cells were induced by IPTG at 37°C for 4
h, then collected and processed for the extraction of RIT from
inclusion bodies. Following denaturation and reduction, the active
RIT was purified from the refolding solution by ion exchange,
polymyxin B endotoxin removal and gel filtration chromatography.
The RIT accumulated in E. coli cells at approximately 20% of
total protein as estimated from the densitometric scanning of
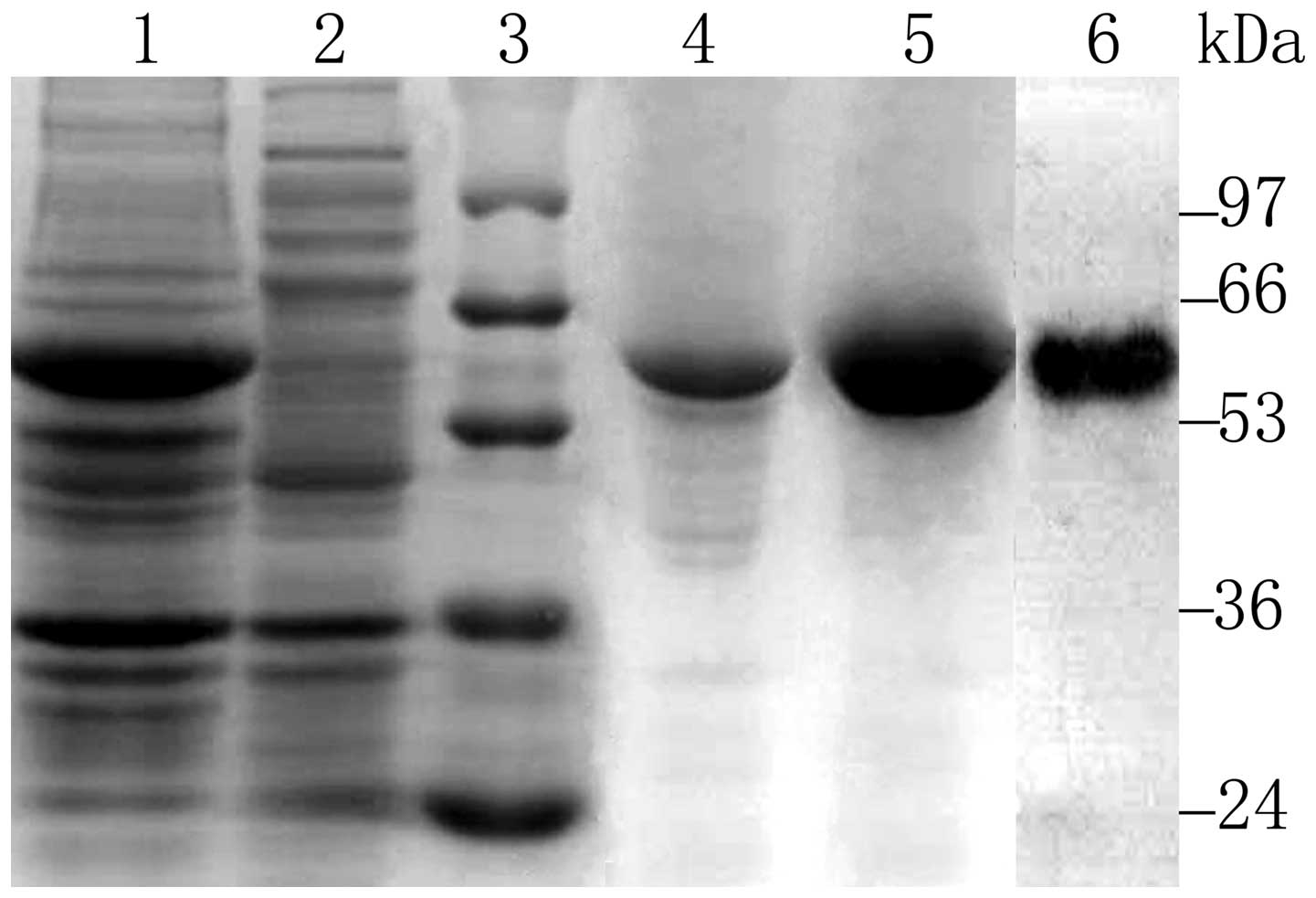
Coomassie blue-stained SDS-PAGE gels. Active IL6(T23)-PE38KDEL was
eluted from a Q column with a Tris-HCl buffer containing 0.20 M
NaCl. The final purified protein showed a single band of 56 kDa
that reacted specifically with rabbit anti-PE38 antibodies as
indicated by western blot analysis (Fig. 1). The endotoxin content of RIT was
2.3 EU/mg.
Cytotoxic specificity
Several control experiments were performed to
determine whether the cytotoxicity of IL6(T23)-PE38KDEL was
specific and required binding to IL6R by IL6(T23). In vitro
cytotoxic studies revealed that U266 with 15,500 IL6R sites/cell
and SP2/0 with 16,500 IL6R sites/cell were highly sensitive to
IL6(T23)-PE38KDEL and IL6-PE40 (Fig.
2). The IC50 values of IL6(T23)-PE38KDEL were 20 and
25 ng/ml, respectively. IL6(T23)-PE38KDEL was 2.0-fold more active
than IL6-PE40 in U266 cells, and IL6(T23)-PE38KDEL was 1.8-fold
more toxic than IL6-PE40 in SP2/0 cells. Conversely, the
IL6R-negative CEM cells were unresponsive to IL6(T23)-PE38KDEL up
to 1,000 ng/ml. The cytotoxicity of IL6(T23)-PE38KDEL was
effectively blocked by IL6 (Fig.
3). Compared with the control groups, U266 and SP2/0 cells
treated with IL6(T23)-PE38KDEL demonstrated shrinkage, cytoplasmic
dark granularity and death. As the negative control cells, CEM
cells were not affected after 30 h treatment with IL6(T23)-PE38KDEL
(Fig. 4). The results indicated
that IL6(T23)-PE38KDEL had better activity and was able to
specifically bind to the human and mouse IL6R, and be targeted to
