Introduction
Despite a declining incidence rate in the United
States and a number of other Western countries, gastric cancer
continues to be a worldwide health problem with more than 600,000
cases reported annually, far higher than pancreatic cancer with
125,000 cases (1). Gastric cancer
is the most common gastrointestinal malignancy in East Asia,
Eastern Europe and parts of Central and South America and is the
second leading cause of cancer-related mortality (2). Despite improvements in surgery,
radiotherapy and cytotoxic chemotherapy, survival rates for
advanced gastric cancer are poor. Five years after multimodal
treatment, less than 40% of Western patients with stage II or III
disease are likely to be alive. At metastatic stage IV disease, the
mean survival is only 10 months (3).
The phosphoinositide-3 kinase (PI3K)-AKT-mammalian
target of rapamycin (mTOR) pathway is an important cellular pathway
involved in cell growth, tumorigenesis, cell invasion and drug
response (4,5). PI3K is a heterodimer of 85- and
110-kDa subunits and has a tyrosine kinase activity, the activation
of which stimulates the production of phosphatidylinositol
3,4,5-triphosphate resulting in activation of the kinases PDK1 and
Akt. Akt is a kinase that phosphorylates a variety of target
molecules to mediate signals, including mTOR, while mTOR
phosphorylates and activates p70 S6 kinase (S6K)-1 and also
inhibits eukaryotic translation initiation factor 4E-binding
protein (4E-BP), resulting in enhanced protein synthesis and cell
proliferation (6–8). This pathway is frequently activated in
numerous types of cancer and uncontrolled PI3K-AKT-mTOR signaling
may also result in a poor clinical outcome in lung, cervical,
ovarian and esophageal cancers (4,6).
mTOR was identified in 1994 by several groups of
investigators as the kinase targeted by rapamycin linked to the
cellular protein FKBP12 (FK506-binding protein) (9). It was therefore also named
FKBP-RAP-associated protein (FRAP), RAP FKBP12 target (RAFT) 1 and
RAP target (RAPT) 1. mTOR is a 289-kDa, ubiquitously expressed,
evolutionarily conserved serine/threonine protein kinase (9) which is important in cellular protein
synthesis and energy balance, affecting numerous aspects of cell
growth and proliferation, including differentiation, cell-cycle
progression, angiogenesis, protein degradation and apoptosis
(10). mTOR is also instrumental in
protein translation initiation (the rate-limiting step of protein
synthesis) by enabling the recruitment of ribosomes to mRNA by
eukaryotic initiation factor. Consequently, mTOR activates its
downstream mediator ribosomal S6K and is responsible for the
progression of the cell from G0/G1 to S phase (11). Consistent with its essential role in
cell growth, aberrant activity of the mTOR pathway is frequently
observed in a number of types of cancer (12).
Phosphatase and tensin homolog deleted on chromosome
10 (PTEN) is critical in cell growth, migration and death. It is
mutated or deleted at a high frequency in various human cancer
tissues to promote tumorigenesis (13). The PI3K-AKT-mTOR pathway is one of
the most upregulated pathways in neoplastic cells through
mechanisms such as PTEN loss of function or PI3K activating
mutations (14). PTEN antagonizes
PI3K pathways by dephosphorylating phosphatidylinositol
3,4,5-triphosphate to convert it back to phosphatidylinositol
4,5-biphosphate. Thus, PTEN is considered to be a negative
regulator of the PI3K-AKT-mTOR pathway (15).
The aim of the present study was to explore the
involvement of the PI3K-AKT-mTOR signaling pathway in the
progression of human gastric cancer. The expression levels of mTOR
and PTEN in human gastric cancer tissues were determined using
immunohistochemical study using biopsies from 33 patients and
correlations with pathological parameters and prognoses were
evaluated.
Materials and methods
Patients and tumor samples
Paraffin-embedded sections were obtained from
patients with gastric cancer who had undergone surgery at Renmin
Hospital of Wuhan University (Wuhan, China) between 2005 and 2008.
Tissues obtained from 30 cases of chronic superficial gastritis
diagnosed by gastroscopic biopsy were used as control samples for
the immunohistochemical staining. General informed consent with
regard to the use of the patients’ tissue specimens and clinical
information was obtained from all patients. None of the patients
recruited in this study had undergone chemotherapy or radiotherapy
prior to surgery. The patients’ clinicopathological data are
summarized in Table I. The
histological diagnosis was determined using hematoxylin and eosin
staining according to the WHO criteria (16). Pathological staging was performed
according to the American Joint Committee on Cancer (AJCC) Cancer
Staging Manual revised in 2010 (17). This study was approved by the
Institutional Ethics Board of the Renmin Hospital of Wuhan
University.
 | Table I.Clinicopathological parameters of the
patients in the study. |
Table I.
