Introduction
Esophageal cancer is a common digestive malignancy.
The latest statistics from the GLOBOCAN project from the World
Health Organization (WHO) showed that 482,000 new cases and 407,000
mortalities worldwide in 2008 were of this type. The mortality rate
was 84.4% (1) Esophageal cancer has
become one of the most threatening malignancies to human health.
Generally, radiotherapy and chemoradiotherapy using conventional
chemotherapy agents are effective against esophageal squamous cell
carcinoma (2). However, the 5-year
survival rate is not satisfactory. A more effective chemotherapy
agent with radiosensitizing effects requires development.
Research showed that a cell’s relative
radiosensitivity is determined by the cell cycle phase. Cells are
most radiosensitive in the G2/M phase, less sensitive in
the G1 phase and least sensitive during the latter part
of the S phase (3). Cell cycle
regulatory proteins, including cyclins and cyclin-dependent kinases
(CDKs), are not well-regulated in tumor cells (4,5).
Flavopiridol, a CDK inhibitor, which has recently entered clinical
trials (6), has been shown to exert
antitumor activity in preclinical tumor models (7,8).
Flavopiridol is a synthetic flavone
(5,7-dihydroxy-8-(4-N-methyl-2-hydroxypyridyl)-6′-chloroflavone
hydrochloride), which is structurally related to a compound derived
from the plant Dysoxylum binectariferum, indigenous to India
and used in Indian folk medicine (9). It inhibits the activity of all CDKs,
but primarily CDK1, 2 and 4; thus, are arrested at the
G2/M phase (10).
Recently, several studies have confirmed that flavopiridol induces
cell cycle arrest in a number of types of cancer (11,12,13).
Given this, flavopiridol may also regulate cycle distribution of
Eca109 (a type of esophageal squamous carcinoma) and affect its
radiosensitivity. The aim of this study is to assess whether
flavopiridol enhances the radiosensitivity of Eca109 cells and to
elucidate its mechanism in vitro.
Materials and methods
Cell lines and treatment
The human esophageal carcinoma cell line Eca109,
provided by the Tumor Cell Library of the Chinese Academy of
Medical Science, was used in this study. The study was approved by
the Ethics Committee of The Second Hospital of Dalian Medical
University, Dalian, China. The cell line was cultured in RPMI-1640
containing 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin and maintained at 37°C in an atmosphere of
5% CO2 and 95% room air.
Reagents
Flavopiridol (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in DMSO to a stock concentration of 1 mg/ml and stored at
−4°C.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells were seeded in a 96-well plate and once
attached they were treated with various concentrations of
flavopiridol ranging from 0 to 517.5 nmol/l. After 48 h, the cells
were stained with 2 mg/ml MTT, lysed in DMSO and the absorbance was
read on an enzyme-labeling instrument at 540 nm. Each concentration
had 3 wells and the experiment was repeated in triplicate.
Clonogenic survival
Cells were trypsinized to single-cell suspension and
200 cells were seeded into each well of a six-well tissue culture
plate. They were divided into 2 groups: radiation only (R) and
flavopiridol with radiation (FR). Each group had 3 wells. After
cells were assigned, group FR received flavopiridol (concentration
0.2×IC50) and group R received DMSO. Graded doses of 0,
2, 4, 6 and 8 Gy of radiation were administered with a 6-MV X-ray.
Colonies were stained with crystal violet 15 days after seeding,
the number of colonies containing at least 50 cells was determined
and the surviving fractions were calculated. This experiment was
repeated in triplicate.
Flow cytometry
Cells were divided into 4 groups: flavopiridol only
(F), radiation only (R), flavopiridol with radiation (FR) and
control (C). Cells in group F were cultured with flavopiridol for
48 h. Group R received 6 Gy radiation. Group FR also received 6 Gy
radiation once the cells had been cultured with flavopiridol for 48
h. Following radiation treatment, the cells were cultured for
another day. The cells were washed with phosphate-buffered saline
(PBS), collected with trypsinization, stained with propidium iodide
(PI) for cell cycle analysis or Annexin V/PI for apoptotic analysis
and analyzed using flow cytometry.
Western blot analysis
The treatment protocols were the same as for the
flow cytometry assay. After treatment, the cells were collected and
washed with PBS. They were lysed with extraction buffer. Cellular
debris was cleared by centrifugation and the protein concentration
was assessed using a BCA protein assay. An equal amount of protein
was subjected to SDS-polyacrylamide gel electrophoresis and
transferred onto a polyvinylidene difluoride (PVDF) membrane. The
membranes were probed with primary and secondary antibodies. The
proteins were visualized using enhanced chemiluminescence (ECL) in
a dark room.
