Introduction
Neuroendocrine tumors (NETs) are relatively rare
(1). Previous World Health
Organization Classification groups NETs into G1 (referred to
synonymously as ‘carcinoid tumor’), G2 and G3 (neuroendocrine
carcinoma) (2). Primary NETs of the
gallbladder comprise only 0.5% of all NETs arising from any tissue
or organ and less than 2% of all cases of gallbladder cancer
(3). Moreover, the majority of
primary gallbladder NETs are G3, making a primary NET G1 in this
organ extremely rare (3,4).
The cytoplasm of NET cells is usually eosinophilic
to amphophilic; however, in rare cases, the cytoplasm appears clear
and is referred to as ‘clear cell variant’. This variant has been
described as a distinctive manifestation of von Hippel-Lindau
disease (VHL) (5) and there are few
reports of clear cell NET in non-VHL patients (6). Only two cases of primary gallbladder
clear cell NET G1 have been reported in the English-language
literature: one was associated with VHL (7) and the other was not (8). This report describes a second case of
clear cell NET G1 of the gallbladder in a patient without VHL and
discusses the clinicopathological features of this extremely rare
lesion.
Patient and methods
Patient
A 71-year-old Japanese male without past or family
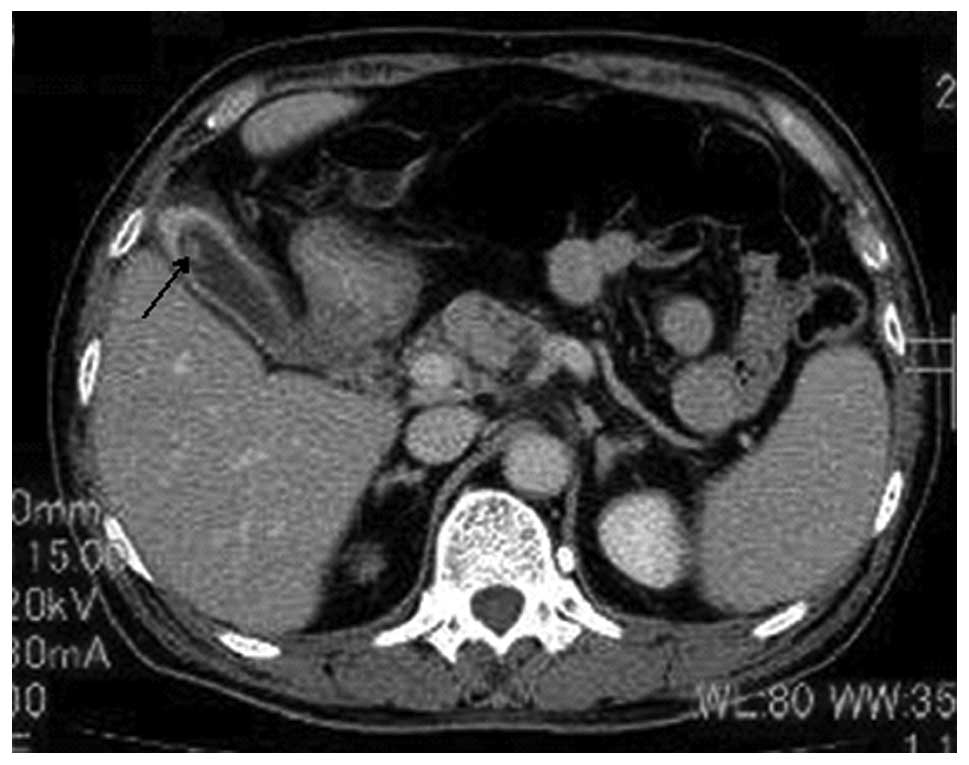
history of VHL, presented with sudden abdominal pain. Computed
tomography demonstrated stones in the common bile duct and
gallbladder, as well as a 9-mm polypoid lesion in the fundus of the
gallbladder (Fig. 1). No tumorous
lesions were detected in the pancreas or kidneys and no symptoms of
carcinoid syndrome were noted. Laparoscopic cholecystectomy was
performed. The study was approved by the ethics committee of Shiga
University of Medical Science, Shiga, Japan. Written informed
patient consent was obtained from the patient.