Clinicopathological parameters of the
patients in the study.
| Factors | Values |
|---|
| Age, years, mean
(range) | 51.6 ±11.6
(24–74) |
| Gender, n (%) | |
| Male | 15 (45.5) |
| Female | 18 (54.5) |
| Invasive depth, n
(%) | |
| Early stage | 6 (18.2) |
| Advanced
stage | 27 (81.8) |
| Differentiation, n
(%) | |
| Well and
moderate | 15 (45.5) |
| Poor | 18 (54.5) |
| Lymph node
metastasis, n (%) | |
| Positive | 16 (48.5) |
| Negative | 17 (51.5) |
| Pathological stage,
n (%) | |
| I+II | 18 (54.5) |
| III+IV | 15 (45.5) |
Immunohistochemical staining
For malin-fixed, paraffin-embedded tissue blocks
obtained from human tissue were cut into 4-μm thick sections and
mounted on adhesive-coated glass slides. mTOR was detected with a
rabbit monoclonal anti-mTOR antibody (Cell Signaling Technology,
Inc., Danvers, MA, USA) and PTEN was observed with a mouse
monoclonal anti-PTEN antibody (Maxim Biotechnology Development Co.
Ltd, Fuzhou, China). Sections were dewaxed in xylene and rehydrated
using graded ethanol and were then incubated in 3% solution of
hydrogen peroxide in methanol for 10 min to inactivate endogenous
peroxidase. This was followed by an antigen retrieval step. The
slides were placed in 0.01 mol/l citrate buffer solution (pH 6.0)
and heated (92–100°C) for 10 min in a microwave oven. Following a
wash with phosphate-buffered saline (PBS; pH 7.2) buffer, the
sections were covered with 2% normal serum for 20 min at room
temperature to eliminate non-specific binding of the antibody and
were then incubated overnight at 4°C with the primary antibodies
diluted in PBS. After washing with PBS, the secondary biotinylated
antibody was added for a 20 min incubation at 37°C. Slides were
then rinsed with PBS and treated with streptavidin-peroxidase
solution for 10 min. Tissue sections were washed once in PBS buffer
and covered with 3,3′-diaminobenzidine solution for 10 min.
Finally, the specimens were counterstained with hematoxylin. Normal
gastric sections served as positive controls while negative control
slides were incubated with the antibody diluents instead of the
primary antibody.
Evaluation of slides
Immunohistochemical staining was evaluated by 3
independent experienced pathologists who were blinded to the
clinicopathological parameters and clinical outcomes of the
patients. In cases of disagreement between the observers slides
were re-evaluated until a consensus was achieved. The sections were
examined at x200 magnification using light microscopy. The
immunostaining was considered to be positive when the neoplastic
cells exhibited specific immunoreactivity in the cytoplasm for mTOR
or in the nucleus for PTEN. The immunostaining results were
assessed semiquantitatively. For each sample the positive rate was
calculated according to the percentage of positive cells of all
counted cells from 5 randomly selected representative fields.
Additionally, the expression was classified according to the
percentage of stained tumor cells as low expression (−, <10%
positive carcinoma cells), intermediate expression (+, ≥10% and
<50% positive carcinoma cells) and high expression (++, ≥50%
positive carcinoma cells) (18).
Statistical analysis
Statistical analysis was performed using the PASW
18.0 software program for Windows. The results for the correlation
between mTOR and PTEN were evaluated using the Chi-squared test.
P<0.05 was considered to indicate statistically significant
differences.
