Introduction
Lung cancer is the leading cause of cancer-related
mortality in the United States and Europe, and 80% of cases are
diagnosed as non-small cell lung cancer (NSCLC). Although NSCLC
frequently develops brain metastasis, effective treatment and
prevention for brain metastasis remain unavailable (1,2).
Metastases form when specialized tumor cells
(‘seeds’) find a suitable environment (‘soil’) to arrest, invade
and grow (3). Metastasis patterns
to different organs are dependent on the tumor cell phenotype and
interactions between the tumor cell and the environment, such as
cellular components, cytokines and organ-derived growth factors
(4).
In the central nervous system, glial cells, which
have traditionally been viewed as providing structural support for
neurons, also play an important role in maintaining homeostasis.
Astrocytes support immune defense in the brain and protect neuronal
cells from waste products and hypoxic damage. Astrocytes also
produce a wide variety of cytokines, including interleukin-1
(IL-1), interleukin-3 (IL-3), interleukin-6 (IL-6), tumor necrosis
factor-α (TNF-α), transforming growth factor-β (TGF-β),
insulin-like growth factor-1 (IGF-1) and platelet-derived growth
factor (PDGF). Cytokines released by activated microglia include
IL-1, IL-6, TNF-α, monocyte chemoattractant protein-1 (MCP-1) and
macrophage inflammatory protein-1 (MIP-1). MCP-1 recruits NK cells
and a subpopulation of T-lymphocytes. MIP-1 attracts
activated/memory T-cells, monocytes/macrophages, and immature
dendritic cells (5–7).
It has recently been postulated that maintaining
E-cadherin expression could potentially block increases in motility
and invasion during epithelial-to-mesenchymal transitions (EMT). It
has further been suggested that the brain microenvironment could be
affected by pre-treatment with peroxisome proliferator-activated
receptor γ (PPARγ)-activating drugs, even before brain metastasis
(8).
Rapamycin, a lipophilic macrolide antibiotic, was
originally identified as a fungicide and immunosuppressant. Studies
have revealed, however, that rapamycin can potently arrest the
growth of cells derived from a broad spectrum of cancers. Rapamycin
has been shown to specifically inhibit mammalian target of
rapamycin (mTOR), which is a key player in tumor development and
progression (9). Rapamycin can
impede tumor metastasis by suppressing tumor angiogenesis and
lymphangiogenesis (10,11).
A dynamic interaction likely exists between cancer
cells and the host microenvironment to support cancerous growth and
spread. The aim of this study was to identify interactions between
astrocytes and rapamycin in brain metastases of NSCLC.
Materials and methods
Apoptosis assay
NCI-H358, a human lung adenocarcinoma cell line, was
obtained from the American Tissue Culture Collection (Manassas, VA,
USA). NCI-H358 tumor cells (1×104 cells/well) were
plated in 96-well flat bottomed tissue culture plates and incubated
at 37°C in a 5% CO2/95% air atmosphere. Rapamycin (10 or 100
μg/ml) was then added to tumor cells cultured alone or
co-cultured with astrocytes. Following 24, 48 or 72 h rapamycin
treatment, 10 μl MTT
(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) stock
solution (EZ Cytox, Daeil Lab Service Co., Seoul, Korea) was added
to each well, and the plates were incubated for 4 h. Plates were
agitated on a plate shaker for 3 sec, and the absorbance at 540 nm
was determined using a scanning multi-well spectrophotometer (VERSA
max, Sunnyvale, CA, USA).
Co-culture of astrocytes and NCI-H358,
and rapamycin administration
Immortalized astrocytes were plated on sterile 0.4
μm cell culture inserts (Becton-Dickinson Labware, Franklin Lakes,
NJ, USA) at a density of 4×105 cells in 1 ml Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% fetal bovine serum
(FBS). The inserts were gently transferred to empty 6-well plates
and placed in at 37°C. NCI-H358 lung adenocarcinoma cells were
plated in 6-well plates at a density of 5×105 cells/well
in 2 ml of RPMI-1640 containing 10% FBS. After incubating
overnight, the medium of both cultures was removed and replaced
with serum-free medium. Inserts containing astrocytes were
transferred to 6-well plates containing tumor cells and co-cultured
for 24, 48 and 72 h. Control samples consisted of cell-free inserts
placed in 6-well plates containing tumor cells. At each time point
the inserts were discarded and the tumor cells were washed with
ice-cold PBS and lysed with buffer. NCI-H358 cells alone and
co-cultured with astrocytes were treated with rapamycin (100 g/ml)
24 h later.