kill IL6R-positive cells.
Non-specific toxicity and treatment doses
of IL6(T23)-PE38KDEL in mice
The non-specific toxicity and treatment doses of
IL6(T23)-PE38KDEL were evaluated in normal mice. Groups of 10 mice
received a one-time i.v. injection with varying doses of RIT and
were observed for 2 weeks. The mortalities occurred within 72 h of
the injection (Table I). The
LD50 of IL6(T23)-PE38KDEL was 0.985 mg/kg (95%
confidence range, 0.839–1.138 mg/kg), and was calculated with SPSS
Statistics 17.0. A single dose of 1 mg/kg IL6(T23)-PE38KDEL by i.v.
injection (100 μl) was lethal to 40% of the mice. A single dose of
0.5 mg/kg IL6(T23)-PE38KDEL was not lethal, but 0.5 mg/kg/day for
10 days was lethal to 20% of the mice. However, treatment with a
total IL6(T23)-PE38KDEL dose up to 4 mg/kg (0.4 mg/kg/day for 10
days) was not lethal. Higher doses of IL6(T23)-PE38KDEL caused
acute death of experimental mice, and the autopsy results showed
that the dose-limiting toxicity of IL6(T23)-PE38KDEL was
hepatotoxicity as the major cause of death.
 | Table IToxicity of IL6(T23)-PE38KDEL
administered to mice by intravenous injection. |
Table I
Toxicity of IL6(T23)-PE38KDEL
administered to mice by intravenous injection.
| Dose (mg/kg) | Treatment
schedule | Mortality |
|---|
| 0.25 | Single dose | 0/10 |
| 0.50 | Single dose | 0/10 |
| 0.75 | Single dose | 3/10 |
| 1.00 | Single dose | 4/10 |
| 1.25 | Single dose | 7/10 |
| 1.50 | Single dose | 10/10 |
| 0.50 | Daily for 10
doses | 2/10 |
| 0.40 | Daily for 10
doses | 0/10 |
To determine the toxic effects of IL6(T23)-PE38KDEL
in normal tissues, normal mice were treated for 10 days
continuously by i.v. injection (0.4 mg/kg/day). Treatment with
IL6(T23)-PE38KDEL had no effect on the absolute number of white
blood cells (WBC), but led to increased platelet numbers and a
slight increase in granulocyte levels, indicative of inflammatory
reactions in the body (Table II).
