Introduction
Colon cancer, one of the most common types of
malignant tumours worldwide (1), is
primarily treated using a chemotherapeutic approach (2). Recently, oxaliplatin (L-OHP) has been
employed as an agent for colon cancer chemotherapy (3); however, drug resistance has posed
problems in the success of this treatment (4). Thus, it is of value and interest to
identify genes that influence chemosensitivity to specific
drugs.
The proapoptotic protein Bcl-2/adenovirus EIB
19-kDa-interacting protein 3 (BNIP3), a member of the Bcl-2 family
of proteins, has been a research topic of interest in recent years.
BNIP3 is a mitochondrial protein that interacts with the E1B 19-kDa
adenovirus protein and Bcl-2 (5),
and its expression is reduced in the majority of tumours, including
pancreatic, haematopoietic and gastric cancers (6–8).
Tumour chemoresistance correlates with the abnormal expression of
BNIP3, and our previous study has demonstrated that BNIP3 may play
a role in the enhancement of radiotherapy efficiency (9). Previous studies have revealed that the
downregulation of BNIP3 in pancreatic cancer cells increased the
resistance to 5-fluorouracil (5-FU) and gemcitabine (10,11).
It has been suggested that the loss of BNIP3 expression, which
occurs late in pancreatic cancer, contributes to chemotherapy
resistance and correlates with poor prognoses (10,11).
Murai et al (7) identified
that the expression of BNIP3 decreased in colon cancer cell lines
that were chemoresistant to 5-FU. Tang et al (12) also demonstrated that colon cancer
cell lines resistant to L-OHP expressed low levels of BNIP3, and
were resistant to 5-FU. Other studies have reached similar
conclusions, indicating that BNIP3 expression correlates with
chemoresistance (11,13,14).
Whether the overexpression of BNIP3 correlates with the reversal of
drug resistance in tumour cells remains unknown. Therefore, this
study investigated the effect of BNIP3 overexpression on the
chemosensitivity of parental and L-OHP-resistant colon cancer cell
lines.
Materials and methods
Cell culture
The human parental colon cancer cell lines (SW620
and colo320) and L-OHP-resistant colon cancer cell lines
(SW620/L-OHP and colo320/L-OHP) were a gift from the Laboratory of
Signal Transduction and Molecular Targeting Therapy of West China
Hospital (Sichuan University, China). SW620 and SW620/L-OHP cells
were cultured in RPMI-1640 medium supplemented with 10% foetal calf
serum and maintained in an atmosphere of 5% CO2 at 37°C.
Colo320 and colo320/L-OHP cells were also cultured in RPMI-1640
medium supplemented with 10% foetal calf serum, but maintained in
an atmosphere under 5% CO2 at 37°C.
Transient transfection
Plasmids, pDsRed-N1 and pDsRed-BNIP3, were acquired
from Dr Chen Ni (Department of Pathology, West China Hospital) and
were sequenced by Invitrogen Life Technologies (Carlsbad, CA, USA).
We extracted and purified plasmid DNA from Escherichia coli
cell lysates using a PureLink™ HiPure Plasmid DNA purification kit
(Invitrogen Life Technologies). The four colon cancer cell lines
(SW620, SW620/L-OHP, colo320 and colo320/L-OHP) were transfected
with pDsRed-N1 or pDsRed-BNIP3 using Lipofectamine™ 2000
(Invitrogen Life Technologies). Cells were briefly trypsinised and
plated onto 6-well plates. The transfection reagent was then added
and incubated at room temperature for 5 min. The appropriate volume
of plasmid DNA was added and the cells were incubated for an
additional 20 min. Fluorescein-labelled pDsRed-N1- or
pDsRed-BNIP3-transfected cells were examined under fluorescence
optics to determine transfection efficiency after 24 h. Cells were
then prepared for western blot analysis, cytotoxicity assays, flow
cytometry or Hoechst 33342 staining.
Western blot analysis
Total protein was extracted using a lysis buffer
containing 50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.1%
sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulphonyl fluoride
(PMSF), 0.5 mM EDTA, 0.6l mM leupeptin and 0.1% pepstatin. Equal
amounts of protein were separated by SDS-PAGE and
electrophoretically transferred onto nitrocellulose membranes. The
membranes were blocked with 5% non-fat milk in TBST and incubated
with BNIP3 and β-actin antibodies (Sigma-Aldrich, St. Louis, MO,
USA) overnight at 4°C. Once the membranes were exposed to the
respective secondary antibody for 2 h, they were analysed by
chemiluminescence detection and autoradiography (Odyssey Imaging
System; LI-COR Biosciences, Lincoln, NE, USA).
Groups
A total of 6 groups were analysed in this study:
control [normal saline (NS)], pDsRed-N1, pDsRed-BNIP3, L-OHP,
pDsRed-N1 + L-OHP and pDsRed-BNIP3 + L-OHP.
Cytotoxicity assays
Cells were seeded into 96-well plates at a density
of 1×103 cells/well in 100 μl culture medium and
allowed to adhere overnight. The cells were transfected with
control (NS) or plasmid DNA (pDsRed-N1 or pDsRed-BNIP3), and after
48 h the cells were exposed to increasing concentrations (0.01-20
μg/ml) of L-OHP (Sanofi Aventis Co., Paris, France) for 24
h. The cells were incubated in L-OHP-free medium for 48 h, and then
in 9% Cell Counting Kit-8 (CCK8; 1:10; Dojindo Laboratories Co.,
Ltd., Kumamoto, Japan) for 4 h at 37°C. Once the absorbance at 450
nm was recorded, the inhibitory concentration of 50% of cells
(IC50) was calculated, and the resistance index (RI) and reversal
resistance ratio were determined using the following equations: RI
= IC50 of drug resistant cell line/IC50 of parent cell line;
reversal resistance ratio = IC50 of cells before reversal/IC50 of
cells after reversal. The assays were conducted in triplicate and
repeated at least 3 times.