This study was approved by the Ethics Committee of
St. Vincent’s Hospital, The Catholic University of Korea, Suwon,
Korea.
Inoculation of intracranial cancer cells
and experimental design
The nude mice were anesthetized with an
intraperitoneal (i.p.) injection of 12 mg/kg xylazine (Rompun;
Cutter Laboratories, Shawnee, KS, USA) and 30 mg/kg ketamine
(Ketalar; Parke-Davis & Co., Morris Plains, NJ, USA). The mice
were then stereotaxically inoculated with 1×106 NCI-H358
cells into the right frontal lobe (2 mm lateral and 1 mm anterior
to the bregma, at a depth of 2.5 mm from the skull) using a sterile
Hamilton syringe fitted with a 26-gauge needle (Hamilton Co., Reno,
NV, USA) and a microinfusion pump (Harvard Apparatus, Holliston,
MA, USA). Intracranial tumors were confirmed by cranial magnetic
resonance imaging (MRI). All MRI experiments were performed on a
4.7 T animal MRI scanner (BioSpec 47/40, Bruker, Germany) with a
quadrature volume coil (diameter, 25 mm) at the Korea Basic Science
Institute in Ochang, Korea.
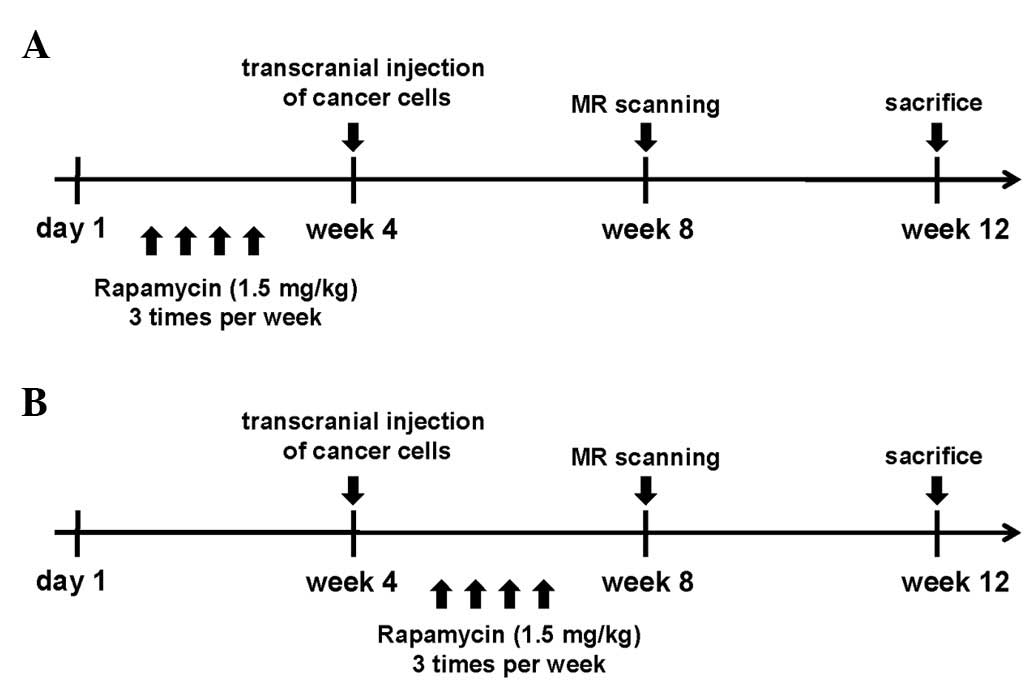
The experimental design is shown in Fig. 1. Both experimental groups contained
five mice initially. In the first group, mice were treated with
rapamycin (1.5 mg/kg) via i.p. injection 3 times a week for 4 weeks
before intracranial inoculation with NCI-H358 cells. In the second
treatment group, mice were treated with rapamycin (1.5 mg/kg) via
i.p. injection 3 times a week for 4 weeks after intracranial
inoculation. Control and treated mice were euthanized 12 weeks
after the intracranial inoculation, and tumor specimens were
obtained for RT-PCR.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from all specimens was extracted using a
commercial kit (RNeasy Mini kit, Qiagen, Hilden, Germany). One
microgram of total RNA was reverse transcribed using the RT-premix
(M-Biotech, Seoul, Korea). RT-PCR was performed on cDNA samples
using a DNA Thermal Cycler (Bio-Rad, Hercules, CA, USA) with Go Taq
Green Master mix (Promega, Madison, WI, USA), RNase-free water and
primers. The primer sequences are summarized in Table I. RT-PCR products were separated on
a 1.5% agarose gel containing ethidium bromide and visualized with
UV light.