The red blood cell (RBC) and hematocrit levels decreased during the
treatment. The levels of hepatic enzymes aspartate aminotransferase
(AST), alanine aminotransferase (ALT) and alkaline phosphatase
(ALP) were elevated to approximately twice those of the untreated
mice, while the renal function was unchanged by the BUN and CK
assay (Table II). These data
indicated that a RIT dose of 0.4 mg/kg/days for 10 days was
well-tolerated with mild hepatotoxicity.
 | Table IIEffect of IL6(T23)-PE38KDEL on
physiological and biochemical parameters of blood after 10 days of
treatment. |
Table II
Effect of IL6(T23)-PE38KDEL on
physiological and biochemical parameters of blood after 10 days of
treatment.
| Items | Control group | Intravenous
group | Intervention
group |
|---|
| WBC
(106/ml) | 8.61±2.06 | 8.99±2.20 | 8.94±2.14 |
| RBC
(109/ml) | 8.90±1.05 | 7.2±1.37 | 7.99±0.85 |
| Platelets
(109/l) | 1246±175 | 1765±189 | 1365±206 |
| Hematocrit (%) | 48.0±2.0 | 36.0±3.0 | 45±5.0 |
| Neutrophil (%) | 40.0±9 | 73.4±11 | 63±10 |
| AST (U/l) | 112.70±19.45 | 191.53±18.38 | 139.83±16.84 |
| ALT (U/l) | 40.08±14.63 | 96.72±16.80 | 55.80±14.66 |
| ALP (U/l) | 88.80±6.05 | 173.45±14.51 | 120.90±11.83 |
| BUN (mM) | 8.42±1.30 | 8.50±0.97 | 8.31±1.53 |
| Creatine kinase
(mM) | 84.70±18.40 | 81.62±17.35 | 83.15±19.86 |
Antitumor activity in mice bearing
multiple myeloma
Following injection of SP2/0 cells into BALB/c mice,
all animals developed MM, characterized by an increase in serum
paraprotein and mild abdominal swelling. The survival time assay
was used as a parameter to evaluate the therapeutic efficacy of
IL6(T23)-PE38KDEL. Following inoculation of 1×107 cells,
untreated MM lead to the gradual death of the negative controls
from day 16 to 19. The gross pathological findings were large
abdominal tumor masses causing ascites and hepatosplenomegaly, and
large numbers of tumor metastases were found in the liver tissue
(Fig. 5A). Five days after
inoculation of the SP2/0 cells, tumor-bearing mice were treated
with three doses of IL6(T23)-PE38KDEL (0.4, 0.2 and 0.1 mg/kg/day)
to determine the anti-myeloma effects. The survival time of animals
treated with the three different doses increased by 1.2, 3.2 and
6.8 days, respectively, when compared to PBS-treated MM controls
(p=0.002, Kaplan-Meier survival analysis; Fig. 6). In the 0.4 mg/kg/day group, 3 of
the 10 mice were alive and in good condition on day 30. When the
animals were sacrificed and autopsied, we found that the three mice
had no visible intra-abdominal tumors. These results demonstrated
that IL6(T23)-PE38KDEL significantly increased the survival times
of treated mice and exhibited a significant dose-dependent
antitumor effect against MM mice.
In a separate experiment, the treatment of MM mice
with 0.4 mg/kg/day of IL6(T23)-PE38KDEL for 10 days (from day 5 to
15) by i.p injection led to a notable effect of tumor regression.
When the mice were sacrificed and dissected, we observed that 8 of
the 10 mice had no visible tumors in the abdominal cavity, and two
mice had significantly smaller tumors than the control group.
Compared to the control group, the liver histopathology from mice
treated with interventional injections revealed an essentially
normal appearance of hepatocytes, without evident tumor metastases
(Fig. 5B). The control group had
large tumors that filled the abdominal cavity, accompanied with
large amounts of ascites. The complete regression rate of
IL6(T23)-PE38KDEL in the mice was significantly higher by
interventional injection compared to i.v. administration.