Results
Differential expression of mTOR and PTEN
in human gastric cancer
The expression levels and cellular distribution of
mTOR and PTEN in the 33 specimens of human gastric cancer and 30
normal gastric tissues were examined by immunohistochemical
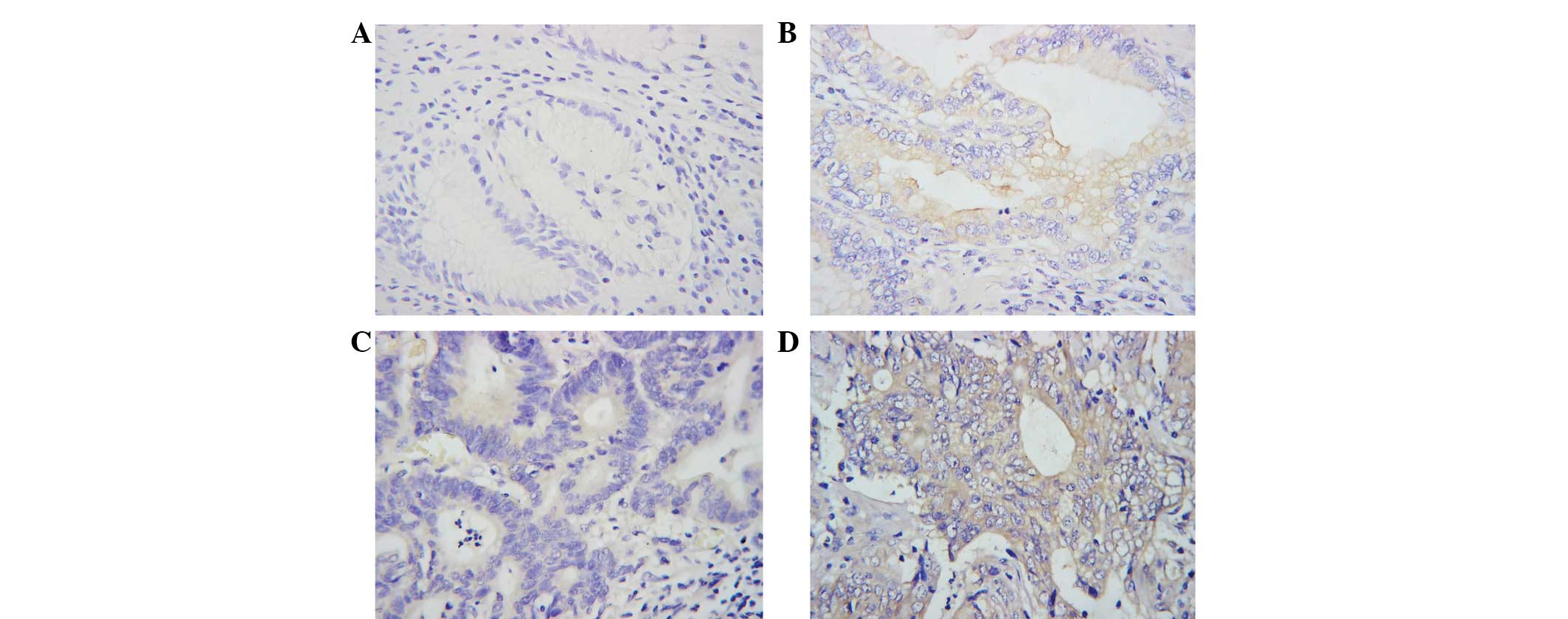
staining. mTOR was distributed mainly in the cytoplasm. Staining
was weaker in low-grade tumors. Positive mTOR expression was
observed in tumor cells in 51.5% (17/33) of the gastric cancer
patients. By contrast, little or no expression of mTOR was observed
in normal gastric tissues (Fig. 1).
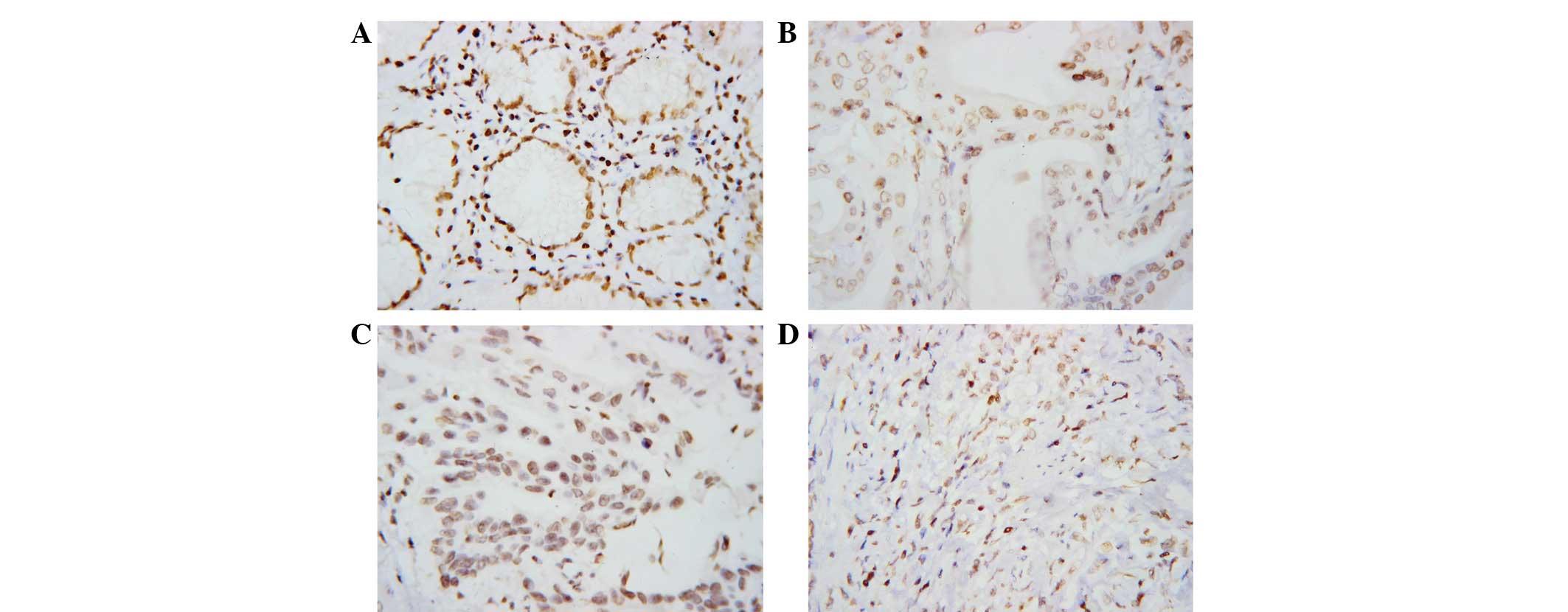
PTEN was distributed mainly in the nuclei. Staining was stronger in
low-grade tumors. Positive PTEN expression was observed in tumor
cells in 54.5% (18/33) of the gastric cancer patients (Fig. 2).