Materials and methods
Formalin-fixed, paraffin-embedded tissue blocks of
the resected specimen of the gallbladder were cut into 3-μm
thick sections, deparaffinized and rehydrated. Each section was
stained with hematoxylin and eosin and then used for
immunostaining. Immunohistochemical analyses were performed using
an autostainer (XT system Benchmark, Ventana Medical System,
Tucson, AZ, USA) according to the manufacturer’s instructions. The
following primary antibodies were used: mouse monoclonal
anti-α-inhibin (R1, Thermo Fisher Scientific, Waltham, MA, USA),
mouse monoclonal anti-α-internexin (2E3, Lab Vision, Waltham, MA,
USA), mouse monoclonal anti-chromogranin A (DAK-A3), rabbit
polyclonal anti-gastrin, rabbit polyclonal anti-glucagon (all
purchased from Dako Cytomation, Glostrup, Denmark), mouse
monoclonal anti-insulin (Z006, Nichirei Bioscience, Tokyo, Japan),
mouse monoclonal anti-Ki-67 (MM1, Novocastra Laboratories, Ltd.,
Newcastle-upon-Tyne, UK), mouse monoclonal anti-peripherin (PJM50,
Novocastra), rabbit polyclonal anti-pancreatic polypeptide (Lab
Vision), rabbit polyclonal anti-S-100 protein (Nichirei), mouse
monoclonal anti-serotonin (5HT-H209, Dako), rabbit polyclonal
anti-somatostatin (Dako) and mouse monoclonal anti-synaptophysin
(27G12, Novocastra).
Results
Macroscopically, we observed three small gallbladder
stones in the lumen, a mild thickening in the wall of the
gallbladder and a 9-mm yellowish polypoid lesion in the fundus.
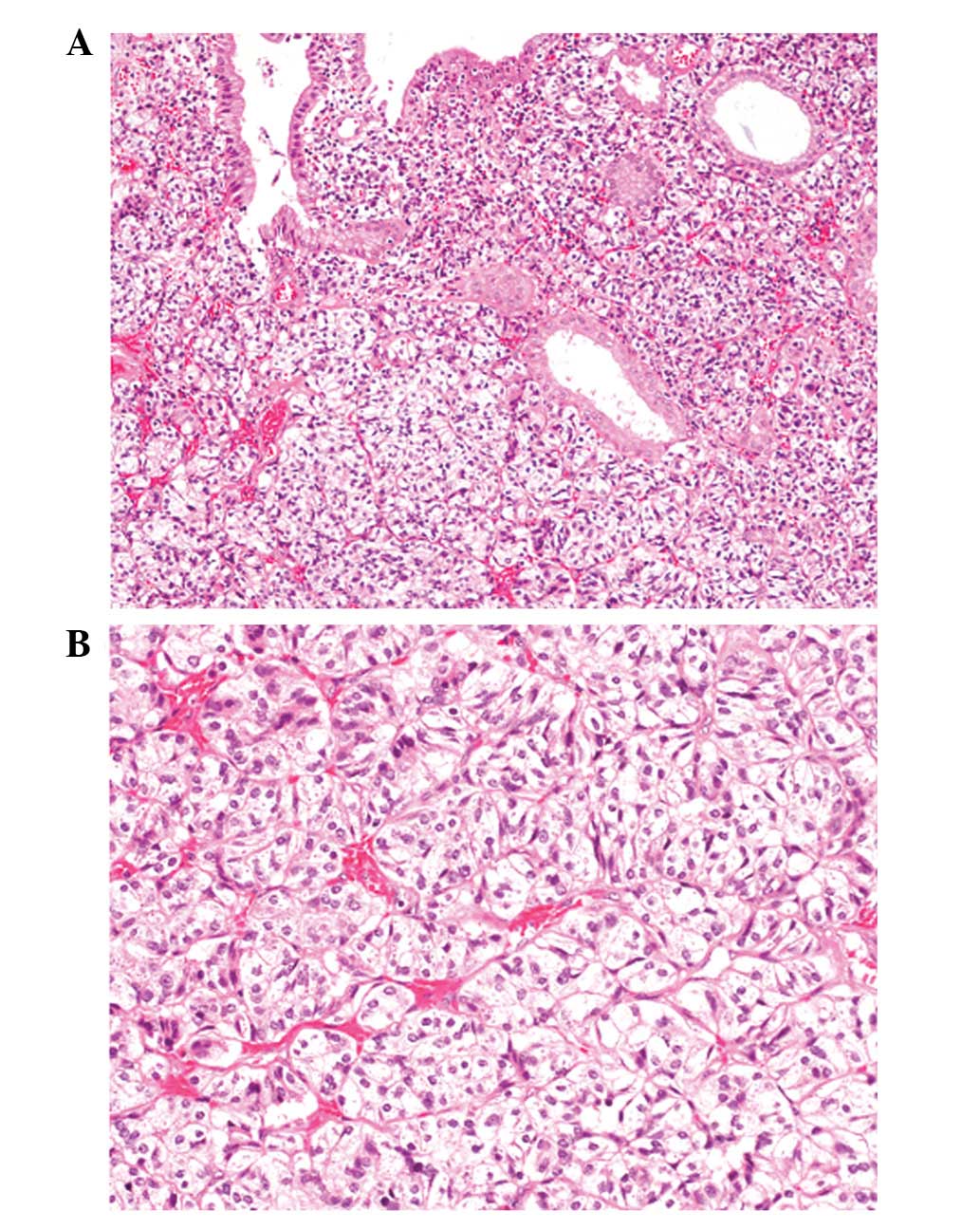
Histopathologically, the polypoid lesion was