The blood assay (Table
II) revealed that IL6(T23)-PE38KDEL did not markedly affect the
main physiological parameters of blood, but caused a slight
increase in liver enzyme activity. Compared with the normal mice
treated, the liver enzyme levels of MM mice treated were
significantly lower.
Discussion
The present study aimed to evaluate the antitumor
activity of the recombinant IL6(T23)-PE38KDEL in vitro and
in vivo. The IL6(T23)-PE38KDEL gene using E. coli
preferred codons was designed to overcome the bottleneck caused by
the low expression level of the natural gene in the pET expression
system. Codon optimization has become a very effective means to
improve protein expression (24,31,32).
In a previous study, we constructed the IL6(T23)-PE38KDEL gene by
overlap PCR using the natural IL6 and PE genes. We attempted to use
the pET28a, pET22b, pQE30 and pKK322 vectors expressing the toxin
in E. coli, but the expression level was only 2–6% of the
total protein concentration (unpublished data). The expression
level of the codon-optimized IL6(T23)-PE38KDEL gene was 5- to
6-fold that of the natural gene. The natural IL6(T23)-PE38KDEL gene
contains a large number of rare codons, thus we reasoned that it
was unsuitable for expressing in E. coli. As an outcome, the
recombinant IL6(T23)-PE38KDEL was expressed and accumulated at
approximately 20–24% of total protein by codon optimization.
The cytotoxic mechanism of cytokine-PE toxins has
previously been elaborated (7,18,33–35).
The cytokine-PE chimeric toxins and derivatives bind to cytokine
receptors on the cell surface and then enter the cell through
receptor-mediated endocytosis. In the endocytic compartment, it is
cleaved into two fragments by a furin enzyme. The C-terminal
fragment, which is composed of PE domain III and a portion of
domain II, is transported to the ER through the KDEL sequence (KDEL
functions as a REDL ER-targeting motif sequence for ER retrieval).
The C-terminal fragment then translocates to the cytosol and
enzymatically inactivates EF2, causing the inhibition of protein
synthesis and leading to cell death. Therefore, the cytotoxicity of
cytokine-PE chimeric proteins depends not only on the cell receptor
number, but also on binding affinity, rate of internalization, rate
of processing into an active form and rate of translocation into
the cytosol (6). The hepatoma cell
line SK-HEP with approximately 100 IL6R sites/cell and the
prostatic carcinoma cell line PC3 with 400 IL6R sites/cell were
both insensitive to IL6-PE4E and IL6-PE40 (13). The cleavage of PE-derived
immunotoxins by furin is a rate-limiting step for cytotoxic
activity (36). IL6R is either
absent or present at very low levels in normal cells (11,12),
and furin is widely expressed at very low levels in normal tissues
(37). Conversely, IL6, IL6R and
furin are overexpressed in certain malignancies (16,37–39).
The key factors of competitive inhibition of IL6 and very low
levels of IL6R and furin limit the efficacy of IL6(T23)-PE38KDEL to
bind to the normal cell surface and be internalized and processed;
this results in insufficient catalytic fragments to kill normal
cells. According to the functional characteristics of tIL6
(40) and IL6-PE40Asp553
(11), and the structural features
of IL6 (15,41), we reasoned that, as with the IL6
ligand, IL6(T23) only has a receptor-binding function, and loses
most of its IL6 transsignaling functionality due to truncation and
PE conjugation.
The cytotoxicity of IL6(T23)-PE38KDEL was greater
than that of IL6-PE40 in vitro. The main reason for the
increased activity may be that PE40 was transformed into PE38KDEL.