Flow cytometry
Cells were seeded onto 6-well plates at a cell
density of 2–3×105 cells/well in 2 ml of culture medium
and allowed to adhere overnight. The cells were transfected with
control (NS) or plasmid DNA (pDsRed-N1 or pDsRed-BNIP3), and after
24 h the cells were treated with NS or L-OHP (1 μg/ml in
SW620 and SW620/L-OHP cells and 2.5 μg/ml in colo320 and
colo320/L-OHP cells). Following a 48-h incubation period, the cells
were harvested and apoptosis was analysed by flow cytometry using
an Annexin V-fluorescein isothiocyanate/propidium iodide (FITC/PI)
apoptosis detection kit (KeyGen Biotechnology Co., Ltd., Nanjing,
China) according to the manufacturer’s instructions. Cells were
briefly harvested using trypsin-EDTA, pelleted by centrifugation,
washed twice with 1X PBS, and resuspended in Annexin V-binding
buffer. FITC-conjugated Annexin V and PI were added to the cells,
incubated for 30 min at room temperature away from light, and then
analysed by flow cytometry (ESP Elite; Beckman Coulter, Inc.,
Fullerton, CA, USA). Annexin V-positive cells were considered in
the early stages of apoptosis, whereas cells in the late stages of
apoptosis were Annexin V- and PI-positive. All experiments were
conducted at least 3 times with similar results.
Hoechst 33342 staining
Cells were treated in the same way as described for
flow cytometry. After 24-h incubation, the supernatant was removed
and the cells were washed twice with 1 ml chilled PBS and stained
with Hoechst 33342 according to the manufacturer’s instructions.
Stained nuclei were visualised under fluorescence optics. The
percentage of apoptotic cells, also referred to as the apoptotic
ratio (AR), was calculated using the formula: AR% = [apoptotic
cells (A)/ total cell count (T)] × l00. Approximately 10 images
from each sample were acquired and analysed in 3 different
experiments.
Statistical analysis
IC50 was calculated using Probit regression
analysis. Results were reported as mean ± standard deviation (SD).
Results were statistically analysed using ANOVA and Student’s
t-test, and all analyses were conducted using the SPSS version 17.0
software (SPSS, Inc., Chicago, IL, USA). All P-values were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
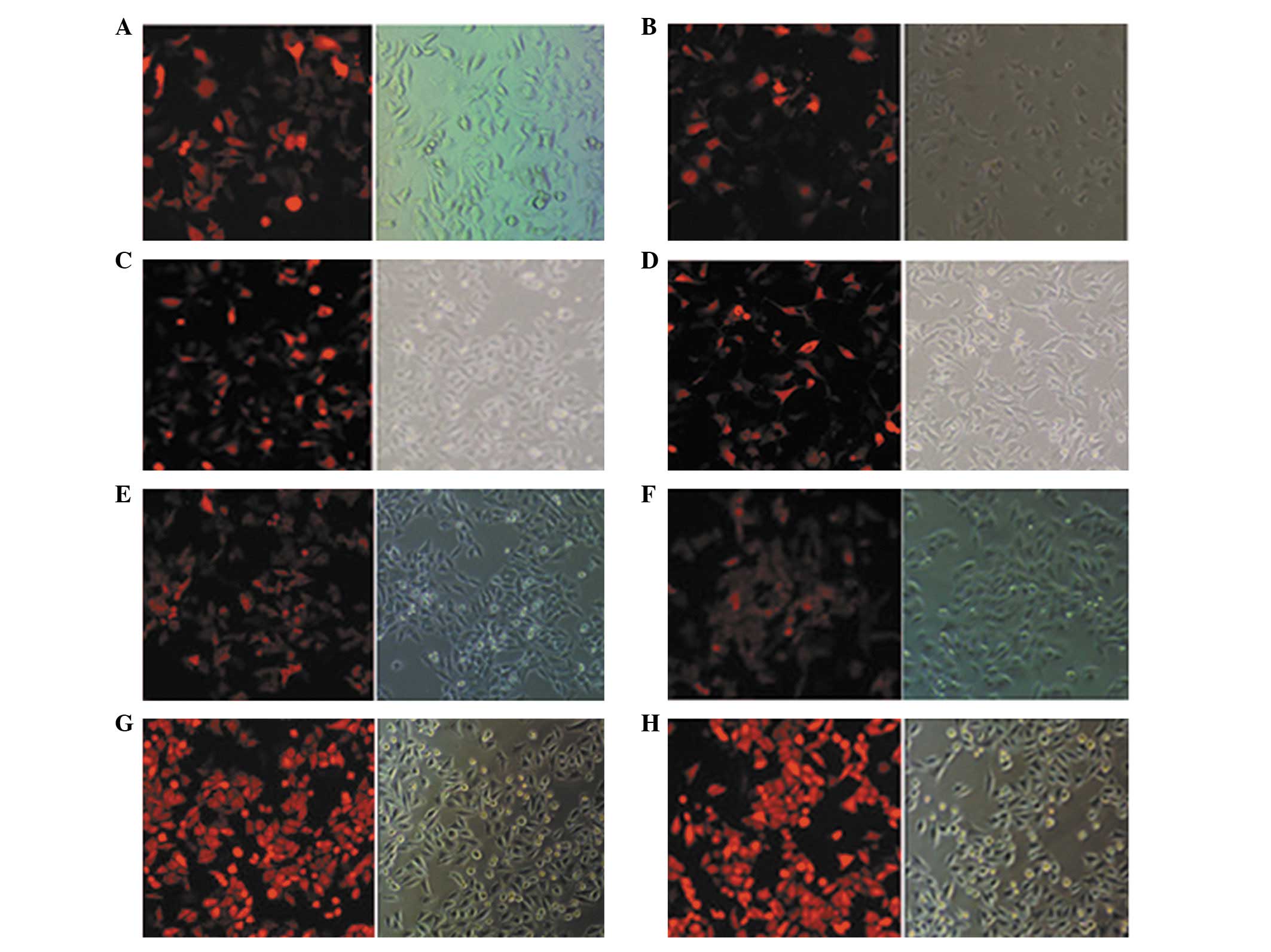
Recombinant BNIP3 expression
The pDsRed-N1 and pDsRed-BNIP3 plasmids were each
transfected into four colon cancer cell lines. The overall
transfection rates were estimated to be >60% following 24 h of
incubation (Fig. 1). Recombinant
plasmid expression was confirmed using western blot analysis. The
results for BNIP3 revealed that cells transfected with pDsRed-BNIP3
expressed higher levels of the 60- and 30-kDa forms of the BNIP3
protein compared with the cells transfected with pDsRed-N1 and
