Introduction
Renal cell carcinoma (RCC), the most common
pathological pattern, accounts for 3% of adult malignancy tumors
and worldwide incidence continues to increase steadily each year
(1). The 5-year survival rate is
80–90% for pT1 and pT2 stage RCC; however, in patients with
metastatic disease, it is only 10% (2,3).
Therefore, the identification of methods to reduce risk factors and
novel therapeutics is extremely important (4). Previous studies have demonstrated that
patients with metabolic diseases, including diabetes mellitus and
obesity, are associated with a higher risk of developing various
types of cancer, including renal (5,6). Lipid
metabolism disorder with high levels of serum free fatty acids
(FFAs) is an important characteristic in metabolic diseases and
FFAs are associated with cell development and invasion in breast
and prostate cancer, indicating the role of FFAs in renal cancer
(7,8).
FFAs have been revealed to stimulate intracellular
signal transduction, functioning as second messengers in vascular
smooth muscle and tumor cells (9,10);
however, the mechanism by which FFAs affect tumor development in
RCC remains unclear. Integrin-linked kinase (ILK) is a
serine/threonine protein kinase, involved in the regulation of cell
growth/survival, cell cycle progression, invasion and migration and
tumor angiogenesis (11). ILK may
activate downstream kinases, including Akt and GSK3β (12), therefore we hypothesized that the
function of FFAs may be markedly associated with the ILK pathway in
RCC. In the present study, the effect of the highest content of
FFAs in serum, namely oleic acid, on human RCC 786-O cells was
investigated in vitro and the mechanism by which FFAs
function was determined.
Materials and methods
Reagents
Oleic acid and de-fatty bovine serum albumin (d-BSA)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Oleic acid
was supplemented with d-BSA, which functioned as a carrier to
ensure sufficient dissolution (mol/mol <2). The annexin V-FITC
apoptosis detection kit was purchased from Biosea Biotechnology Co.
Ltd. (Beijing, China). ILK small interfering RNA (siRNA) and
control non-silencing siRNA were purchased from Cell Signaling
Technology Inc. (Danvers, MA, USA). Polyclonal anti-ILK, anti-Akt,
anti-p-Akt ser473 and anti-G protein-coupled receptor 40 (GPR40)
antibodies were purchased from Cell Signaling Technology.
Cell culture and oleic acid
treatment
Human RCC cell line, 786-O, was obtained from
American Type Culture Collection (Manassas, VA, USA) and routinely
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS) and 100 U/ml penicillin and streptomycin. For
treatment, cells were cultured in growth medium for 24 h and then
the medium was replaced with oleic acid-enriched medium at various
concentrations of oleic acid (0.05, 0.1, 0.2 mmol/l). The control
group received d-BSA alone at an equal concentration.
The study was approved by the Ethics Committee of
Peking University People’s Hospital, Beijing, China.
MTT assay
Cells (2×103 cells/well) were seeded in
96-well microtitre plates and incubated for 24 h in 100 μl
culture medium. Cells in the experimental group were then treated
with 0.05, 0.1 or 0.2 mmol/l oleic acid for 48 h. MTT [20 μl
(5 g/l)] was added to the cells which were then cultivated for a
further 4 h. Following the removal of the supernatant fluid, 150
μl/well DMSO was added to the cells which were agitated for
15 min. Absorbance was measured at 490 nm by an ELISA reader.
Untreated 786-O cells served as controls. Each assay was repeated
three times. The relative growth rate of 786-O cells was calculated
using the following equation: cell viability rate (%) =
(ODoleic acid/ODcontrol) × 100.
Apoptosis assessed by flow cytometry
The extent of apoptosis was evaluated by annexin
V-FITC and flow cytometry. Cells were grown at a density of
1×106 cells in 6-well culture dishes and were treated
with 0.05, 0.1 or 0.2 mmol/l oleic acid for 48 h. Following
treatment, cells were harvested, washed twice with pre-chilled PBS
and resuspended in 1X binding buffer at a concentration of
1×106 cells/ml. The solution (100 μl) was mixed
with 5 μl annexin V-FITC and 5 μl PI for 15 min, then
400 μl 1X binding buffer was added. Analysis was performed
using a FACStar cytofluorometer with CellQuest Pro software.