In addition, the recombinant immunotoxin exhibited specific
antitumor activity against both human IL6R and murine IL6R. The
ID50 value showed that the sensitivity of U266 cells to
IL6(T23)-PE38KDEL was slightly higher than that of SP2/0, therefore
we adapted the mouse SP2/0 tumor model to evaluate the antitumor
effects and side effects of IL6 (T23)-PE38KDEL. The repeat
administration of IL6(T23)-PE38KDEL by i.v. injection, at a dose of
0.4 mg/kg/day for 10 days, resulted in a 30% tumor regression and a
significant increase in the survival time. IL6(T23)-PE38KDEL (0.4
mg/kg/day for 10 days) by i.p. injection resulted in a notable
response of 80% tumor regression. The complete regression rate
using i.v. administration was significantly lower than that using
interventional injection; this result indicated that certain
factors in the blood acted as a counteractant which cleared
IL6(T23)-PE38KDEL before binding to the tumor cells. Our data
indicated that IL6(T23)-PE38KDEL may be suitable to target tumors
by interventional therapy. MM liver metastases may be associated
with IL6. In the MM mouse model, a large amount of myeloma cell
growth was bound to produce a large amount of IL6 in an autocrine
manner. The pleiotropic cytokine IL6, a major mediator of
inflammation (42) and an activator
of STAT3 (43), may serve to
promote the dissemination of myeloma cells into the blood
circulation, and planting and developing in the liver. Even if
there is competitive inhibition between IL6 and IL6(T23)-PE38KDEL,
sufficient IL6(T23)-PE38KDEL binding to IL6R kills IL6R-bearing
cancer cells and inhibits tumor metastasis. The liver pathology
revealed that MM liver metastasis in the high dose group was
significantly lower than that in the control group. Therefore, we
suggest that IL6(T23)-PE38KDEL may be suitable for use after
surgery to inhibit IL6R-bearing tumor cell metastasis.
Since human IL6 crossreacts with murine IL6R, we
used normal mice to evaluate the side effects of IL6(T23)-PE38KDEL.
The results indicated that IL6(T23)-PE38KDEL did not markedly
affect the absolute WBC and RBC numbers, which indicates that
IL6(T23)-PE38KDEL does not kill the myeloid progenitor cells. In
normal mice, the high dose of IL6(T23)-PE38KDEL caused mild liver
cell damage. Such hepatotoxicity may be attributed to non-specific
internalization of the drug or lower IL6R levels in liver cells
(44,45). However, since the nonspecific toxic
effects were significantly reduced in the tumor-bearing mice
treated, it may be the large amount of IL6(T23)-PE38KDEL binding to
IL6-overexpressing on tumor that causes the reduction of
IL6(T23)-PE38KDEL concentration in liver, and results in lower
liver toxicity.
Hepatotoxicity of IL6(T23)-PE38KDEL was the major
cause of death in the treated mice. Similar results have also been
observed in mice treated with IL6-PE4E and
IL2-PE4E (26,46). However, hepatotoxicity of PE-derived
immunotoxins is commonly dose-limiting in mice, but only rarely in
patients (7). By comparing the ID50
value in mice, the side effects of IL6(T23)-PE38KDEL were lower
than those of IL6-PE4E, which may be attributed to the
conversion of PE4E to PE38KDEL. Certain studies have
shown that high-dose injection of IL6 induces anemia and leads to a
reduced RBC in mice (47–49).
The antitumor activity of IL6(T23)-PE38KDEL depends
not only on the IL6R number, but also on the levels of IL6 and
sIL6R. High levels of IL6 and sIL6R in the blood have been found in
malignant conditions (12,50,51).
The antitumor activity of IL6(T23)-PE38KDEL can be blocked by an
excess of IL6 and sIL6R. Therefore, we cannot rule out the
possibility that IL6 and sIL6R act as competitors for
IL6(T23)-PE38KDEL, which leads to the poor effects observed in the
low-dose group. Specifically, sIL6R binds to the toxin forming
complexes that may lead to non-specific effects. IL6(T23)-PE38KDEL
is a protein antigen in mice, and long-term injection of the toxin
is likely to produce neutralizing antibodies to decrease the
antitumor activity. However, more studeis are required to identify
whether polyethylene glycol modification could decrease the side
effects and immunogenicity of IL6(T23)-PE38KDEL(52).
In conclusion, evaluation of IL6(T23)-PE38KDEL
indicated that the recombinant toxin has selective cytotoxicity
against IL6R-overexpressing cancer cells in vitro and in
vivo. At a dose of 0.4 mg/kg/day for 10 days, IL6(T23)-PE38KDEL
caused significant tumor regression in mice. These results make
IL6(T23)-PE38KDEL a potential candidate for further development as
an anticancer drug for IL6R-overexpressing tumors.
Acknowledgements
We would like to thank Mrs. Wang Xin Rui and Miss
Peng Chao for their technical assistance, and to Li Le and Yan Dong
Ming for their assistance with animal care and management. We also
thank Dr Yu Lu for his revision of this manuscript.