controls following a 24-h treatment (Fig. 2). Additionally, SW620/L-OHP and
colo320/L-OHP cells expressed lower levels of BNIP3 protein
compared with SW620 and colo320 cells, respectively (Fig. 2).
 | Figure 2.Western blot analysis identified
BNIP3 expression in colon cancer cells after 24-h transfection. (A)
Lane 1, SW620; lane 2, SW620-N1; lane 3, SW620-BNIP3; lane 4,
SW620/L-OHP; lane 5, SW620/L-OHP-N1; lane 6, SW620/L-OHP-BNIP3. (B)
Lane 1, colo320; lane 2, colo320-N1; lane 3, colo320-BNIP3; lane 4,
colo320/L-OHP; lane 5, colo320/L-OHP-N1; lane 6,
colo320/L-OHP-BNIP3. Recombinant human BNIP3 was expressed as 2
bands of approximately 30 and 60 kDa in pDsRed-BNIP3-transfected
(lanes 3 and 6) colon cancer cells. Both forms of BNIP3 revealed a
visible increase compared with the empty plasmid
(pDsRed-N1)-transfected (lanes 1 and 4) or untreated (lanes 2 and
5) colon cancer cells. BNIP3 expression in the L-OHP-resistant
colon cancer cell lines (SW620/L-OHP and colo320/L-OHP) was lower
compared with that of the parental human colon cancer cell lines
(SW620 and colo320). BNIP3, Bcl-2/adenovirus EIB 19-kDa-interacting
protein 3; L-OHP, oxaliplatin. |
BNIP3 expression enhances the sensitivity
of cells to L-OHP
The sensitivity of colon cancer cells to L-OHP was
evaluated using the CCK8 assay. Results revealed that at the same
concentration of L-OHP, the surviving fraction of the
pDsRed-BNIP3-transfected cells was significantly reduced
(P<0.01) compared with the pDsRed-N1-transfected cells and the
control groups; while that of the pDsRed-N1-transfected cells
remained unchanged (P>0.05) compared with the control groups
(Fig. 3). The surviving fraction of
the pDsRed-BNIP3-transfected L-OHP-resistant cells
(SW620/L-OHP-BNIP3 and colo320/L-OHP-BNIP3) did not significantly
differ (P>0.05) from that of the pDsRed-BNIP3-transfected
parental cells (SW620-BNIP3 and colo320-BNIP3). As shown in
Table I, the IC50 values of the
pDsRed-BNIP3-transfected cells were significantly reduced
(P<0.01) compared with those of the pDsRed-N1-transfected cells
and the control groups. By contrast, the pDsRed-BNIP3-transfected
L-OHP-resistant cells (SW620/L-OHP-BNIP3 and colo320/L-OHP-BNIP3)
did not significantly differ in this regard (P>0.05) from the
pDsRed-BNIP3-transfected parental cells (SW620-BNIP3 and
colo320-BNIP3). BNIP3 was revealed to reverse the drug
resistance of SW620/L-OHP and colo320/L-OHP to L-OHP by 9.67 and
4.44 times, respectively. These results indicate that the BNIP3
protein not only enhanced the sensitivity of parental colon cancer
cells, but also reversed drug resistance in L-OHP-resistant colon
cancer cells.
 | Table I.L-OHP sensitivities as detected by
CCK8 (n=3). |
Table I.
L-OHP sensitivities as detected by
CCK8 (n=3).