The expression of the mTOR and PTEN antigens was
assessed by immunohistochemical staining in sections obtained from
33 gastric cancer patients with various histological diagnoses and
pathological staging according to the AJCC handbook (Table II). In early and advanced cases, the
respective positive expression rates were 16.7% (1/6) and 59.3%
(16/27) for mTOR and 100.0% (6/6) and 44.4% (12/27) for PTEN. In
well- and moderately differentiated tissues and in poorly
differentiated specimens, the respective positive expression rates
were 26.7% (4/15) and 72.2% (13/18) for mTOR and 73.3% (11/15) and
38.9% (7/18) for PTEN. In patients with or without lymph node
metastasis, the respective positive expression rates were 81.3%
(13/16) and 23.5% (4/17) for mTOR and 18.8% (3/16) and 88.2%
(15/17) for PTEN. In stage I+II and stage III+IV, the respective
positive expression rates were 16.7% (3/18) and 93.3% (14/15) for
mTOR and 94.4% (17/18) and 6.7% (1/15) for PTEN.
 | Table II.Correlations between mTOR and PTEN
expression and clinicopathological characteristics in gastric
carcinoma cases. |
Table II.
Correlations between mTOR and PTEN
expression and clinicopathological characteristics in gastric
carcinoma cases.
| mTOR expression
| PTEN expression
|
|---|
| Factors | Positive (%) | Negative (%) | P-value | Positive (%) | Negative (%) | P-value |
|---|
| Gender | | | 0.849 | | | 0.407 |
| Male | 8 (53.3) | 7 (46.7) | | 7 (46.7) | 8 (53.3) | |
| Female | 9 (50.0) | 9 (50.0) | | 11 (61.1) | 7 (38.9) | |
| Age (years) | | | 0.221 | | | 0.009 |
| <54 | 10 (62.5) | 6 (37.5) | | 5 (31.3) | 11 (68.8) | |
| ≥54 | 7 (41.2) | 10 (58.8) | | 13 (76.5) | 4 (23.5) | |
| Invasive depth | | | 0.085 | | | 0.021 |
| Early stage | 1 (16.7) | 5 (83.3) | | 6 (100.0) | 0 (0) | |
| Advanced
stage | 16 (59.3) | 11 (40.7) | | 12 (44.4) | 15 (55.6) | |
|
Differentiation | | | 0.009 | | | 0.048 |
| Well and
moderate | 4 (26.7) | 11 (73.3) | | 11 (73.3) | 4 (26.7) | |
| Poor | 13 (72.2) | 5 (27.8) | | 7 (38.9) | 11 (61.1) | |
| Lymph node
metastasis | | | 0.001 | | | 0.000 |
| Positive | 13 (81.3) | 3 (18.8) | | 3 (18.8) | 13 (81.3) | |
| Negative | 4 (23.5) | 13 (76.5) | | 15 (88.2) | 2 (11.8) | |
| Pathological
stage | | | 0.000 | | | 0.000 |
| I+II | 3 (16.7) | 15 (83.3) | | 17 (94.4) | 1 (5.6) | |
| III+IV | 14 (93.3) | 1 (6.7) | | 1 (6.7) | 14 (93.3) | |
The expression of mTOR had a significant positive
correlation with differentiation, lymph node metastasis and
clinical staging (P<0.01), but was independent of gender, age
and invasive depth (P>0.05). The expression of PTEN was
negatively correlated with invasive depth and differentiation
(P<0.05) and significantly negatively correlated with age, lymph
node metastasis and clinical pathological staging (P<0.01), but
was not associated with gender (P>0.05).