 | Table IPrimer pairs for reverse
transcription-polymerase chain reaction (RT-PCR). |
Table I
Primer pairs for reverse
transcription-polymerase chain reaction (RT-PCR).
| mRNA | Direction | Sequence | Length (bp) |
|---|
| IL-1 | Forward |
GAGAGCCGGGTGACAGTATC | |
| Reverse |
ACTTCTGCCTGACGAGCTTC | 202 |
| IL-3 | Forward |
TGCCTACATCTGCGAATGAC | |
| Reverse |
TTAGGTGCTCTGCCTGCTG | 203 |
| IL-6 | Forward |
ACTTCCATCCAGTTGCCTTC | |
| Reverse |
CAGAATTGCCATTGCACAAC | 201 |
| TNF-α | Forward |
CCCATGGATGTCCCATTTAG | |
| Reverse |
CCAGGATTCTGTGGCAATC | 193 |
| ATGF-β | Forward |
GTGGTTTCTGTGACCCTTGG | |
| Reverse |
CCAGTCACACAGGCAACAAG | 201 |
| IGF-1 | Forward |
CTCTTCTACCTGGCGCTCTG | |
| Reverse |
GCAACACTCATCCACAATGC | 195 |
| PDGF-A | Forward |
CCCCTGCCCATTCGGAGGAAGAG | |
| Reverse |
TTGGCCACCTTGACGCTGCGGTG | 203 |
| MCP-1 | Forward |
CTCACCTGCTGCTACTCATTC | |
| Reverse |
GCTTGAGGTGGTTGTGGAAAA | 650 |
| MIP-1 | Forward |
TCAGCACCATGAAGGTCTCCAC | |
| Reverse |
CTCAGGCATTTAGTTCCAGCTC | 450 |
Statistical analysis
All data are shown as the means ± SEM. Comparisons
between groups were made using unpaired Student’s t-tests, and
among multiple groups by ANOVA. P<0.05 was considered to
indicate a statistically significant result.
Results
Astrocytes and rapamycin induce apoptosis
of NCI-H358 cells
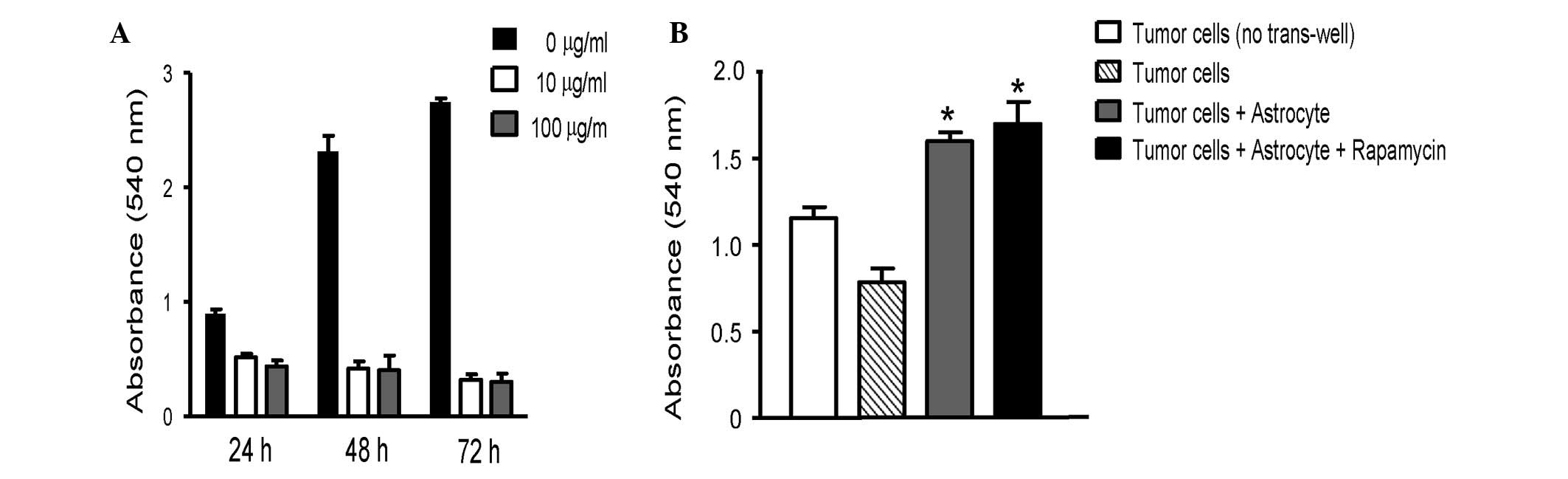
Rapamycin inhibits survival of NCI-H358 lung cancer
cells in a dose- and time-dependent manner. To determine whether
astrocytes affect tumor cell survival, astrocytes were co-cultured
with NCI-H358 lung cancer cells. Co-culture with astrocytes induced
NCI-H358 cell survival, compared with NCI-H358 cells alone.