A, SW620 and
SW620/L-OHP cells
|
| IC50 (μg/ml)
| |
| Group | SW620 | SW620/L-OHP | RI |
|
| NS | 0.038±0.004 | 0.648±0.023 | 17.053 |
| pDsRed-N1 | 0.036±0.003 | 0.647±0.028 | 17.972 |
| pDsRed-BNIP3 | 0.020±0.002a | 0.067±0.005b | 3.35 |
|
B, Colo320 and
colo320/L-OHP cells
|
| IC50 (μg/ml)
| |
| Group | Colo320 | Colo320/L-OHP | RI |
|
| NS | 0.294±0.014 | 2.668±0.333 | 9.075 |
| pDsRed-N1 | 0.295±0.020 | 2.631±0.267 | 8.919 |
| pDsRed-BNIP3 | 0.114±0.009b | 0.601±0.005b | 5.272 |
BNIP3 expression enhances L-OHP-induced
apoptosis
Cell apoptosis was detected using Annexin V-FITC/PI.
Fig. 4 and Table II show that the
pDsRed-BNIP3-transfected cells exhibited significantly higher
(P<0.01) apoptosis rates compared with the pDsRed-N1-transfected
cells and control groups. Compared with the untransfected cells
treated with L-OHP and pDsRed-BNIP3-transfected cells, BNIP3 in
combination with L-OHP resulted in significantly higher rates of
apoptosis (P<0.01) in the parental and L-OHP-resistant colon
cancer cell lines. By contrast, the rates of apoptosis of the
pDsRed-BNIP3-transfected L-OHP-resistant cells (SW620/L-OHP-BNIP3
and colo320/L-OHP-BNIP3) did not significantly differ (P>0.05)
from those of the pDsRed-BNIP3-transfected parental cells
(SW620-BNIP3 and colo320-BNIP3). These results indicate that the
expression of the BNIP3 protein not only exerted a proapoptotic
effect on colon cancer cells but also conferred chemosensitivity on
drug-resistant colon cancer cells. Additionally, the results
indicate that the expression of BNIP3 combined with L-OHP resulted
in a higher apoptosis index relative to that of cells treated with
BNIP3 or L-OHP alone.
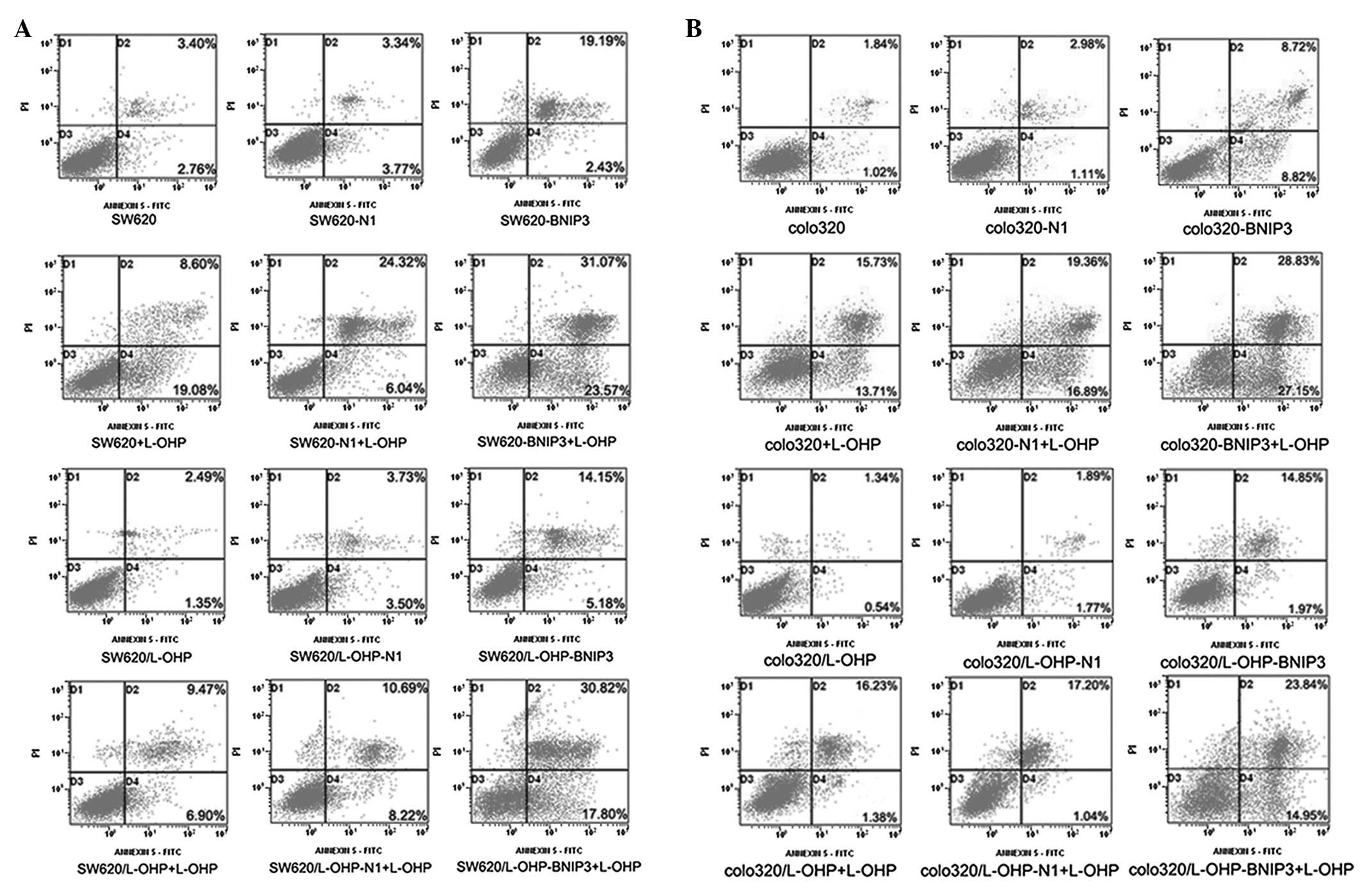 | Figure 4.Apoptosis of colon cancer cells as
detected by Annexin V-FITC/PI. Quadrant 1, PI(+) (cells undergoing
necrosis); Quadrant 2, Annexin V-FITC(+) PI(+) (cells in the late
period of apoptosis and undergoing secondary necrosis); Quadrant 3,
Annexin V-FITC(−) PI(−) (living cells); Quadrant 4, Annexin
V-FITC(+) PI(−) (cells in the early period of apoptosis). The total
AR was calculated as Quadrant 2 + Quadrant 4. The
pDsRed-BNIP3-transfected cells exhibited higher apoptotic rates
compared with the pDsRed-N1-transfected cells and the control.