Correlation between mTOR and PTEN in
human gastric cancer
When the expression of mTOR was analyzed with regard
to PTEN expression, the staining pattern was divided into 4 groups:
mTOR−/PTEN−, mTOR+/PTEN+,
mTOR+/PTEN− and mTOR−/ PTEN+. A
comparison of the mTOR−/PTEN+ and
mTOR+/PTEN− groups (Table III) revealed that that in early
gastric cancer, the size of the former group was larger than the
that of latter and that the differences between the 2 groups were
statistically significant with regard to invasive depth (P=0.041).
In well- and moderately differentiated gastric cancer, the
mTOR−/PTEN+ group was larger than the
mTOR+/PTEN− group and the difference between the 2
groups was statistically significant with regard to differentiation
(P=0.012). In patients without lymph node metastasis, the
mTOR−/PTEN+ group was larger than the
mTOR+/PTEN− group and the difference between
the 2 groups was statistically significant with regard to lymph
node metastasis (P=0.000). In stage I+II, the
mTOR−/PTEN+ group was larger than the
mTOR+/PTEN− group and the difference between
two groups was statistically significant with regard to
pathological stage (P=0.000).
 | Table III.Co-expression of mTOR and PTEN and
clinicopathological characteristics. |
Table III.
Co-expression of mTOR and PTEN and
clinicopathological characteristics.
| Factors |
mTOR−/PTEN+ |
mTOR+/PTEN− | P-value |
|---|
| Invasive depth | | | |
| Early stage | 5 | 0 | 0.041 |
| Advanced
stage | 9 | 13 | |
|
Differentiation | | | |
| Well and
moderate | 10 | 3 | 0.012 |
| Poor | 4 | 10 | |
| Lymph node
metastasis | | | |
| Positive | 2 | 12 | 0.000 |
| Negative | 12 | 1 | |
| Pathological
stage | | | |
| I+II | 14 | 0 | 0.000 |
| III+IV | 0 | 13 | |
Discussion
Previous studies have suggested that the
PI3K-AKT-mTOR pathway is frequently activated in various types of
cancer and that this pathway is considered to be important for
cancer cell survival, proliferation, angiogenesis and resistance to
chemotherapy (19–21). However, the activated molecule of
the PI3K-AKT-mTOR pathway in gastric cancer has not yet been
studied. In the present study, 33 cases of gastric cancer were
investigated and statistical analyses were performed concerning the
correlation between the clinicopathological parameters in gastric
cancer and the immunohistochemical expression levels of mTOR and
PTEN. The results indicated that mTOR and PTEN were negatively
correlated in the pathogenesis of gastric cancer. The
overexpression of mTOR and low expression of PTEN proteins were
strongly correlated with the pathological staging. These results
suggested that mTOR and PTEN may be clinically useful prognostic
markers and may provide additional information for the histological
diagnosis and pathological staging of gastric cancer.
Possible correlations between mTOR expression in
gastric cancer and pathological parameters were investigated. The
present study demonstrated that mTOR was activated in human gastric
cancer and was significantly correlated with invasive depth,
differentiation and lymph node metastasis, suggesting that the high
expression of mTOR contributes to the progression and metastasis of
gastric cancer. Positive mTOR expression was detected in tumor
cells in 51.5% (17/33) of gastric cancer patients, while little or
no expression was observed in normal gastric tissues. Furthermore,
93.3% (14/15) of gastric cancer patients had positive expression of
mTOR at stage III+IV suggesting that the hyperactivation of mTOR
kinase was a late event in the development of gastric cancer.
Similarly, the positive expression rate of mTOR was high for those
patients with lymph node metastasis (81.3%, 13/16). mTOR
immunoreactivity intensity data revealed that the high expression
levels of mTOR significantly increased with the tumor
progression.
It has been reported that mTOR is a powerful
oncoprotein overexpressed in numerous types of cancer, including
hepatocellular carcinoma (22),
lung cancer (23), esophageal
squamous cell carcinoma (24) and
breast cancer (25,26). Furthermore, patients with breast
cancer and mTOR overexpression had a risk of recurrence 3 times
greater than that of patients without mTOR overexpression (25,27).