Moreover, rapamycin (100 μg/ml) enhanced the survival of
lung cancer cells in co-culture (Fig.
2).
Modulation of cytokines and growth
factors by rapamycin in brain metastasis model
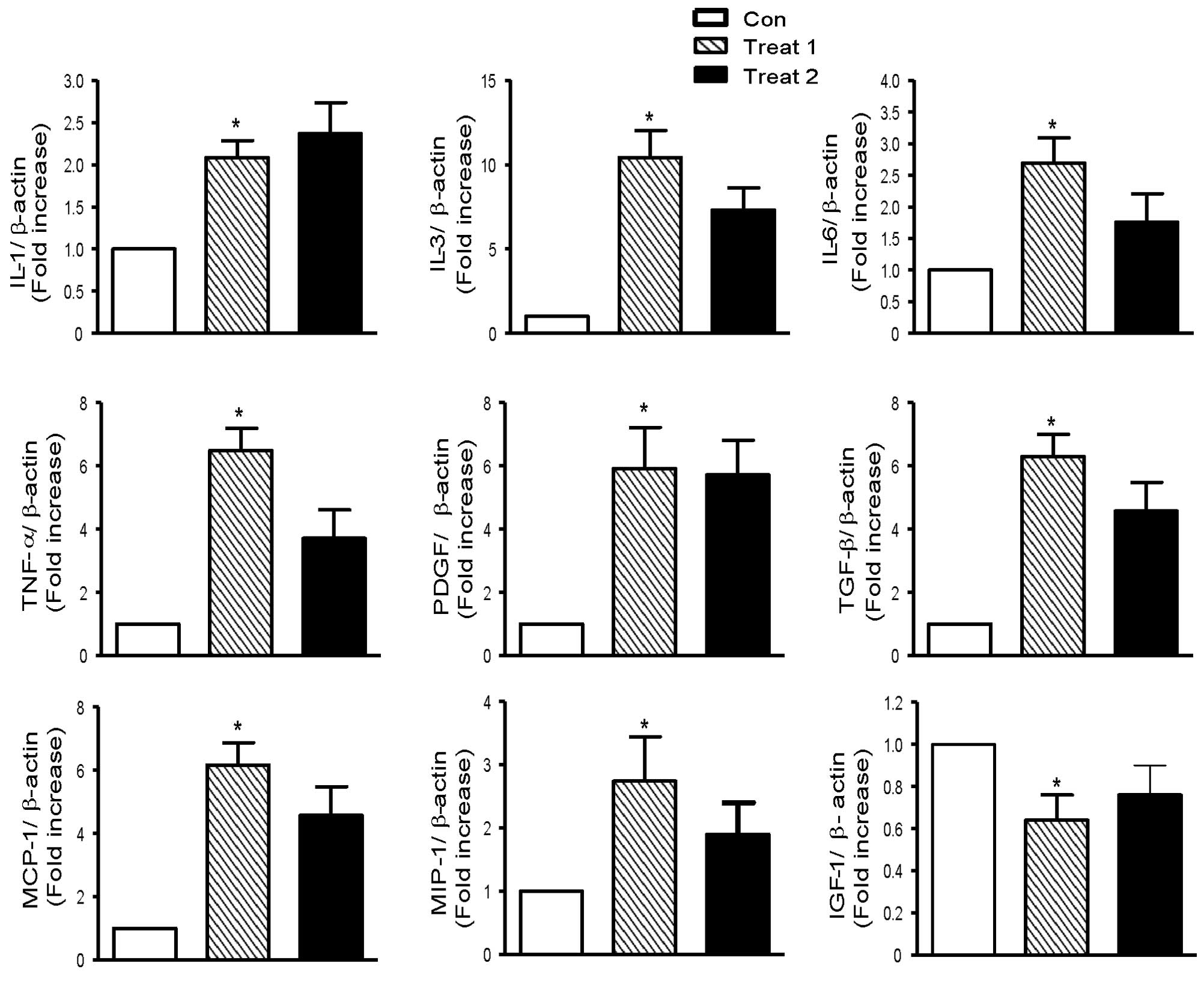
Activated astrocytes produce a wide variety of
cytokines, including IL-1, IL-3, IL-6, TNF-α, TGF-β, IGF-1 and
PDGF. Cytokines released by activated microglia include IL-1, IL-6,
TNF-α, MCP-1, and MIP-1. IL-1, IL-3, IL-6, TNF-α, TGF-β, PDGF,
