Introduction
Pathological proliferation of the plasma cell
population produces a wide spectrum of disorders, ranging from
benign solitary plasmacytoma to malignant multiple myeloma
(1). Myeloma is a type of clonal
hematopathy. Solitary plasmacytoma of the skull is a rare plasma
cell tumor which represents the proliferation of monoclonal plasma
cells and produces monoclonal immunoglobulin. Osteolytic skull
lesions are commonly observed in routine clinical work. The
appearance of a single osteolytic plasmacytoma of the skull without
signs of systemic myelomatosis is extremely rare (2,3). The
prognosis for solitary plasmacytoma of the cranial vault appears to
be good when it is diagnosed on strict criteria, which is based on
a radiologically solitary bone lesion, neoplastic plasma cells in
the biopsy specimen, <5% plasma cells in bone marrow, <2.0
g/dl monoclonal protein in the serum when present and negative
urine test for Bence Jones protein (monoclonal light chain)
(4). Hence, making the appropriate
diagnosis is critical. The present study describes two cases of
skull solitary plasmacytoma, discusses the relevant literature
concerning this disease and raises the issue of newer diagnosis and
therapy modalities for this disease. The study was approved by the
ethics committee of the Clinical Medical College of Yangzhou
University, Yangzhou, China and informed consent was obtained from
each patient’s family.
Case reports
Case 1
A 70-year-old female with a 10-year history of
diabetes mellitus first noted a rubbery swelling 4×5 cm in diameter
in the parietal region in June 2003. Neurological examination
identified a relatively fixed mass with no tenderness and no
abnormalities. Computed tomography (CT) showed a large extradural
mass with homogeneous enhancement following intravenous
administration of contrast material, and bone CT revealed a
solitary osteolytic lesion involving the whole layer of the skull.
Cranial magnetic resonance imaging (MRI) scan revealed that the
occupying lesion in the right frontal and parietal skull was mostly
isointense with the brain parenchyma on both T1- and T2-weighted
images and was homogeneously enhanced. Laboratory examinations
showed a red blood cell count of 3.15×1012/l; hemoglobin
(HGB), 109 g/l; white blood cell (WBC) count,
4.8×109/l; neutrophils, 49.5%; lymphocytes, 39.5%; which
were all within the normal range. A urine test for Bence Jones
protein was negative. Renal function showed abnormal levels of urea
nitrogen (BUN; 9.38 mmol/l) and creatinine (CRE; 203.6
μmol/l).
The patient underwent a craniectomy under general
anesthesia on June 6, 2003. An ∼28-cm horseshoe-shaped incision was
made in the right frontal-parietal bone. The tumor extended to the
subcutaneous and the subdural space through the dura mater with
skull defects ∼4×4 cm. The tumor was an enhancing extracranial mass
∼4×4×1.5 cm. It was purple, soft, had a rich blood supply and was
easily separated from the skull. The marginal bone around the tumor
was rongeured out to ensure the complete removal of the tumor. The
tumor was completely resected, including the marginal bone but
without dural lesions, forming a bone window ∼7×8 cm in size. The
tumor was ∼5×6×3 cm.
Pathological diagnosis of the tumor was plasmacytoma
(right frontoparietal; Fig. 1).