Compared with the untransfected cells treated with L-OHP and the
pDsRed-BNIP3-transfected cells, the apoptosis regulator in
combination with L-OHP significantly increased the apoptosis rates
in parental and L-OHP-resistant colon cancer cell lines. FITC,
fluorescein isothiocyanate; PI, propidium iodide; BNIP3,
Bcl-2/adenovirus EIB 19-kDa-interacting protein 3; L-OHP,
oxaliplatin; AR, apoptosis rate. |
 | Table II.Apoptosis rates detected by Annexin
V-FITC/PI (n=3). |
Table II.
Apoptosis rates detected by Annexin
V-FITC/PI (n=3).
A, SW620 and
SW620/L-OHP cells
|
| Group | L-OHP (0
μg/ml) | L-OHP (1
μg/ml) |
|
| SW620 | 4.28±0.47 | 33.43±2.68 |
| SW620-N1 | 6.12±1.03 | 35.8±4.6 |
| SW620-BNIP3 | 24.30±3.61a | 53.50±2.86b |
| SW620/L-OHP | 4.15±0.39 | 20.74±3.11 |
| SW620/L-OHP-N1 | 6.32±1.08 | 21.06±2.62 |
|
SW620/L-OHP-BNIP3 | 17.34±0.39a | 47.93±4.82b |
|
B, Colo320 and
colo320/L-OHP cells
|
| Group | L-OHP (0
μg/ml) | L-OHP (2.5
μg/ml) |
|
| Colo320 | 4.44±0.63 | 33.22±0.85 |
| Colo320-N1 | 6.68±0.88 | 34.40±0.72 |
| Colo320-BNIP3 | 17.81±2.47a | 51.98±1.84b |
| Colo320/L-OHP | 4.11±1.02 | 20.53±2.36 |
|
Colo320/L-OHP-N1 | 6.09±1.85 | 22.24±1.20 |
|
Colo320/L-OHP-BNIP3 | 16.59±2.35a | 49.79±1.13b |
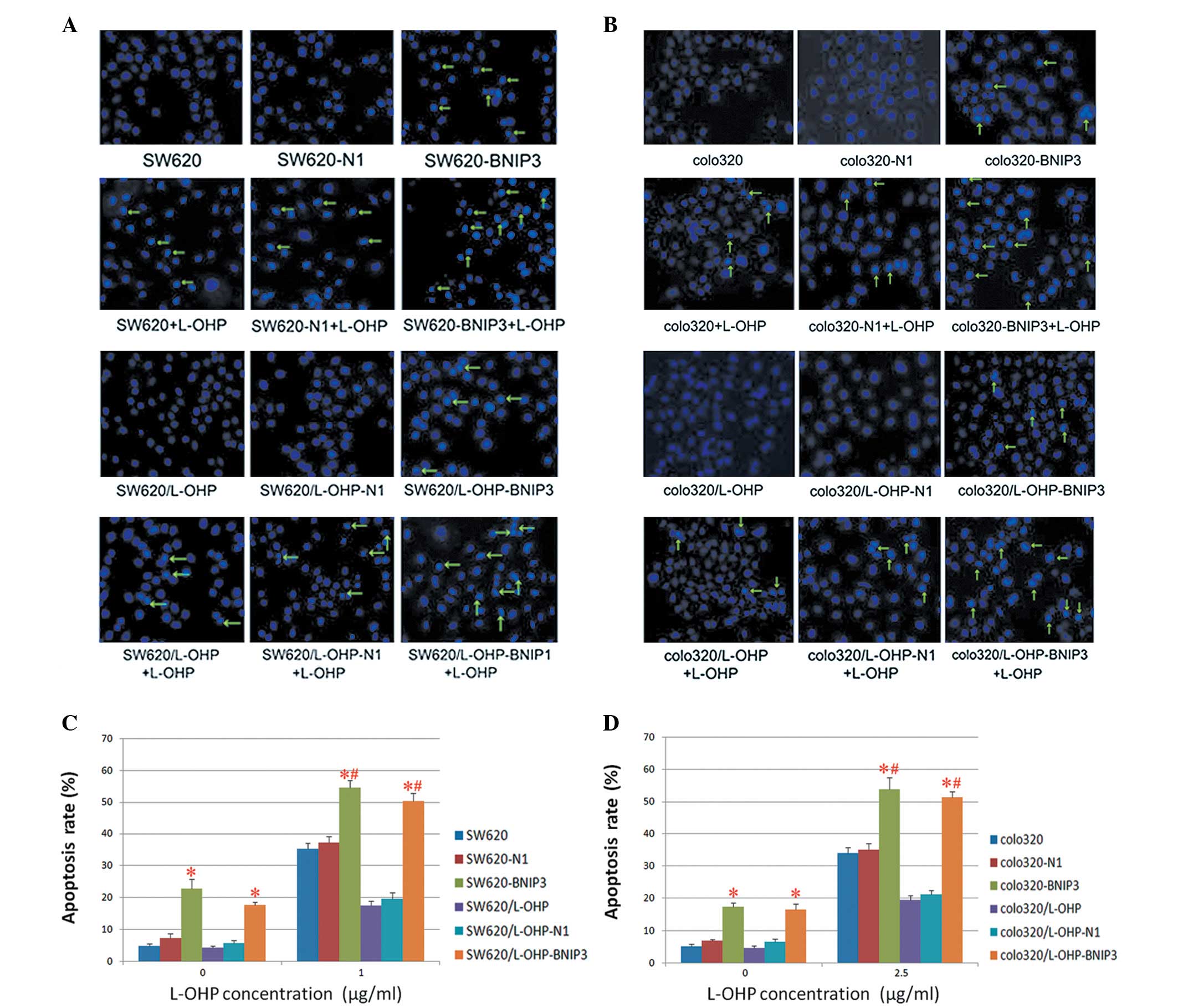
Cell apoptosis as detected by Hoechst
33342 staining
The effects of BNIP3 expression on the
chemosensitivity of colon cancer cells were verified further by
Hoechst 33342 staining. Once the cells were stained with Hoechst
33342, typical morphological findings of apoptosis, including
chromatin condensation and apoptotic bodies, were visualised under
fluorescence optics (Fig. 5A and
B). The percentage of apoptotic cells (AR%) was calculated as
previously described, and the results were confirmed using flow
cytometry. The expression of BNIP3 not only exerted a proapoptotic
effect on colon cancer cells but also conferred chemosensitivity on
drug-resistant colon cancer cells. Additionally, the results
indicate that expression of BNIP3 combined with L-OHP resulted in a
higher apoptosis index relative to that of the cells treated with
BNIP3 or L-OHP alone (Fig. 5C
and D).
Discussion
Drug resistance remains a significant obstacle for
successful chemotherapy in colon cancer. Mechanisms of resistance
to platinum agents, such as L-OHP, include increased DNA repair,
overexpression of copper transporters, enhanced drug detoxification
and increased tolerance for DNA damage (15,16).
However, despite the fact that the mechanisms influencing treatment
responses are well known, it appears that the major process leading
to chemotherapy resistance are associated with the lack or
inhibition of proapoptotic regulators (17–19).
Apoptosis is the predominant mechanism of cell death
induced by chemotherapy and radiation treatments (20). Regulation of apoptosis is a delicate