The mTOR inhibitor everolimus has demonstrated promising clinical
efficacy in a phase III randomized and double-blind trial in
patients with metastatic renal cell cancer (28). The exact mechanisms for the
overexpression of mTOR in carcinoma remain unclear. As a highly
conserved, ubiquitously expressed signaling molecule, mTOR is
activated downstream of multiple distinct growth factor receptors
and is crucial for mediating cell proliferation and survival
(29). Several mTOR inhibitors,
including rapamycin/sirolimus (Wyeth) and derivatives, such as
RAD001/everolimus (Novartis), CCI-779/temsirolimus (Wyeth) and
AP23573 (Ariad), are being developed as anti-cancer agents against
various types of malignancies (30).
By contrast, PTEN was shown by immunohistochemistry
to be expressed in normal gastric tissues and almost all the early
gastric cancer cases. The underexpression of PTEN was significantly
correlated with invasive depth, differentiation and lymph node
metastasis. Approximately one-half of the gastric cancer patients
exhibited a loss of PTEN expression. Additionally, the loss of PTEN
expression was correlated with invasive depth and differentiation
(P<0.05) and closely correlated with lymph node metastasis and
clinical pathological staging (P<0.01). PTEN immunoreactivity
intensity data were also analyzed and the results revealed that the
high expression levels of PTEN significantly decreased with the
tumor progression.
PTEN, which is located at human chromosome 10q23,
has been identified as a tumor suppressor gene and an important
negative regulator of the PI3K-AKT-mTOR signaling pathway that
promotes cell proliferation and inhibits apoptosis (31). Inactivation of PTEN by mutation,
deletion and promoter hypermethylation has been demonstrated in a
range of cancer types, including lung, breast, prostate and
esophageal carcinomas (31–35). Langlois et al observed that
PTEN controls the cellular polarity, establishment of cell-cell
junctions, paracellular permeability, migration and tumorigenic
potential of human colorectal cancer cells (36). Varied analysis of colorectal
carcinomas suggested that the patients without PTEN expression had
shorter survival times than the patients with PTEN expression
(P=0.003) (37). Abnormal
expression of PTEN may predict the metastasis and prognosis of
gastric cancer (21,38,39).
We concluded that mTOR facilitated the development
of gastric cancer while PTEN, a tumor suppressor gene, was able to
inhibit tumor invasion and metastasis. mTOR and PTEN co-regulate
the progression of tumors and participate in proliferation,
invasion and metastasis in gastric cancer. Bakarakos et al
discovered that the loss of PTEN and activation of mTOR was closely
correlated with breast cancer (40). In vitro studies suggested
that PTEN is capable of inhibiting cell proliferation and promoting
apoptosis via inhibition of the activity of the PI3K-Akt-mTOR
pathway (41). The combined
deletion of PTEN and Lkb1 in the mouse bladder significantly
activated the mTOR pathway and increased bladder epithelial cell
proliferation and tumorigenesis (42). When we compared the
mTOR-/PTEN+ and mTOR+/PTEN− groups, the
differences between them were statistically significant with regard
to invasive depth, histological type, lymph node metastasis and
pathological stage. Consequently, collaborative detection of mTOR
and PTEN expression may be more useful in the diagnosis of gastric
cancer.
In summary, upregulated expression of mTOR and
downregulated expression of PTEN were involved in carcinogenesis
and progression of gastric cancer. A negative correlation between
mTOR and PTEN expression implied that their modified expression may
be important in the pathogenesis, invasion and metastasis of
carcinoma tissue. Combined detection of mTOR and PTEN expression
may be used to evaluate the degree of malignancy in gastric cancer,
which may be a useful marker for the early diagnosis of gastric
cancer. Further studies with more patients, including follow-up and
different molecular biomarkers in addition to these two molecules,
would aid the clarification of the disease pathogenesis and
identification of potential therapeutic approaches.
Acknowledgements
The author Min Li gratefully
acknowledges the assistance of his elder sister Li Li for her
critical reading of the manuscript before its submission. We also
gratefully acknowledge the assistance of Xinyu Qin, Huawen Sun and
Lujun Song in the preparation of this study.
References
|
1.
|
K Washington7th edition of the AJCC cancer
staging manual: stomachAnn Surg
Oncol1730773079201010.1245/s10434-010-1362-z20882416
|
|
2.
|
S LiangL HeX ZhaoMicroRNA let-7f inhibits
tumor invasion and metastasis by targeting myh9 in human gastric
cancerPloS One6e18409201110.1371/journal.pone.001840921533124
|
|
3.
|
DH RoukosTargeting gastric cancer with
trastuzumab: new clinical practice and innovative developments to
overcome resistanceAnn Surg
Oncol171417201010.1245/s10434-009-0766-019841980
|
|
4.
|
N BalNE KocerME ErtorerET CanpolatF
KayaselcukMaspin, E-selectin, and P-selectin expressions in
papillary thyroid carcinomas and their correlation with prognostic
parametersPathol Res
Pract204743750200810.1016/j.prp.2008.04.01618597952
|
|
5.