Treatment with ILK siRNA
siRNA duplexes specific for human ILK were used to
specifically knock down ILK. For the transfection procedure, cells
were grown to 60% confluence and ILK and control siRNA were
transfected using the Oligofectamine reagent according to the
manufacturer’s instructions. Briefly, Oligofectamine reagent was
incubated with serum-free medium for 10 min. Subsequently, a
mixture of respective siRNA was added. Following incubation for 15
min at room temperature, the mixture was diluted with medium and
added to each well. The final concentration of siRNA in each well
was 100 nmol/l. Following culture for 32 h, cells were washed and
resuspended in oleic acid-enriched culture medium for an additional
48 h for cell growth assays and western blot analysis.
Western blot analysis
Cell lysates were boiled with 5X loading buffer and
then fractionated by SDS-PAGE. Proteins were transferred to PVDF
membrane and incubated with primary specific antibodies against
GPR40, ILK, Akt and phosphor-Akt in 5% milk. Blots were washed and
horseradish peroxidase-conjugated anti-mouse or anti-rabbit
antibodies were applied. Blots were washed, transferred to freshly
made enhanced chemiluminescence solution for 5 min and images were
captured using the ImageQuant Gel imaging system.
Statistical analysis
Data are expressed as mean ± SD for groups and
analyzed using the Student’s t-test to determine differences
between the oleic acid-treated and control groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Oleic acid increases 786-O cell
proliferation
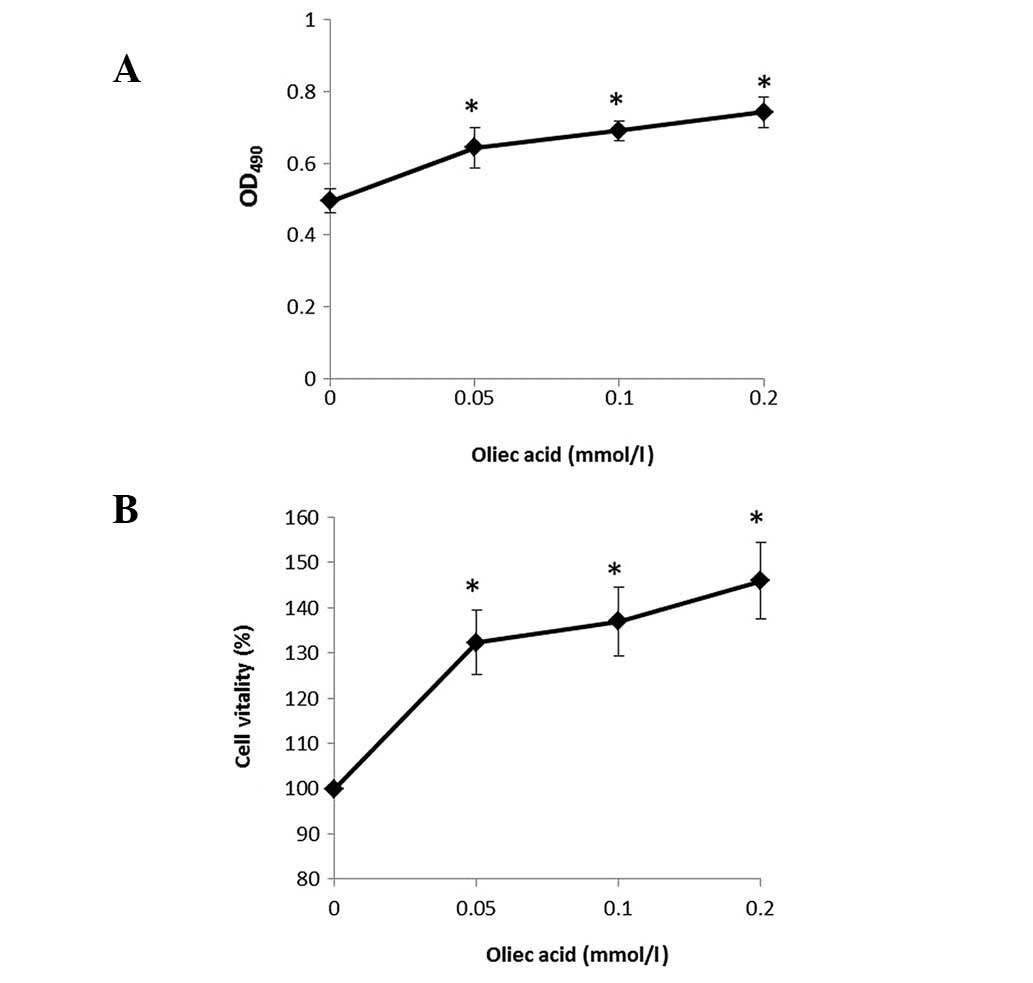
To examine the function of oleic acid on the human
RCC cell line, 786-O cells were cultured and treated with various
concentrations of oleic acid and cell viability was detected using
the MTT assay. Percentages of viable cells when cultured with oleic
acid (0.05, 0.1 and 0.2 mmol/l) relative to the control-treated
cells were determined at 4 h. The results indicate that oleic acid
treatment induced a marked increase in the proliferation of 786-O
cells in a dose-dependent manner (Fig.