MCP-1 and MIP-1 expression was higher in rapamycin-treated mice
compared with controls (Fig. 3).
IGF-1 expression, however, was lower in rapamycin-treated mice than
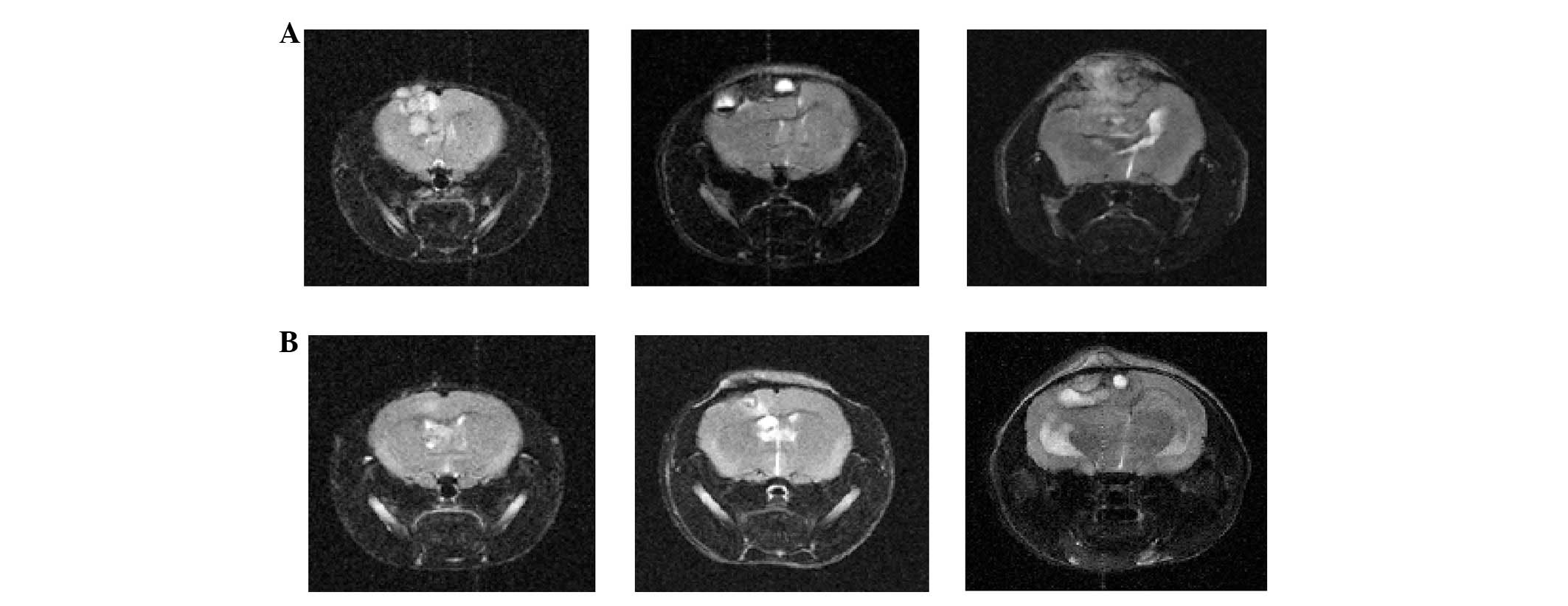
control mice. Rapamycin treatment before tumor cell inoculation
affected the later cytokine expression in the tumor. The tumor
progressively increased in size, and compressed the brain
parenchyma at 4, 8 and 12 weeks after inoculation in control mice.