|
X ChenJ LiaoY LuX DuanW SunActivation of
the PI3K Akt pathway mediates bone morphogenetic protein 2-induced
invasion of pancreatic cancer cells Panc-1Pathol Oncol
Res17257261201110.1007/s12253-010-9307-120848249
|
|
6.
|
Z XuY ZhangJ JiangEpidermal growth factor
induces HCCR expression via PI3K/Akt/mTOR signaling in PANC-1
pancreatic cancer cellsBMC
Cancer10161201010.1186/1471-2407-10-16120423485
|
|
7.
|
AP BhattPM BhendeSH SinD RoyDP DittmerB
DamaniaDual inhibition of PI3K and mTOR inhibits autocrine and
paracrine proliferative loops in PI3K/Akt/mTOR-addicted
lymphomasBlood11544554463201010.1182/blood-2009-10-25108220299510
|
|
8.
|
M SajiMD RingelThe PI3K-Akt-mTOR pathway
in initiation and progression of thyroid tumorsMol Cell
Endocrinol3212028201010.1016/j.mce.2009.10.01619897009
|
|
9.
|
S VignotS FaivreD AguirreE
RaymondmTOR-targeted therapy of cancer with rapamycin
derivativesAnn Oncol16525537200510.1093/annonc/mdi11315728109
|
|
10.
|
D MahalingamK SankhalaA MitaFJ GilesMM
MitaTargeting the mTOR pathway using deforolimus in cancer
therapyFuture Oncol5291303200910.2217/fon.09.919374536
|
|
11.
|
CB ChingDE HanselExpanding therapeutic
targets in bladder cancer: the PI3K/Akt/mTOR pathwayLab
Invest9014061414201010.1038/labinvest.2010.13320661228
|
|
12.
|
JS CarewKR KellyST NawrockiMechanisms of
mTOR inhibitor resistance in cancer therapyTarget
Oncol61727201110.1007/s11523-011-0167-821547705
|
|
13.
|
A YoshimiS GoyamaN Watanabe-OkochiEvi1
represses PTEN expression and activates PI3K/AKT/mTOR via
interactions with polycomb
proteinsBlood11736173628201110.1182/blood-2009-12-26160221289308
|
|
14.
|
M MireutaA DarnelM PollakIGFBP-2
expression in MCF-7 cells is regulated by the PI3K/AKT/mTOR pathway
through Sp1-induced increase in transcriptionGrowth
Factors28243255201010.3109/0897719100374547220370577
|
|
15.
|
C LiuJL WuK XuNeuroprotection by baicalein
in ischemic brain injury involves PTEN/AKT pathwayJ
Neurochem11215001512201010.1111/j.1471-4159.2009.06561.x20050973
|
|
16.
|
SR HamiltonLA AaltonenPathology and
Genetics of Tumors of the Digestive SystemWHO PressGeneva2000
|
|
17.
|
SB EdgeDR ByrdCC ComptonAG FritzF GreeneA
TrottiAJCC cancer staging manual7th editionSpringerNew York2010
|
|
18.
|
X HeQ WeiX ZhangImmunohistochemical
expression of CXCR4 in thyroid carcinomas and thyroid benign
lesionsPathol Res
Pract206712715201010.1016/j.prp.2010.05.00320646838
|
|
19.
|
HY NiuJH WangH LiP HeRapamycin potentiates
cytotoxicity by docetaxel possibly through downregulation of
Survivin in lung cancer cellsJ Exp Clin Canc
Res3028201110.1186/1756-9966-30-2821392382
|
|
20.
|
M ChenJ GuGL DelclosGenetic variations of
the PI3KAKT-mTOR pathway and clinical outcome in muscle invasive
and metastatic bladder cancer
patientsCarcinogenesis3113871391201010.1093/carcin/bgq11020530239
|
|
21.
|
M LiL SongX QinGlycan changes: cancer
metastasis and anti-cancer vaccinesJ
Biosci35665673201010.1007/s12038-010-0073-821289447
|
|
22.
|
F SahinR KannangaiO AdegbolaJZ WangG SuM
TorbensonmTOR and P70 S6 kinase expression in primary liver
neoplasmsClin Cancer
Res1084218425200410.1158/1078-0432.CCR-04-094115623621
|
|
23.
|
K SchmidZ Bago-HorvathW BergerDual
inhibition of EGFR and mTOR pathways in small cell lung cancerBr J
Cancer103622628201010.1038/sj.bjc.660576120683448
|
|
24.