1A and 1B).
Oleic acid-delayed apoptosis of 786-O
cells
To determine the effect of oleic acid on the
apoptosis of 786-O cells, annexin V/PI was used. It was observed
that, following treatment of 786-O cells for 48 h with oleic acid
(0.05, 0.1 and 0.2 mmol/l), the total percentage of apoptotic cells
was not directly associated with oleic acid concentration,
decreasing from 2.42 to 2.33 and 2.25%, compared with the control
cells (2.04%) (0.05, 0.1 and 0.2 mmol/l oleic acid, respectively;
Fig. 2); consistent with results of
the MTT assay. The results revealed that oleic acid delayed 786-O
cell apoptosis in a concentration-dependent manner.
ILK is upregulated by oleic acid
treatment in 786-O cells
Since oleic acid treatment induced 786-O cell
proliferation and delayed cell apoptosis, the effect of oleic acid
on specific cell proliferation regulatory molecules was analyzed.
Western blot analysis revealed that oleic acid treatment induced
upregulation of ILK and phospho-Akt protein, a key regulator of
cell proliferation, in a dose-dependent manner (Fig. 3). To determine the mechanims by
which oleic acid activates intra-cellular signaling, the expression
of GPR40 protein, a specific receptor of long chain fatty acids,
was determined. GRP40 protein expression was also found to be
higher following oleic acid treatment in 786-O cells (Fig. 3).
ILK siRNA reverses the effects of oleic
acid on cell growth and gene expression
To determine the role of ILK on 786-O cell
proliferation, siRNA tranfection was performed to downregulate ILK
expression. Cell viability was suppressed following transfection
with ILK siRNA and treatment with oleic acid (Fig. 4B) and the expression of ILK and
phospho-Akt was decreased (Fig.
4A). The results indicated that cell proliferation is markedly
associated with the expression of ILK.
Discussion
Previous epidemiological and animal studies have
identified an association between dietary fatty acids and metabolic
diseases characterized by hyperlipidemia and an elevation in
circulating FFAs and have indicated that this association also
correlates with enhanced cancer risk (13–16).
The long chain polyunsaturated fatty acids (PUFA) in daily diets
include n-3 PUFA, n-6 PUFA and others, and exhibit various roles in
cancer. Previous studies have demonstrated that n-3 PUFA suppresses
tumor cell proliferation and induces cell apoptosis, while n-6 PUFA
promotes tumor development (17,18).
Oleic acid is an n-9 monounsaturated fatty acid,
which activates G protein-coupled receptors and phosphorylates
ERK1/2 to induce cancer cell proliferation in breast cancer
(19). The concentration of oleic
acid in normal serum is ∼0.05 mmol/l; however, a number of studies
have reported that oleic acid may affect tumor cell development
when its concentration is >0.025 mmol/l (20). In the present study, high
concentration levels of oleic acid were used to imitate the effects
of abnormal levels of FFAs on tumor growth. The results of the MTT
assay indicated that specific concentrations of oleic acid
stimulate 786-O cell viability in a dose-dependent manner. In
addition, flow cytometry assay revealed that oleic acid delayed
786-O cell apoptosis in a concentration-dependent manner. Following
this, specific signaling molecules identified in previous studies
to be involved in this mechanism were investigated. Western blot
analysis revealed that oleic acid treatment upregulated ILK
expression in a concentration-dependent manner. Overexpression of
ILK in tumor cells has been found to result in
anchorage-independent cell growth, cell cycle progression and
tumorigenicity (21). In the
current study, overexpression of ILK was found to increase the
phosphorylation of Akt on Ser-473, which was consistent with
previous observations reporting that the activity of ILK is
regulated in a PI3-kinase-dependent manner. In addition, when siRNA
was used to target and knock down ILK, the effects of oleic acid on
786-O cells growth were weakened and the expression of ILK was
suppressed. These results indicate that oleic acid may regulate RCC
cell development through the PI3K/ILK/Akt pathway.