and complex process, and two major genes (p53 and Bcl-2) have been
identified to be involved in this process. As one of the downstream
genes of p53, the gene encoding the proapoptotic BNIP3 protein
should participate in the apoptotic process following chemotherapy
and radiotherapy (21) during which
BNIP3 forms heterodimers and interacts with antiapoptotic
molecules, including Bcl-2 or Bcl-xl. The heterodimer form of BNIP3
prompts activated Bax and Bak to adhere to the outer mitochondrial
membrane, forming a tetramer channel, which in turn induces the
mitochondria to release cytochrome c and activate
caspase-9-dependent apoptosis (22). As a mitochondrial protein, BNIP3
induces apoptosis when transiently overexpressed, opening the
mitochondrial permeability transition pore (MPTP) and releasing
cytochrome c from the mitochondrial intermembranous space into the
cytoplasm, subsequently promoting apoptosis (5,23–27).
However, we observed that BNIP3 expression was reduced in most
tumour tissues compared with healthy tissues, yet similar to
pancreatic, haematopoietic and gastric cancers (6–8).
Our previous study revealed that BNIP3 may play a
role in the enhancement of radiotherapy efficiency (9). Tumour chemoresistance correlates with
abnormal expression of BNIP3, and previous studies have revealed
that the downregulation of BNIP3 increased resistance to 5-FU and
gemcitabine in pancreatic cancer cells (10,11).
Loss of BNIP3 expression, which occurs late in pancreatic cancer,
contributes to chemotherapy resistance and correlates with poor
prognosis (10,11). Murai et al (7) revealed that the expression of BNIP3
was reduced in colon cancer cell lines that were chemoresistant to
5-FU. Tang et al (12)
identified that colon cancer cell lines resistant to L-OHP
expressed low levels of BNIP3, and were also resistant to 5-FU.
Other studies reached similar conclusions, indicating that BNIP3
expression correlates with chemoresistance (11,13,14).
Whether the expression of BNIP3 correlates with the reversal of
drug resistance in tumour cells remains unknown.
To determine the potential of BNIP3 as a therapeutic
target for chemosensitisation, we overexpressed BNIP3 in parental
and L-OHP-resistant colon cancer cell lines by transfection with
pDsRed-BNIP3 in vitro. The results revealed that the
L-OHP-resistant colon cancer cells expressed lower levels of BNIP3
compared with the parental cells. As confirmed by fluorescence
microscopy and western blot analysis, BNIP3 expression was
successfully increased in parental and L-OHP-resistant colon cancer
cells using the pDsRed-BNIP3 treatment strategy. Furthermore, CCK8
demonstrated that the overexpression of BNIP3 could not only
enhance the sensitivity of parental colon cancer cells but also
reverse the drug resistance of L-OHP-resistant colon cancer cells.