In mice treated with rapamycin before inoculation, the tumor formed
and thrived slowly (Fig. 4).
Discussion
Rapamycin caused apoptosis of tumor cells alone.
However, co-cultures of astrocytes with NCI-H358 lung cancer cells
prevented apoptosis of tumor cells even after rapamycin treatment.
In the experimental mouse model, IL-1, IL-3, IL-6, TNF-α, TGF-β,
PDGF, MCP-1 and MIP-1 expression in the inoculated tumors increased
in rapmycin-treated mice. IGF-1 expression, however, decreased.
Co-culturing breast or lung cancer cells with
astrocytes led to upregulated survival genes, including GSTA5,
BCL2L1 and TWIST1, in tumor cells (12). Brain metastases are surrounded and
infiltrated by activated astrocytes and are highly resistant to
chemotherapy. Astrocytes in co-culture were activated by tumor
cell-oriented factors, including macrophage migration inhibitory
factor (MIF), IL-8 and plasminogen activator inhibitor-1 (PAI-1).
Interactions between metastatic tumor cells and activated
astrocytes are important in creating a favorable microenvironment
for the tumor cells in the brain (13).
The expression of cytokines and growth factors in
the tumor-bearing mouse model was measured to investigate the role
of rapamycin in the microenvironment of brain metastases.
IL-1 is a macrophage-derived proinflammtory ‘alarm’
cytokine that mediates inflammation. Balanced IL-1 levels have been
associated with inducing antitumor immunity (14). IL-3 gene expression within tumors
leads to host-cell infiltration, particularly by macrophages,
slower tumor growth and enhanced immunogenicity (15). IL-6 has been shown to inhibit growth
and enhance motility in breast cancer cell lines (16). TNF-α has an antitumor effect in
murine tumors and human tumor xenografts in vivo and appears
to be cytotoxic to many human tumor cell lines in
vitro(17). TGF-β is an
important suppressor of primary tumorigenesis. The role of TGF-β in
apoptosis and cell cycle arrest depends on the microenvironment
(18). PDGF induces important
cellular processes including chemotaxis, survival, apoptosis and
transformation in vitro(19). MCP-1, also known as chemokine ligand
2 (CCL2), is a pro-inflammatory chemokine that recruits and
activates monocytes during the inflammatory response. MCP-1 is a
pivotal regulator of tumor growth, progression and metastasis
(20,21). MCP-1 and MIP-1 have been reported to
regulate immunity to melanoma by promoting lymphocyte infiltration
into tumors and subsequent cytokine production (22,23).
MCP-1 or MIP-1 loss significantly promotes primary tumor growth and
lung metastasis by inhibiting IL-6, TNF-α and TGF-β expression. In
this study, IGF-1 levels decreased in rapamycin-treated mice. IGF-1
is critical to activate and sustain an inflammatory response in the
liver, which is needed for hepatic metastasis, not only through
direct, paracrine effects on tumor cell growth, but also through
indirect effects involving the tumor microenvironment (24). IGF-1 receptor expression in
neuroblastoma cells has been reported to increase tumor cell
interaction with the bone microenvironment, resulting in greater
metastasis formation (25).
Rapamycin is highly lipophilic and thus penetrates
the blood brain barrier (BBB) (26). Combined treatment with rapamycin and
brain penetrant MEK inhibitor significantly reduces brain
metastasis by prohibiting perivascular invasion of tumor cells and
tumor angiogenesis in triple-negative breast cancer models
(27). Rapamycin effectively
inhibits cancer metastasis in various preclinical models (28–31).
The results demonstrated that several cytokines and
growth factors, except for IGF-1, were increased in mice treated
with rapamycin before inoculation, compared with control mice.