GPR40 is a membrane-bound receptor paired with
medium- and long-chain fatty acids as endogenous ligands (22). Briscoe et al found that GPR40
was highly expressed in ob/ob mice and may be involved in cell
proliferation (23). In the breast
cancer cell line, MCF-7, GPR40 was found to be significantly
increased at the start and end of cell proliferation and silencing
the GPR40 gene using RNA interference was found to suppress
oleate-induced cell proliferation (24,25).
In the current study, GPR40 was also upregulated by oleic acid
treatment and GPR40 was hypothesized to activate the signals
associated with cell growth, including ILK and Akt. Akt kinase is
activated by phosphorylation at S473 in the regulatory tail by
phosphoinositide-dependent kinase, PDK-2, the identity of which is
cell or tissue-specific and its activity is highly regulated
(26). To date, ∼10 kinases have
been demonstrated to function as a PDK-2, including ILK, PKC, PKA
and the mTOR complex (27).
Consistent with these observations, ILK is activated by GPR40
combined with oleic acid treatment and functions as a PDK-2 to
regulate the Akt pathway in RCC.
In summary, the results of this study indicate the
following cascade of events in response to oleic acid in 786-O
cells (Fig. 5). Unsaturated FFA
binds to GPR40 and may also bind other FFA receptors, resulting in
the activation of PI3K, ILK, Akt and subsequent promotion of cell
growth. These results provide a novel mechanism for the action of
oleic acid in RCC cells on cell growth by demonstrating that this
monounsaturated FFA functions as an extracellular signaling
molecule to regulate 786-O cell proliferation via the GPR40/ILK/Akt
pathway. This pathway may represent a potential therapeutic target
and link between insulin resistance, obesity, type 2 diabetes and
cancer.
 | Figure 5Schematic representation of oleic acid
signaling in human renal cell carcinoma (RCC). Oleic acid activates
the Akt pathway through stimulation of ILK, identified as one of
the kinases with PDK-2 activity in human RCC. The mechanisms
involved in this action are not well understood but may be
GPR40-dependent. FFA, free fatty acid; GPR40, G protein-coupled
receptor 40; ILK, integrin-linked kinase; PTEN, phosphatase and
tensin homolog deleted on chromosome 10; P(4,5)P2,
phosphatidylinositol-4,5-bisphosphate; P(3,4,5)P3,
phosphatidylinositol-3,4,5-trisphosphate. |
Acknowledgements
The authors thank Dr. Zhang Xiaowei
for his English editorial assistance. The present study was
supported by the National Natural Science Foundation of China (no.
31171341).
References
|
1
|
Sourbier C and Massfelder T: Parathyroid
hormone-related protein in human renal cell carcinoma. Cancer Lett.
240:170–182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schrader AJ, Varga Z, Hegele A, Pfoertner
S, Olbert P and Hofmann R: Second-line strategies for metastatic
renal cell carcinoma: classics and novel approaches. J Cancer Res
Clin Oncol. 132:137–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tolle A, Jung M, Lein M, et al: Brain-type
and liver-type fatty acid-binding proteins: new tumor markers for
renal cancer? BMC Cancer. 9:2482009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escudier B: Advanced renal cell carcinoma:
current and emerging management strategies. Drugs. 67:1257–1264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Habib SL, Prihoda TJ, Luna M and Werner
SA: Diabetes and risk of renal cell carcinoma. J Cancer. 3:42–48.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drabkin HA and Gemmill RM: Obesity,
cholesterol and clear-cell renal cell carcinoma (RCC). Adv Cancer
Res. 107:39–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Navarro-Tito N, Soto-Guzman A,
Castro-Sanchez L, Martinez-Orozco R and Salazar EP: Oleic acid
promotes migration on MDA-MB-231 breast cancer cells through an
arachidonic acid-dependent pathway. Int J Biochem Cell Biol.