Abbreviations:
|
RIT
|
recombinant immunotoxin
|
|
PE
|
Pseudomonas exotoxin
|
|
IL6
|
human interleukin-6
|
|
IL6R
|
interleukin-6 receptor
|
|
sIL6
|
soluble interleukin-6
|
|
MM
|
multiple myeloma
|
|
scFv
|
single-chain variable fragment
|
|
IL6(T23)
|
N-terminal 23 amino acids deleted form
of human interleukin-6
|
|
E. coli
|
Escherichia coli
|
|
IPTG
|
isopropyl-β-D-1-thiogalactopyranoside
|
|
PBS
|
phosphate-buffered saline
|
|
WBC
|
white blood cells
|
|
RBC
|
red blood cells
|
|
AST
|
aspartate aminotransferase
|
|
ALT
|
alanine aminotransferase
|
|
MTS
|
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
|
References
|
1
|
Durie BG: Role of new treatment approaches
in defining treatment goals in multiple myeloma - the ultimate goal
is extended survival. Cancer Treat Rev. 36:S18–S23. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shain KH and Dalton WS:
Environmental-mediated drug resistance: a target for multiple
myeloma therapy. Expert Rev Hematol. 2:649–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajkumar SV: Treatment of multiple
myeloma. Nat Rev Clin Oncol. 8:479–491. 2011. View Article : Google Scholar
|
|
4
|
Mohindru M and Verma A: Engineered
antibodies act as targeted therapies in cancer treatment. Indian J
Pediatr. 72:943–947. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Binyamin L, Borghaei H and Weiner LM:
Cancer therapy with engineered monoclonal antibodies. Update on
cancer therapeutics. 1:147–157. 2006. View Article : Google Scholar
|
|
6
|
Pastan I: Immunotoxins containing
Pseudomonas exotoxin A: a short history. Cancer Immunol
Immunother. 52:338–341. 2003.PubMed/NCBI
|
|
7
|
Kreitman RJ: Recombinant immunotoxins
containing truncated bacterial toxins for the treatment of
hematologic malignancies. BioDrugs. 23:1–13. 2009. View Article : Google Scholar
|
|
8
|
Li J and Zhang JK: LHRH-PE40-induced
vascular leak syndrome. Toxicol Mech Methods. 16:473–476. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Sun Y and Zhang J: A recombinant
protein LHRH-PE40 for tumour therapy: preclinical safety studies.
Basic Clin Pharmacol Toxicol. 99:398–404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kreitman RJ: Recombinant immunotoxins for
the treatment of chemoresistant hematologic malignancies. Curr
Pharm Des. 15:2652–2664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegall CB, Chaudhary VK, FitzGerald DJ
and Pastan I: Cytotoxic activity of an interleukin
6-Pseudomonas exotoxin fusion protein on human myeloma
cells. Proc Natl Acad Sci USA. 85:9738–9742. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kreitman RJ, Siegall CB, FitzGerald DJ,
Epstein J, Barlogie B and Pastan I: Interleukin-6 fused to a mutant
form of Pseudomonas exotoxin kills malignant cells from
patients with multiple myeloma. Blood. 79:1775–1780.
1992.PubMed/NCBI
|
|
13
|
Siegall CB, Schwab G, Nordan RP,
FitzGerald DJ and Pastan I: Expression of the interleukin 6
receptor and interleukin 6 in prostate carcinoma cells. Cancer Res.
50:7786–7788. 1990.PubMed/NCBI
|
|
14
|
Siegall CB, FitzGerald DJ and Pastan I:
Cytotoxicity of IL6-PE40 and derivatives on tumor cells expressing
a range of interleukin 6 receptor levels. J Biol Chem.
265:16318–16323. 1990.PubMed/NCBI
|
|
15
|
Simpson RJ, Hammacher A, Smith DK,
Matthews JM and Ward LD: Interleukin-6: structure-function
relationships. Protein Sci. 6:929–955. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong DS, Angelo LS and Kurzrock R:
Interleukin-6 and its receptor in cancer: implications for
translational therapeutics. Cancer. 110:1911–1928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siegall CB, Kreitman RJ, FitzGerald DJ and
Pastan I: Antitumor effects of interleukin 6-Pseudomonas
exotoxin chimeric molecules against the human hepatocellular
carcinoma, PLC/PRF/5 in mice. Cancer Res. 51:2831–2836.