Brain MRIs of tumor-bearing mice showed that tumor growth was
slower in pre-treated mice than in control mice. These results
suggest that rapamycin administration influences the brain
microenvironment before brain metastases develop. Accumulating
evidence indicates that metastatic progression is regulated, in
large part, by interactions between tumor cells and non-tumor cells
in the brain microenvironment (32,33).
Astrocytes could contribute to the microenvironment associated with
metastatic cell growth, and they are a source of cytokines and
growth factors, which may modulate metastatic-cell growth. The
results suggest that modulating the brain microenvironment could be
worthy of further research as a new target to prevent brain
metastasis of NSCLC.
Acknowledgements
This study was supported by the BumSuk
Academic Research Fund of 2011. Normal human astrocyte cell lines
were donated by Dr Yong-Wan Kim, MD Anderson Cancer Center,
University of Texas, USA.
References
|
1
|
Jemal A, Siegal R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Robnett TJ, Machtay M, Stevenson JP,
Algazy KM and Hahn SM: Factors affecting the risk of brain
metastases after definitive chemoradiation for locally advanced
non-small-cell lung carcinoma. J Clin Oncol. 19:1344–1349.
2001.PubMed/NCBI
|
|
3
|
Rusciano D and Burger MM: Why do cancer
cells metastasize into particular organs? Bioessays. 14:185–194.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nicolson GL: Cancer progression and
growth: relationship of paracrine and autocrine growth mechanisms
to organ preference of metastasis. Exp Cell Res. 204:171–180. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sierra A, Price JE, García-Ramirez M,
Méndez O, López L and Fabra A: Astrocyte-derived cytokines
contribute to the metastatic brain specificity of breast cancer
cells. Lab Invest. 77:357–368. 1997.PubMed/NCBI
|
|
6
|
Yang I, Han SJ, Kaur G, Crane C and Parsa
AT: The role of microglia in central nervous system immunity and
glioma immunology. J Clin Neurosci. 17:6–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Langley RR, Fan D, Guo L, Zhang C, Lin Q,
Brantley EC, McCarty JH and Fidler IJ: Generation of an
immortalized astrocyte cell line from H-2Kb-tsA58 mice to study the
role of astrocytes in brain metastasis. Int J Oncol. 35:665–672.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoo JY, Yang SH, Lee JE, Cho DG, Kim HK,
Kim SH, Kim IS, Hong JT, Sung JH, Son BC and Lee SW: E-cadherin as
a predictive marker of brain metastasis in non-small-cell lung
cancer, and its regulation by pioglitazone in a preclinical model.
J Neurooncol. 109:219–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bjornsti MA and Houghton PJ: The TOR
pathway: a target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guba M, von Breitenbuch P, Steinbauer M,
Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S,
Anthuber M, Jauch KW and Geissler EK: Rapamycin inhibits primary
and metastatic tumor growth by antiangiogenesis: involvement of
vascular endothelial growth factor. Nat Med. 8:128–135. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobayashi S, Kishimoto T, Kamata S, Otsuka
M, Miyazaki M and Ishikura H: Rapamycin, a specific inhibitor of
the mammalian target of rapamycin, suppresses lymphangiogenesis and
lymphatic metastasis. Cancer Sci. 98:726–733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SJ, Kim JS, Park ES, Lee JS, Lin Q,
Langley RR, Maya M, He J, Kim SW, Weihua Z, Balasubramanian K, Fan
D, Mills GB, Hung MC and Fidler IJ: Astrocytes upregulate survival
genes in tumor cells and induce protection from chemotherapy.
Neoplasia. 13:286–298. 2011.PubMed/NCBI
|
|
13
|
Seike T, Fujita K, Yamakawa Y, Kido MA,
Takiguchi S, Teramoto N, Iguchi H and Noda M: Interaction between
lung cancer cells and astrocytes via specific inflammatory
cytokines in the microenvironment of brain metastasis. Clin Exp
Metastasis. 28:13–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carmi Y, Rinott G, Dotan S, Elkabets M,
Rider P, Voronov E and Apte RN: Microenvironment-derived IL-1 and
IL-17 interact in the control of lung metastasis. J Immunol.
186:3462–3471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu YZ, Hong JH, Huang HH, Dougherty GJ,
McBride WH and Chiang CS: Mechanisms mediating the effects of IL-3
gene expression on tumor growth. J Leukoc Biol. 68:890–896.