Subsequently, we identified the presence of apoptotic cells using
flow cytometry and Hoechst 33342 staining, indicating that BNIP3
overexpression not only exerts a proapoptotic effect on colon
cancer cells but also confers chemosensitivity on drug-resistant
colon cancer cells. Additionally, the results revealed that the
expression of BNIP3 combined with L-OHP resulted in a higher
apoptosis index relative to the apoptosis index of cells treated
with BNIP3 or L-OHP alone.
The exact mechanism by which BNIP3 overexpression
reverses the resistance of drug-resistant cancer cells and
increases drug-induced apoptosis has not been fully elucidated.
BNIP3 is a downstream gene of p53, with a probable
chemosensitisation mechanism involving BNIP3. Firstly, the
overexpression of BNIP3 enhances the proapoptotic activity via the
intrinsic apoptotic pathways. The heterodimer form of BNIP3 prompts
the activated Bax and Bak to adhere and consequently open MPTP,
releasing cytochrome c from the mitochondrial intermembranous space
into the cytoplasm, and ultimately promoting apoptosis (5,23–27).
Secondly, BNIP3 inhibits the effects of antiapoptotic molecules.
BNIP3 forms heterodimers and interacts with antiapoptotic
molecules, including Bcl-2 or Bcl-xl, and suppresses the
antiapoptotic effects. Bcl-2, originally identified as a universal
inhibitor of apoptotic cell death, has since been implicated in
suppressing autophagy, functioning as a promoter of oncogenic
growth, synergistically promoting tumour growth and promoting
cancer development and drug resistance (28). As a Bcl-2 family member with similar
antiapoptotic features to Bcl-2, Bcl-xl displayed the same pattern
of combinatorial interactions with Bcl-2 family proteins. The
suppression of Bcl-xl, which is involved in the process of drug
resistance, could improve chemotherapy efficacy in colon tumours
(29). Lastly, BNIP3 interacts with
other drug resistance-related molecules, including P-gp, MRP, MDR
and LRP. Additionally, BNIP3 facilitates mitochondrial autophagy
(30).
In conclusion, BNIP3 expression not only enhances
the sensitivity of parental colon cancer cells but also reverses
the resistance of L-OHP-resistant colon cancer cells. Furthermore,
the combined treatment with BNIP3 and L-OHP exerted a
synergistic effect on apoptosis rates in L-OHP-resistant colon
cancer cells. However, the exact mechanism by which BNIP3
expression reversed the resistance of drug-resistant cancer cells
and increased drug-induced apoptosis requires further study.
Subsequent clinical trials aimed at evaluating the
chemosensitisation effect of BNIP3 expression on colon cancer are
required.
References
|
1.
|
A JemalF BrayMM CenterJ FerlayE WardD
FormanGlobal cancer statisticsCA Cancer J
Clin616990201110.3322/caac.20107
|
|
2.
|
NH SegalLB SaltzEvolving treatment of
advanced colon cancerAnnu Rev
Med60207219200910.1146/annurev.med.60.041807.132435
|
|
3.
|
C TournigandT AndréE AchilleG LledoM
FleshD Mery-MignardE QuinauxC CouteauM BuyseG GanemB LandiP ColinC
LouvetA de GramontFOLFIRI followed by FOLFOX6 or the reverse
sequence in advanced colorectal cancer: a randomized GERCOR studyJ
Clin Oncol22229237200410.1200/JCO.2004.05.113
|
|
4.
|
DL WuF HuangHZ LuDrug-resistant proteins
in breast cancer: recent progress in multidrug resistanceChin J
Cancer22441444200312704006
|
|
5.
|
C Vande VeldeJ CizeauD DubikJ AlimontiT
BrownS IsraelsR HakemAH GreenbergBNIP3 and genetic control of
necrosis-like cell death through the mitochondrial permeability
transition poreMol Cell Biol2054545468200010891486
|
|
6.
|
M MuraiM ToyotaH SuzukiA SatohY SasakiK
AkinoM UenoF TakahashiM KusanoH MitaK YanagiharaT EndoY HinodaT
TokinoK ImaiAberrant methylation and silencing of the BNIP3 gene in
colorectal and gastric cancerClin Cancer
Res1110211027200515709167
|
|
7.
|
M MuraiM ToyotaA SatohH SuzukiK AkinoH
MitaY SasakiT IshidaL ShenG Garcia-ManeroJP IssaY HinodaT TokinoK
ImaiAberrant DNA methylation associated with silencing BNIP3 gene
expression in haematopoietic tumoursBr J
Cancer9211651172200510.1038/sj.bjc.660242215756280
|
|
8.
|
J OkamiDM SimeoneCD LogsdonSilencing of
the hypoxia-inducible cell death protein BNIP3 in pancreatic
cancerCancer
Res6453385346200410.1158/0008-5472.CAN-04-008915289340
|
|
9.
|
Z WangCM HuangQ DengH ZengX WangS ZhangF
BiQL TangRM ZhongAJ LiYB HeN ChenZP LiW WangEffects of the
proapoptotic regulator Bcl2/adenovirus EIB 19 kDa-interacting
protein 3 on radiosensitivity of cervical cancerCancer Biother
Radiopharm26279286201110.1089/cbr.2010.089821711117
|
|
10.
|
M AkadaT Crnogorac-JurcevicS LattimoreP
MahonR LopesM SunamuraS MatsunoNR LemoineIntrinsic chemoresistance
to gemcitabine is associated with decreased expression of BNIP3 in
pancreatic cancerClin Cancer
Res1130943101200510.1158/1078-0432.CCR-04-178515837765
|
|
11.