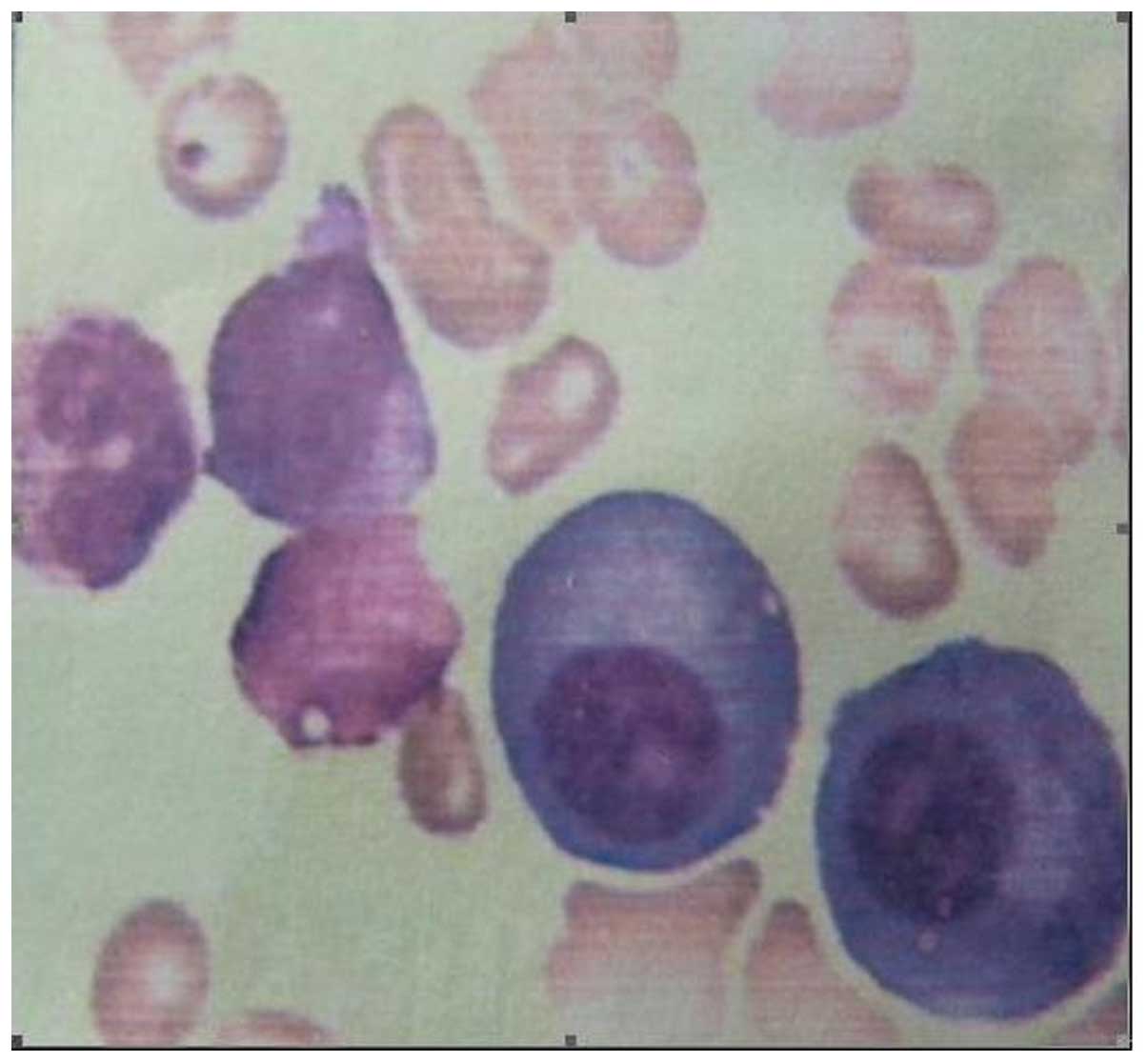
Bone marrow aspiration revealed multiple systemic myelomatosis, and
displayed an increasing quantity of plasmacytes after each period
of time (Fig. 2). X-ray revealed
signs of multiple myeloma in the skull and pelvis.Chemotherapy
(melphalan) treatment was administered in the Department of
Hematology and started on June 13, 2003. Immunohistochemistry
showed embryonal membrane antigen (EMA)(−), GFAP(−) and an
erythrocyte sedimentation rate of 120 mm/h. The
immunoelectrophoresis of serum proteins revealed that the levels of
immunoglobulins (Igs) were: IgG, 4.54 g/l; IgM, 0.17 g/l; IgA 14.9
g/l, β-2 microglobulin, 2.35 mg/l; all were within the normal
range. The patient received postoperative chemotherapy. Two years
later, CT review was unable to identify the tumor.
Case 2
A 75-year-old female first noted a rubbery swelling
in the right parietal bone for half a month in April 2010. There
was a subcutaneous lump ∼3×3 cm in size at the right parietal bone.
The border of the mass was clear, smooth and soft, and there was no
tenderness. The skull around the lump was defective. The patient
had a history of diabetes and renal disease. Cranial CT and MRI
scan and enhancement showed the osteolytic defects in the right
parietal bone (Figs. 3 and 4). Chest CT scan showed no abnormality.