comprised of nests or trabecular growths of cuboidal cells. These
cells had rich clear cytoplasm and small round nuclei with
inconspicuous nucleoli separated by delicate capillary networks
(Fig. 2A and B). Mitotic figures
were rarely observed (<1/20 high power fields). The neoplastic
growth of clear cells was restricted to the lamina propria of the
polypoid lesion. The surface of the polyp was composed of biliary
epithelium without atypia and non-neoplastic biliary glands were
entrapped among the nests of clear cells (Fig. 2B). Neither vascular nor lymphatic
invasion were present. Chronic cholecystitis with
Rokitansky-Aschoff sinuses, as well as gastric metaplasia and
adenomyosis, was observed in the surrounding gallbladder tissue,
however, intestinal metaplasia was not noted.
Immunohistochemical analyses revealed diffuse
expression of synaptophysin and chromogranin A in the neoplastic
clear cells (Fig. 3), but no S-100
protein or α-inhibin. While somatostatin was focally expressed in
clear cells, we did not detect any immunoreactivity for gastrin,
glucagon, insulin, serotonin or pancreatic polypeptide. The Ki-67
labeling index was 0.8%. The expression of peripherin and
α-internexin was not observed in the neoplastic cells. In addition,
we detected focal expression of chromogranin A and synaptophysin in
the non-neoplastic glands with gastric metaplasia (Fig. 3, inset).
According to the histopathological and
immunohistochemical findings of the present study, diagnosis of
clear cell NET G1 of the gallbladder was made.