2000.PubMed/NCBI
|
|
16
|
Jiang XP, Yang DC, Elliott RL and Head JF:
Down-regulation of expression of interleukin-6 and its receptor
results in growth inhibition of MCF-7 breast cancer cells.
Anticancer Res. 31:2899–2906. 2011.PubMed/NCBI
|
|
17
|
Suarez Pestana E, Björklund G, Larsson R,
Nygren P, Nilsson K and Bergh J: Effects of interferons and tumour
necrosis factor-alpha on human lung cancer cell lines and the
development of an interferon-resistant lung cancer cell line. Acta
Oncol. 35:473–478. 1996.PubMed/NCBI
|
|
18
|
Tse JC and Kalluri R: Mechanisms of
metastasis: epithelialto-mesenchymal transition and contribution of
tumor microenvironment. J Cell Biochem. 101:816–829. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu J, Ustach C and Kim HR:
Platelet-derived growth factor signaling and human cancer. J
Biochem Mol Biol. 36:49–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raffaghello L, Cocco C, Corrias MV,
Airoldi I and Pistoia V: Chemokines in neuroectodermal tumour
progression and metastasis. Semin Cancer Biol. 19:97–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Patel L and Pienta KJ: CC
chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis
and metastasis. Cytokine Growth Factor Rev. 21:41–48. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakasone Y, Fujimoto M, Matsushita T,
Hamaguchi Y, Huu DL, Yanaba M, Sato S, Takehara K and Hasegawa M:
Host-derived MCP-1 and MIP-1α regulate protective anti-tumor
immunity to localized and metastatic B16 melanoma. Am J Pathol.
180:365–374. 2012.
|
|
23
|
van Deventer HW, Serody JS, McKinnon KP,
Clements C, Brickey WJ and Ting JP: Transfection of macrophage
inflammatory protein 1 alpha into B16 F10 melanoma cells inhibits
growth of pulmonary metastases but not subcutaneous tumors. J
Immunol. 169:1634–1639. 2002.PubMed/NCBI
|
|
24
|
Wu Y, Brodt P, Sun H, Mejia W, Novosyadlyy
R, Nunez N, Chen X, Mendoza A, Hong SH, Khanna C and Yakar S:
Insulin-like growth factor-I regulates the liver microenvironment
in obese mice and promotes liver metastasis. Cancer Res. 70:57–67.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Y, Yakar S, Zhao L, Hennighausen L and
LeRoith D: Circulating insulin-like growth factor-I levels regulate
colon cancer growth and metastasis. Cancer Res. 62:1030–1035.
2002.PubMed/NCBI
|
|
26
|
Sehgal SN, Baker H and Vézina C: Rapamycin
(AY-22,989), a new antifungal antibiotic. II Fermentation,
isolation and characterization. J Antibiot. 28:727–732. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao H, Cui K, Nie F, Wang L, Brandl MB,
Jin G, Li F, Mao Y, Xue Z, Rodriguez A, Chang J and Wong ST: The
effect of mTOR inhibition alone or combined with MEK inhibitors on
brain metastasis: an in vivo analysis in triple-negative
breast cancer models. Breast Cancer Res Treat. 131:425–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luan FL, Ding R, Sharma VK, Chon WJ,
Lagman M and Suthanthiran M: Rapamycin is an effective inhibitor of
human renal cancer metastasis. Kidney Int. 63:917–926. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Z, Lei Z, Li B, Zhou Y, Zhang GM,
Feng ZH, Zhang B, Shen GX and Huang B: Rapamycin inhibits lung
metastasis of B16 melanoma cells through down-regulating alphav
integrin expression and up-regulating apoptosis signaling. Cancer
Sci. 101:494–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel V, Marsh CA, Dorsam RT, Mikelis CM,
Masedunskas A, Amornphimoltham P, Nathan CA, Singh B, Weigert R,
Molinolo AA and Gutkind JS: Decreased lymphangiogenesis and lymph
node metastasis by mTOR inhibition in head and neck cancer. Cancer
Res. 71:7103–7112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hussein O, Tiedemann K, Murshed M and
Komarova SV: Rapamycin inhibits osteolysis and improves survival in
a model of experimental bone metastases. Cancer Lett. 314:176–184.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langley RR and Fidler IJ: Tumor cell-organ
microenvironment interactions in the pathogenesis of cancer
metastasis. Endocr Rev. 28:297–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gupta GP and Massagué J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|