|
M ErkanJ KleeffI EspositoT GieseK
KettererMW BüchlerNA GieseH FriessLoss of BNIP3 expression is a
late event in pancreatic cancer contributing to chemoresistance and
worsened
prognosisOncogene2444214432200510.1038/sj.onc.120864215856026
|
|
12.
|
H TangYJ LiuM LiuX LiEstablishment and
gene analysis of an oxaliplatin-resistant colon cancer cell line
THC8307/L-OHPAnticancer
Drugs18633639200710.1097/CAD.0b013e328020042817762391
|
|
13.
|
BC ValdezD MurrayL RamdasM de LimaR JonesS
KornblauD BetancourtY LiRE ChamplinBS AnderssonAltered gene
expression in busulfan-resistant human myeloid leukemiaLeukemia
Research3216841697200810.1016/j.leukres.2008.01.01618339423
|
|
14.
|
M IshiguroS IidaH UetakeS MoritaH MakinoK
KatoY TakagiM EnomotoK SugiharaEffect of combined therapy with
low-dose 5-Aza-2′-deoxycytidine and irinotecan on colon cancer cell
line HCT-15Ann Surg Oncol14175217622007
|
|
15.
|
B DesoizeC MadouletParticular aspects of
platinum compounds used at present in cancer treatmentCrit Rev
Oncol Hematol42317325200210.1016/S1040-8428(01)00219-012050023
|
|
16.
|
CC ChenLT ChenTC TsouWY PanCC KuoJF LiuSC
YehFY TsaiHP HsiehJY ChangCombined modalities of resistance in an
oxaliplatin-resistant human gastric cancer cell line with enhanced
sensitivity to 5-fluorouracilBr J
Cancer97334344200510.1038/sj.bjc.660386617609664
|
|
17.
|
N MitsiadesWH YuV PoulakiM TsokosI
StamenkovicMatrix metalloproteinase-7-mediated cleavage of Fas
ligand protects tumor cells from chemotherapeutic drug
cytotoxicityCancer Res61577581200111212252
|
|
18.
|
YG AssarafL RothemJH HooijbergM StarkI
IferganI KathmannBA DijkmansGJ PetersG JansenLoss of multidrug
resistance protein 1 expression and folate efflux activity results
in a highly concentrative folate transport in human leukemia cellsJ
Biol Chem27866806686200310.1074/jbc.M209186200
|
|
19.
|
ME PeterP LegembreBC BarnhartDoes CD95
have tumor promoting activities?Biochim Biophys
Acta17552536200515907590
|
|
20.
|
S FuldaKM DebatinExtrinsic versus
intrinsic apoptosis pathways in anticancer
chemotherapyOncogene2547984811200610.1038/sj.onc.120960816892092
|
|
21.
|
P FeiW WangSH KimS WangTF BurnsJK SaxM
BuzzaiDT DickerWG McKennaEJ BernhardWS El-DeiryBnip3L is induced by
p53 under hypoxia, and its knockdown promotes tumor growthCancer
Cell6597609200410.1016/j.ccr.2004.10.01215607964
|
|
22.
|
G van LooX SaelensM van GurpM MacFarlaneSJ
MartinP VandenabeeleThe role of mitochondrial factors in apoptosis:
a Russian roulette with more than one bulletCell Death
Differ910311042200212232790
|
|
23.
|
SA SusinHK LorenzoN ZamzamiI MarzoBE
SnowGM BrothersJ MangionE JacototP CostantiniM LoefflerN
LarochetteDR GoodlettR AebersoldDP SiderovskiJM PenningerG
KroemerMolecular characterization of mitochondrial
apoptosis-inducing
factorNature397441446199910.1038/171359989411
|
|
24.
|
JY KimJJ ChoJ HaJH ParkThe carboxy
terminal C-tail of BNip3 is crucial in induction of mitochondrial
permeability transition in isolated mitochondriaArch Biochem
Biophys398147152200210.1006/abbi.2001.267311831844
|
|
25.
|
S LoveApoptosis and brain ischemiaProg
Neuropsych-ophermacol Biol
Psychiatry27267282200310.1016/S0278-5846(03)00022-8
|
|
26.
|
A BurlacuRegulation of apoptosis by Bcl-2
family proteinJ Cell Mol
Med7249257200310.1111/j.1582-4934.2003.tb00225.x14594549
|
|
27.
|
JC ReedMechanisms of apoptosisAm J
Pathol15714151430200010.1016/S0002-9440(10)64779-7
|
|
28.
|
S OhSD PiroozD NiZ ZhaoC
LiangAnti-autophagic Bcl-2: not just an innocent
bystanderAutophagy7231232201110.4161/auto.7.2.1419821099349
|
|
29.
|
F D’AnselmiA CucinaPM BiavaS ProiettirP
ColucciaL FratiM BizzarriZebrafish stem cell differentiation stage
factors suppress Bcl-xL release and enhance 5-Fu-mediated apoptosis
in colon cancer cellsCurr Pharm Biotechnol122612672011
|
|
30.
|
H ZhangM Bosch-MarceLA ShimodaYS TanJH
BaekJB WesleyFJ GonzalezGL SemenzaMitochondrial autophagy is an
HIF-1-dependent adaptive metabolic response to hypoxiaJ Biol
Chem2831089210903200810.1074/jbc.M80010220018281291
|