42:306–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suburu J and Chen YQ: Lipids and prostate
cancer. Prostaglandins Other Lipid Mediat. 98:1–10. 2012.
View Article : Google Scholar
|
|
9
|
Yun MR, Lee JY, Park HS, et al: Oleic acid
enhances vascular smooth muscle cell proliferation via
phosphatidylinositol 3-kinase/Akt signaling pathway. Pharmacol Res.
54:97–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vinciguerra M, Carrozzino F, Peyrou M, et
al: Unsaturated fatty acids promote hepatoma proliferation and
progression through downregulation of the tumor suppressor PTEN. J
Hepatol. 50:1132–1141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hannigan G, Troussard AA and Dedhar S:
Integrin-linked kinase: a cancer therapeutic target unique among
its ILK. Nat Rev Cancer. 5:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McDonald PC, Oloumi A, Mills J, et al:
Rictor and integrin-linked kinase interact and regulate Akt
phosphorylation and cancer cell survival. Cancer Res. 68:1618–1624.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soto-Guzman A, Navarro-Tito N,
Castro-Sanchez L, Martinez-Orozco R and Salazar EP: Oleic acid
promotes MMP-9 secretion and invasion in breast cancer cells. Clin
Exp Metastasis. 27:505–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stemmer K, Perez-Tilve D, Ananthakrishnan
G, et al: High-fat-diet-induced obesity causes an inflammatory and
tumor-promoting microenvironment in the rat kidney. Dis Model Mech.
5:627–635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartsch H, Nair J and Owen RW: Dietary
polyunsaturated fatty acids and cancers of the breast and
colorectum: emerging evidence for their role as risk modifiers.
Carcinogenesis. 20:2209–2218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willett WC: Specific fatty acids and risks
of breast and prostate cancer: dietary intake. Am J Clin Nutr.
66:1557S–1563S. 1997.PubMed/NCBI
|
|
17
|
Han S, Sun X, Ritzenthaler JD and Roman J:
Fish oil inhibits human lung carcinoma cell growth by suppressing
integrin-linked kinase. Mol Cancer Res. 7:108–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hammamieh R, Chakraborty N, Miller SA, et
al: Differential effects of omega-3 and omega-6 Fatty acids on gene
expression in breast cancer cells. Breast Cancer Res Treat.
101:7–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soto-Guzman A, Robledo T, Lopez-Perez M
and Salazar EP: Oleic acid induces ERK1/2 activation and AP-1 DNA
binding activity through a mechanism involving Src kinase and EGFR
transactivation in breast cancer cells. Mol Cell Endocrinol.
294:81–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gorjao R, Hirabara SM, de Lima TM,
Cury-Boaventura MF and Curi R: Regulation of interleukin-2
signaling by fatty acids in human lymphocytes. J Lipid Res.
48:2009–2019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Persad S and Dedhar S: The role of
integrin-linked kinase (ILK) in cancer progression. Cancer
Metastasis Rev. 22:375–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Del GS, Bugliani M, D’Aleo V, et al:
G-protein-coupled receptor 40 (GPR40) expression and its regulation
in human pancreatic islets: the role of type 2 diabetes and fatty
acids. Nutr Metab Cardiovasc Dis. 20:22–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Briscoe CP, Tadayyon M, Andrews JL, et al:
The orphan G protein-coupled receptor GPR40 is activated by medium
and long chain fatty acids. J Biol Chem. 278:11303–11311. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yonezawa T, Katoh K and Obara Y: Existence
of GPR40 functioning in a human breast cancer cell line, MCF-7.
Biochem Biophys Res Commun. 314:805–809. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hardy S, St-Onge GG, Joly E, Langelier Y
and Prentki M: Oleate promotes the proliferation of breast cancer
cells via the G protein-coupled receptor GPR40. J Biol Chem.
280:13285–13291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanada M, Feng J and Hemmings BA:
Structure, regulation and function of PKB/AKT - a major therapeutic
target. Biochim Biophys Acta. 1697:3–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong LQ and Liu F: PDK2: the missing piece
in the receptor tyrosine kinase signaling pathway puzzle. Am J
Physiol Endocrinol Metab. 289:E187–E196. 2005. View Article : Google Scholar : PubMed/NCBI
|