1991.PubMed/NCBI
|
|
18
|
Rozemuller H, Rombouts WJ, Touw IP, et al:
Treatment of acute myelocytic leukemia with interleukin-6
Pseudomonas exotoxin fusion protein in a rat leukemia model.
Leukemia. 10:1796–1803. 1996.PubMed/NCBI
|
|
19
|
Proudfoot AE, Brown SC, Bernard AR,
Bonnefoy JY and Kawashima EH: Recombinant human IL-6 expressed in
E. coli undergoes selective N-terminal degradation: evidence
that the protein consists of a stable core and a nonessential
flexible N-terminal. J Protein Chem. 12:489–497. 1993.PubMed/NCBI
|
|
20
|
Hammacher A, Ward LD, Weinstock J,
Treutlein H, Yasukawa K and Simpson RJ: Structure-function analysis
of human IL-6: identification of two distinct regions that are
important for receptor binding. Protein Sci. 3:2280–2293. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ehlers M, Grötzinger J, deHon FD, et al:
Identification of two novel regions of human IL-6 responsible for
receptor binding and signal transduction. J Immunol. 153:1744–1753.
1994.PubMed/NCBI
|
|
22
|
Weldon JE and Pastan I: A guide to taming
a toxin - recombinant immunotoxins constructed from
Pseudomonas exotoxin A for the treatment of cancer. FEBS J.
278:4683–4700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kreitman RJ and Pastan I: Importance of
the glutamate residue of KDEL in increasing the cytotoxicity of
Pseudomonas exotoxin derivatives and for increased binding
to the KDEL receptor. Biochem J. 307(Pt 1): 29–37. 1995.PubMed/NCBI
|
|
24
|
Grote A, Hiller K, Scheer M, et al: JCat:
a novel tool to adapt codon usage of a target gene to its potential
expression host. Nucleic Acids Res. 33:W526–531. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brinkmann U, Pai LH, FitzGerald DJ,
Willingham M and Pastan I: B3(Fv)-PE38KDEL, a single-chain
immunotoxin that causes complete regression of a human carcinoma in
mice. Proc Natl Acad Sci USA. 88:8616–8620. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kreitman RJ and Pastan I: Purification and
characterization of IL6-PE4E, a recombinant fusion of interleukin 6
with Pseudomonas exotoxin. Bioconjug Chem. 4:581–585. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olson BJ and Markwell J: Assays for
determination of protein concentration. Curr Protoc Protein Sci.
Chapter 3(Unit 3): 42007.PubMed/NCBI
|
|
28
|
Tsuchiya M, Takaoka A, Tokioka N and
Matsuura S: Development of an endotoxin-specific Limulus amebocyte
lysate test blocking beta-glucan-mediated pathway by
carboxymethylated curdlan and its application. Nihon Saikingaku
Zasshi. 45:903–911. 1990.(In Japanese).
|
|
29
|
Malich G, Markovic B and Winder C: The
sensitivity and specificity of the MTS tetrazolium assay for
detecting the in vitro cytotoxicity of 20 chemicals using human
cell lines. Toxicology. 124:179–192. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Croucher PI, Shipman CM, Lippitt J, et al:
Osteoprotegerin inhibits the development of osteolytic bone disease
in multiple myeloma. Blood. 98:3534–3540. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burgess-Brown NA, Sharma S, Sobott F,
Loenarz C, Oppermann U and Gileadi O: Codon optimization can
improve expression of human genes in Escherichia coli: A
multi-gene study. Protein Expr Purif. 59:94–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu X, Jornvall H, Berndt KD and Oppermann
U: Codon optimization reveals critical factors for high level
expression of two rare codon genes in Escherichia coli: RNA
stability and secondary structure but not tRNA abundance. Biochem
Biophys Res Commun. 313:89–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kreitman RJ and Pastan I: Immunobiological
treatments of hairy-cell leukaemia. Best Pract Res Clin Haematol.