Laboratory examinations revealed a red blood cell count of
2.59×1012/l; WBC count, 4.1×109/l;
neutrophils, 74.5%; lymphocytes, 19.6%; HGB, 79 g/l; which were all
within the normal range. Renal function tests showed renal
dysfunction and abnormal levels of BUN (18.36 mmol/l) and CRE
(381.6 μmol/l). A test for Bence Jones protein in the urine
was weakly positive. The tumor markers carcinoembryonic antigen
(CEA; 1.65 ng/ml) and rapid plasma reagin (RPR; negative) were both
within the normal range, but the level of serum β-2 microglobulin
(5.31 mg/l) was abnormal. Bone marrow aspiration revealed evidence
of multiple myeloma. The surgery was abandoned, and the patient was
treated with chemotherapy and radiation therapy. After 18 months,
CT review was unable to identify the tumor.
Discussion
Myeloma is a malignant tumor which originates from
the reticulocytes of the bone marrow. The tumor cells have the
characteristics of an increasing quantity of plasmacytes, so the
disease was known as plasma cell myeloma (2). Plasma cell myeloma mostly occurs in
the elderly over 40 years of age; the median age of individuals
with the disease in the United States was 62 years old (2). Only 2–3% of patients with the disease
are younger than 30 years old (3).
The two patients described in the present study were over the age
of 70, and this is similar to the literature. Bone destruction due
to myeloma may occur in any area of the body. Its incidence is as
follows: spine, 49%; skull, 35%; pelvis, 34%; ribs, 33%; humerus,
22%; femur, 13%; mandible, 10% (2).
Tumors which occur in the skull are called cranial myelomas and are
also known as cranial plasma cell tumors. Single tumors are rarely
seen in the clinic (3). The
preoperative diagnosis of Case 1 was meningioma, the postoperative
pathological diagnosis was plasma cell tumor and the bone marrow
examination made a definite diagnosis of multiple myeloma. The
imaging diagnosis of Case 2 was metastatic tumors, but during the
preoperative discussion the anemia and renal dysfunction were found
and skull myeloma was suspected. The surgery was abandoned, as the
diagnosis from the bone marrow examination was multiple
myeloma.
The diagnosis of skull myeloma also needs to be
differentiated from eosinophilic granuloma, osteosarcoma and
metastatic carcinoma (3).Eosinophilic granuloma is mainly
observed in children and young individuals, and most occur singly
(85%), which aids the identification of skull myeloma. Eosinophilic
granuloma may mostly exhibit bone lesions or a large number of
eosinophilic granulomas. The skull is the predilection site, with
tumors often located in the frontal, temporal and parietal bones.
Tumors have a rich blood supply but clear boundaries from the
surrounding tissues. The size of eosinophilic granuloma is usually
small, rarely more than 2–3 cm. The edge of the tumor often has a
‘clivus-like’ or ‘bilateral-like’ appearance, and a sequestrum of
the ‘button’ type may be observed. Osteosarcoma is a common primary
maligant bone tumor which is mostly found in long bones, with few
cases in the skull. Osteosarcoma may be divided into osteolytic
type, bone and mixed type. The osteolytic type mainly shows bone
destruction with a round or irregular shape, with blurred contours.
The bone destruction mainly occurs in the outer cranial plate and
large extracranial soft tissue is often observed, in which the bone
tumor may be found. Osteolytic metastasis is the most common type
of metastasis, and the metastases are often multiple and of a range
of sizes, with osteolytic bone destruction. The edges of metastases
may be blurred and there may be an associated small adjacent soft
tissue mass. A small number of osteolytic metastases show single
bone destruction with a larger soft tissue mass in which residual
bone chips may be observed.
Naganuma et al suggested that laboratory
examination should include bone marrow examination, serum protein
electrophoresis, serum immunoglobulins, blood, urine Bence Jones
protein and kidney function (5).
The International Myeloma Working Group proposed new
criteria for the diagnosis and classification of myeloma based on
routinely available examinations. According to the criteria,
symptomatic myeloma requires evidence of an M-protein in the serum
and urine, bone marrow plasmacytosis and related end-organ damage
(6). The criteria for asymptomatic,
or smouldering, myeloma are M-protein levels ≥30 g/l and/or bone
marrow clonal cells ≥10%, but no related organ or tissue impairment
(ROTI; end-organ damage). Cases with ROTI typically present with
increased calcium levels, renal insufficiency, anemia or bone
lesions, which are attributed to the proliferation of plasma cells.