|
K HirashimaY BabaM WatanabePhosphorylated
mTOR expression is associated with poor prognosis for patients with
esophageal squamous cell carcinomaAnn Surg
Oncol1724862493201010.1245/s10434-010-1040-120339946
|
|
25.
|
G YuJ WangY ChenOverexpression of
phosphorylated mammalian target of rapamycin predicts lymph node
metastasis and prognosis of Chinese patients with gastric
cancerClin Cancer
Res1518211829200910.1158/1078-0432.CCR-08-213819223493
|
|
26.
|
J AnH JeongY LeeSU WooJH SeoA
KimPhosphorylated Akt and phosphorylated mTOR expression in breast
invasive carcinomas: analysis of 530 casesJ Breast
Cancer13337348201010.4048/jbc.2010.13.4.337
|
|
27.
|
S BoseS ChandranJM MirochaN BoseThe Akt
pathway in human breast cancer: a tissue-array-based analysisMod
Pathol19238245200610.1038/modpathol.3800525
|
|
28.
|
RJ MotzerB EscudierS OudardEfficacy of
everolimus in advanced renal cell carcinoma: a double-blind,
randomised, placebo-controlled phase III
trialLancet372449456200810.1016/S0140-6736(08)61039-918653228
|
|
29.
|
M MarinovA ZiogasOE PardoAKT/mTOR pathway
activation and BCL-2 family proteins modulate the sensitivity of
human small cell lung cancer cells to RAD001Clin Cancer
Res1512771287200910.1158/1078-0432.CCR-08-216619228731
|
|
30.
|
KH TamZF YangCK LauCT LamRW PangRT
PoonInhibition of mTOR enhances chemosensitivity in hepatocellular
carcinomaCancer
Lett273201209200910.1016/j.canlet.2008.08.01818824293
|
|
31.
|
KS JangYS SongSH JangClinicopathological
significance of nuclear PTEN expression in colorectal
adenocarcinomaHistopathology56229239201010.1111/j.1365-2559.2009.03468.x20102402
|
|
32.
|
T AndjelkovicJ BankovicJ
StojsicCoalterations of p53 and PTEN tumor suppressor genes in
non-small cell lung carcinoma patientsTransl
Res1571928201110.1016/j.trsl.2010.09.00421146147
|
|
33.
|
AM Gonzalez-AnguloJ Ferrer-LozanoK
Stemke-HalePI3K pathway mutations and PTEN levels in primary and
metastatic breast cancerMol Cancer
Ther1010931101201110.1158/1535-7163.MCT-10-108921490305
|
|
34.
|
DJ MulhollandLM TranY LiCell autonomous
role of PTEN in regulating castration-resistant prostate cancer
growthCancer Cell19792804201110.1016/j.ccr.2011.05.00621620777
|
|
35.
|
G HouZ LuM LiuH LiuL XueMutational
analysis of the PTEN gene and its effects in esophageal squamous
cell carcinomaDig Dis
Sci5613151322201110.1007/s10620-010-1474-021116717
|
|
36.
|
MJ LangloisS BergeronG BernatchezThe PTEN
phosphatase controls intestinal epithelial cell polarity and
barrier function: role in colorectal cancer progressionPloS
One5e15742201010.1371/journal.pone.001574221203412
|
|
37.
|
XH LiHC ZhengH TakahashiS MasudaXH YangY
TakanoPTEN expression and mutation in colorectal carcinomasOncol
Rep22757764200919724853
|
|
38.
|
CY GuoXF XuJY WuSF LiuPCR-SSCP-DNA
sequencing method in detecting PTEN gene mutation and its
significance in human gastric cancerWorld J
Gastroenterol1438043811200810.3748/wjg.14.380418609703
|
|
39.
|
M LiL SongX GaoW ChangX QinToll-like
receptor 4 on islet β cells senses expression changes in
high-mobility group box 1 and contributes to the initiation of type
1 diabetesExp Mol Med442602672012
|
|
40.
|
P BakarakosI TheohariA
NomikosImmunohistochemical study of PTEN and phosphorylated mTOR
proteins in familial and sporadic invasive breast
carcinomasHistopathology56876882201010.1111/j.1365-2559.2010.03570.x20636791
|
|
41.
|
ZY ChengXL GuoXY YangPTEN and rapamycin
inhibiting the growth of K562 cells through regulating mTOR
signaling pathwayJ Exp Clin Cancer
Res2787200810.1186/1756-9966-27-8719115995
|
|
42.
|
BY ShorningD GriffithsAR ClarkeLkb1 and
Pten synergise to suppress mTOR-mediated tumorigenesis and
epithelial-mesenchymal transition in the mouse bladderPloS
One6e16209201110.1371/journal.pone.001620921283818
|