16:117–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pastan I, Hassan R, Fitzgerald DJ and
Kreitman RJ: Immunotoxin therapy of cancer. Nat Rev Cancer.
6:559–565. 2006. View Article : Google Scholar
|
|
35
|
Pastan I, Beers R and Bera TK: Recombinant
immunotoxins in the treatment of cancer. Methods Mol Biol.
248:503–518. 2004.
|
|
36
|
Chiron MF, Fryling CM and FitzGerald D:
Furin-mediated cleavage of Pseudomonas exotoxin-derived
chimeric toxins. J Biol Chem. 272:31707–31711. 1997.PubMed/NCBI
|
|
37
|
Bassi DE, Lopez De Cicco R, Mahloogi H,
Zucker S, Thomas G and Klein-Szanto AJ: Furin inhibition results in
absent or decreased invasiveness and tumorigenicity of human cancer
cells. Proc Natl Acad Sci USA. 98:10326–10331. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thomas G: Furin at the cutting edge: from
protein traffic to embryogenesis and disease. Nat Rev Mol Cell
Biol. 3:753–766. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bassi DE, Fu J, Lopez de Cicco R and
Klein-Szanto AJ: Proprotein convertases: ‘master switches’ in the
regulation of tumor growth and progression. Mol Carcinog.
44:151–161. 2005.
|
|
40
|
Alberti L, Bachelot T, Duc A, Biota C and
Blay JY: A spliced isoform of interleukin 6 mRNA produced by renal
cell carcinoma encodes for an interleukin 6 inhibitor. Cancer Res.
65:2–5. 2005.PubMed/NCBI
|
|
41
|
Scheller J and Rose-John S: Interleukin-6
and its receptor: from bench to bedside. Med Microbiol Immunol.
195:173–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wong VW, Yu J, Cheng AS, et al: High serum
interleukin-6 level predicts future hepatocellular carcinoma
development in patients with chronic hepatitis B. Int J Cancer.
124:2766–2770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hodge DR, Hurt EM and Farrar WL: The role
of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer.
41:2502–2512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song S, Xue J, Fan K, et al: Preparation
and characterization of fusion protein truncated Pseudomonas
Exotoxin A (PE38KDEL) in Escherichia coli. Protein Expr
Purif. 44:52–57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nesbitt JE and Fuller GM: Dynamics of
interleukin-6 internalization and degradation in rat hepatocytes. J
Biol Chem. 267:5739–5742. 1992.PubMed/NCBI
|
|
46
|
Lorberboum-Galski H, Garsia RJ, Gately M,
et al: IL2- PE664Glu, a new chimeric protein cytotoxic to
human-activated T lymphocytes. J Biol Chem. 265:16311–16317.
1990.PubMed/NCBI
|
|
47
|
Mori K, Fujimoto-Ouchi K, Onuma E, et al:
Novel models of cancer-related anemia in mice inoculated with
IL-6-producing tumor cells. Biomed Res. 30:47–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jongen-Lavrencic M, Peeters HR, Rozemuller
H, et al: IL-6-induced anaemia in rats: possible pathogenetic
implications for anemia observed in chronic inflammations. Clin Exp
Immunol. 103:328–334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Raj DS: Role of interleukin-6 in the
anemia of chronic disease. Semin Arthritis Rheum. 38:382–388. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wierzbowska A, Urbanska-Rys H and Robak T:
Circulating IL-6-type cytokines and sIL-6R in patients with
multiple myeloma. Br J Haematol. 105:412–419. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Usnarska-Zubkiewicz L: Level of
interleukin-6 (IL-6), soluble interleukin-6 receptors (sIL-6R) and
tumor necrosis factor alpha (TNF-alpha) in untreated and
progressing multiple myeloma. Pol Arch Med Wewn. 99:30–37. 1998.(In
Polish).
|
|
52
|
Tsutsumi Y, Onda M, Nagata S, Lee B,
Kreitman RJ and Pastan I: Site-specific chemical modification with
polyethylene glycol of recombinant immunotoxin anti-Tac(Fv)-PE38
(LMB-2) improves antitumor activity and reduces animal toxicity and
immunogenicity. Proc Natl Acad Sci USA. 97:8548–8553. 2000.
View Article : Google Scholar
|