Discussion
In the present study we describe the second reported
case of clear cell NET G1 of the gallbladder in a patient without
VHL. Table I summarizes the
clinicopathological features of all three reported cases of clear
cell NET G1 of the gallbladder (7,8). All
patients were male and middle-aged to elderly (average age, 57.7
years; range, 38–71). Lesions were located in the neck in the two
previous cases and in the fundus in the present study. Only one
previous case was associated with VHL and the others, including the
present case, were not associated with VHL. Cases of clear cell NET
G1 of the gallbladder all occurred in males, contrasting with
general cases of clear cell NET G1 of the pancreas and gallbladder
NETs, which are more prevalent in females (60–70%) (3,5).
 | Table I.Clinicopathological features of clear
cell neuroendocrine tumor G1 of the gallbladder. |
Table I.
Clinicopathological features of clear
cell neuroendocrine tumor G1 of the gallbladder.
| Case | Age/Gender | Location | VHL | Cholecystitis | Gallbladder
stone | Reference |
|---|
| 1 | 38/Male | Neck | + | Not available | − | 7 |
| 2 | 64/Male | Neck | − | + | + | 8 |
| Present | 71/Male | Fundus | − | + | + | |
While normal gallbladder mucosa has no
neuroendocrine cells, gallbladder mucosa with gastric and/or
intestinal metaplasia contains neuroendocrine cells that express
peptides, including gastrin, somatostatin, serotonin and glucagon
(9–11). Moreover, the majority of previous
reports of NETs of the gallbladder describe cases with gallbladder
stones and cholecystitis (3,12). In
one study, intestinal metaplasia was found in 11.7% of gallbladders
with cholelithiasis and 83.3% also contained chromogranin
A-positive neuroendocrine cells (11). These data suggest that the
occurrence of gallbladder NETs is associated with chronic
cholecystitis induced by gallbladder stones and that this
association may explain the predilection for gallbladder NETs in
females.
Cholecystitis was also evident in two cases of clear
cell NET G1 of the gallbladder. While gastric metaplasia with
chromogranin A-positive neuroendocrine cells was observed in the
present case, it is unclear whether neuroendocrine cells were
present in gallbladder mucosa in the case reported by Konishi et
al (8). Although reported cases
are extremely limited, gallbladder stones were also found in two of
three clear cell NET G1 cases reviewed here (the case without
gallbladder stone was VHL-related; Table I). Therefore, chronic cholecystitis
induced by gallbladder stones may be associated with clear cell NET
G1 (particularly non-VHL-related cases) as well as conventional
gallbladder NETs. It is plausible that gallbladder NETs, including
the clear cell variant, may originate from neuroendocrine cells of
intestinal and/or gastric metaplastic mucosa induced by chronic
cholecystitis (3).
α-inhibin is expressed in NET G1 of the gallbladder
and pancreas associated with VHL. However, classical NET G1 of the
gallbladder and non-VHL associated NET of the pancreas do not
exhibit positive immunoreactivity for this marker and α-inhibin has
subsequently been reported as a marker for VHL (7). In the present case of clear cell NET
G1, consistent with the previous report without VHL (8), no α-inhibin was observed in the tumor
cells. These results suggest that surveillance of α-inhibin
expression may be a useful criterion for distinguishing whether
clear cell NET is associated with VHL.
We previously characterized the expression of
neuronal intermediate filament proteins in NETs of various organs
(13,14). While peripherin (a type III
intermediate filament protein expressed in normal peripheral
nerves) is expressed in all NET G1 of the rectum, its expression
incidence is low in NET G2 of the rectum (13). By contrast, expression of
α-internexin (a type IV intermediate filament protein normally
found in the central nervous system) is observed in all NET G1 of
the appendix and approximately half of rectal NET G1. All
appendiceal NET G1 cases co-express peripherin and α-internexin
(14). Since neither peripherin nor
α-internexin expression was observed in this case of clear cell NET
G1 of the gallbladder, it appears that intermediate filament
protein expression varies with NET origin.
In conclusion, while clear cell NET is a rare
histopathological variant often described as a distinct
manifestation of VHL, we report a rare case of clear cell NET G1 of
the gallbladder without VHL. Surveillance immunohistochemistry for
α-inhibin may prove useful as a determinant of whether a clear cell
NET is VHL-associated or not.