Symptomatic myeloma requires evidence of ROTI. Solitary
plasmacytoma of bone, extramedullary plasmacytoma and multiple
solitary plasmacytomas (+/− recurrent) are also defined as distinct
entities. The use of these criteria should facilitate the
comparison of therapeutic trial data (7). The results of the bone marrow
examination confirmed the diagnosis in Case 2.
Prior to 2011, there were only hundreds of cases of
solitary plasmacytoma reported in the English literature (8). In cases with no lesions in other parts
of the body, the patients have good prognosis following surgical
resection and radiotherapy. Chemotherapy is being increasingly used
in the treatment of plasma cell myeloma, but radiotherapy is being
used less. The prognosis of multiple myeloma is not as good as
solitary plasmacytoma (9).
The patient described in Case 1 underwent a
frontal-temporal bone craniectomy. During the surgery it is
important to control bleeding, and new methods different from the
conventional procedure for craniotomy should be selected. If the
bone milling cutters or wire sawing are used for craniotomy, it is
difficult to stop the bleeding in time and greatly increases the
risk of surgery. Since the blood supply of myeloma tumors is mainly
taken from the surrounding skull, the process of blocking the blood
supply should be performed during surgery on the calvaria. Using
the rongeur, while cutting the skull along the edge of tumor up to
the normal tissue, discontinuous dural suspension should be carried
out. Using the method above, we may effectively reduce blood loss.
We should also pay more attention to myeloma in the skull base,
since it is more difficult to control bleeding there.
The characteristics of myeloma are complicated.
Plasmacytoma of the skull has a wide spectrum of pathology,
including a quite benign, solitary plasmacytoma (SPC), and an
extremely malignant, multiple myeloma (MM) at the two ends of the
spectrum. The clinical features are complex and not easily
identified, leading to the high misdiagnosis rate. A comprehensive
examination and analysis is needed for correct diagnosis, which
includes immunoglobulin, biochemistry, urine Bence Jones protein
and bone marrow (10). If the CT
scan shows changes in the bone and cartilage, the meta-analysis
should be performed to identify the diagnosis.
References
|
1.
|
Joshi A, Jiang D, Singh P and Moffat D:
Skull base presentation of multiple myeloma. Ear Nose Throat J.
90:E6–E9. 2011.PubMed/NCBI
|
|
2.
|
George ED and Sadovsky R: Multiple
myeloma: recognition and management. Am Fam Physician.
59:1885–1894. 1999.PubMed/NCBI
|
|
3.
|
Wein RO, Popat SR, Doerr TD and Dutcher
PO: Plasma cell tumors of the skull base: four case reports and
literature review. Skull Base. 12:77–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Tanaka M, Shibui S, Nomura K and Nakanishi
Y: Solitary plasmacytoma of the skull: a case report. Jpn J Clin
Oncol. 28:626–30. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Naganuma H, Sakatsume S, Sugita M, Satoh
E, Asahara T and Nukui H: Solitary plasmacytoma of the skull:
immunohistochemical study of angiogenic factors and syndecan-1 -
two case reports. Neurol Med Chir (Tokyo). 44:195–200. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hotta T: Classification, staging and
prognostic indices for multiple myeloma. Nihon Rinsho.
65:2161–2166. 2007.(In Japanese).
|
|
7.
|
International Myeloma Working Group:
Criteria for the classification of monoclonal gammopathies,
multiple myeloma and related disorders: a report of the
International Myeloma Working Group. Br J Haematol. 121:749–757.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lorsbach RB, Hsi ED, Dogan A and Fend F:
Plasma cell myeloma and related neoplasms. Am J Clin Pathol.
136:168–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kyle RA, Therneau TM, Rajkumar SV, et al:
Incidence of multiple myeloma in Olmsted County, Minnesota: Trend
over 6 decades. Cancer. 101:2667–2674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shaheen SP, Talwalkar SS and Medeiros LJ:
Multiple myeloma and immunosecretory disorders: an update. Adv Anat
Pathol. 15:196–210. 2008. View Article : Google Scholar : PubMed/NCBI
|