References
|
1.
|
S MassironiV SciolaM PeracchiC
CiafardiniMP SpampattiD ConteNeuroendocrine tumors of the
gastro-entero-pancreatic systemWorld J
Gastroenterol1453775384200810.3748/wjg.14.537718803349
|
|
2.
|
P KomminothR ArnoldC CapellaNeuroendocrine
neoplasms of the gallbladder and extrahepatic bile ductsWHO
Classification of Tumours of the Digestive SystemFT BosmanF
CarneiroRH HrubanND TheiseIARC PressLyon2742762010
|
|
3.
|
KM EltawilBI GustafssonM KiddIM
ModlinNeuroendocrine tumors of the gallbladder: an evaluation and
reassessment of management strategyJ Clin
Gastroenterol44687695201020375728
|
|
4.
|
V AnjaneyuluG Shankar-SwarnalathaSC
RaoCarcinoid tumor of the gall bladderAnn Diagn
Pathol11113116200710.1016/j.anndiagpath.2005.12.00317349570
|
|
5.
|
MP HoangRH HrubanJ Albores-SaavedraClear
cell endocrine pancreatic tumor mimicking renal cell carcinoma: a
distinctive neoplasm of von Hippel-Lindau diseaseAm J Surg
Pathol25602609200110.1097/00000478-200105000-0000611342771
|
|
6.
|
S NunobeN FukushimaS YachidaK ShimadaT
KosugeM SakamotoClear cell endocrine tumor of the pancreas which is
not associated with von Hippel-Lindau disease: report of a caseSurg
Today33470474200310.1007/s10595-002-2508-x12768377
|
|
7.
|
PA SinkreL MurakataL RabinMP HoangJ
Albores-SaavedraClear cell carcinoid tumor of the gallbladder:
another distinctive manifestation of von Hippel-Lindau diseaseAm J
Surg
Pathol2513341339200110.1097/00000478-200110000-0001711688471
|
|
8.
|
E KonishiY NakashimaTC SmyrkS MasudaClear
cell carcinoid tumor of the gallbladder. A case without von
Hippel-Lindau diseaseArch Pathol Lab Med127745747200312741904
|
|
9.
|
J Albores-SaavedraM NadjiDE HensonJ
Ziegels-WeissmanJM MonesIntestinal metaplasia of the gallbladder: a
morphologic and immunocytochemical studyHum
Pathol17614620198610.1016/S0046-8177(86)80134-42872152
|
|
10.
|
M YamamotoS NakajoN MiyoshiS NakaiE
TaharaEndocrine cell carcinoma (carcinoid) of the gallbladderAm J
Surg Pathol13292302198910.1097/00000478-198904000-000042648878
|
|
11.
|
H SakamotoH MutohK IdoK SatohH HayakawaK
SuganoA close relationship between intestinal metaplasia and Cdx2
expression in human gallbladders with cholelithiasisHum
Pathol386671200710.1016/j.humpath.2006.06.01016996572
|
|
12.
|
A MaitraM TascilarRH HrubanGJ OfferhausJ
Albores-SaavedraSmall cell carcinoma of the gallbladder: a
clinicopathologic, immunohistochemical, and molecular pathology
study of 12 casesAm J Surg
Pathol25595601200110.1097/00000478-200105000-0000511342770
|
|
13.
|
M IshidaR KushimaT ChanoH
OkabeImmunohistochemical demonstration of the type III intermediate
filament peripherin in human rectal mucosae and well-differentiated
endocrine neoplasmsOncol Rep186336372007
|
|
14.
|
M IshidaR KushimaM BrevetD ChatelainH
OkabeCo-expression of neuronal intermediate filaments, peripherin
and α-internexin in human well-differentiated endocrine neoplasms
(carcinoid tumors) of the appendixMol Med Report11911952008
|