Results
MTT assay
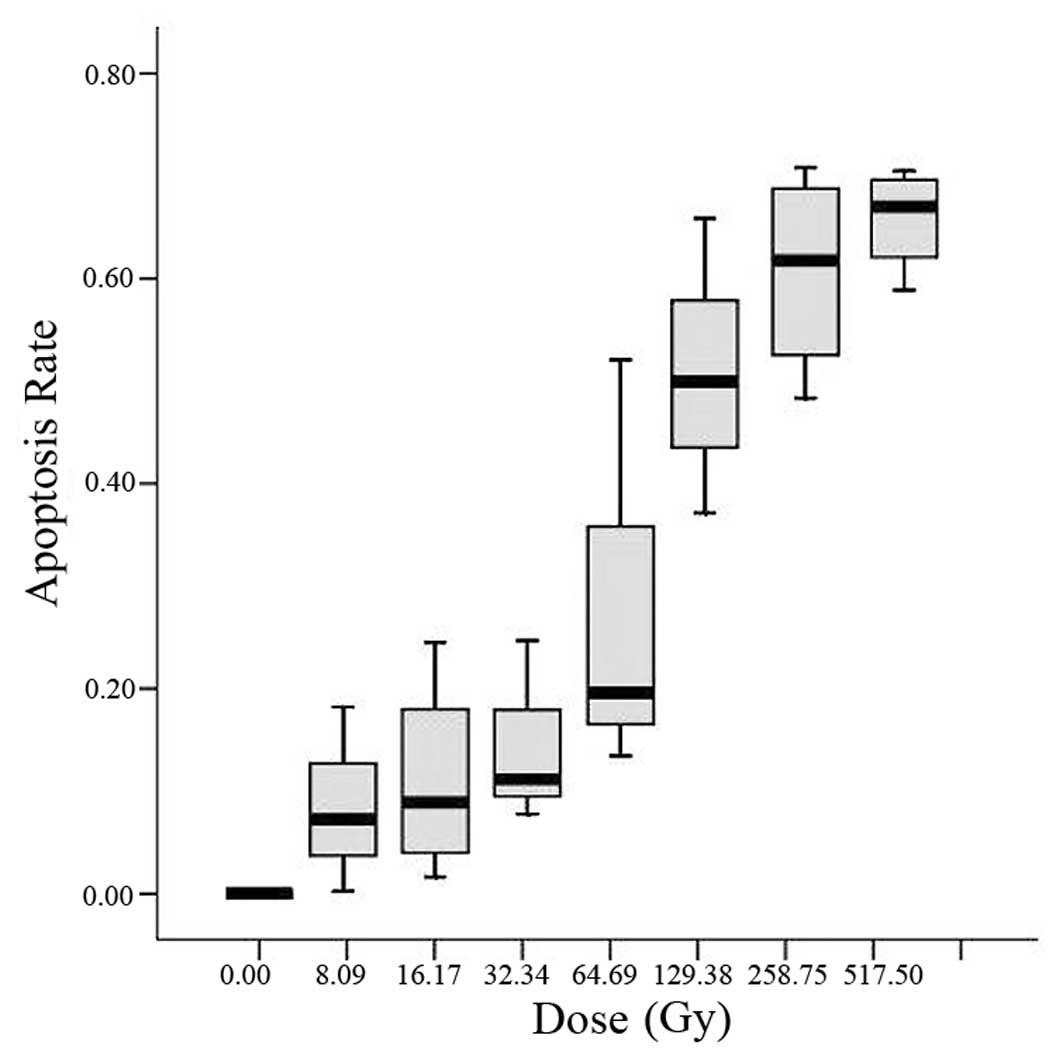
The dose-dependent cytotoxicity of flavopiridol
alone was determined using an MTT assay. Cells were incubated in
the presence of gradient concentrations of flavopiridol (ranging
from 0 to 517.5 nmol/l) for 48 h. Flavopiridol alone reduced cell
survival and the effect was dose dependent (Fig. 1). Its IC50 was 193.3
nmol/l.
Clonogenic survival
To determine the effects of flavopiridol on Eca109
radiosensitivity, a clonogenic survival analysis was performed. As
shown in Fig. 2, the number of
colonies was decreased with increasing irradiation dose in both
groups (R and FR). The number of FR group decreased more
significantly. Flavopiridol enhanced the radiosensitivity of Eca109
cells. Multi-target single-hit model fitting survival curves
resulted in a sensitization enhancement ratio (SER) of 1.194.
Flow cytometry
To determine whether flavopiridol influences the
cell cycle distribution of Eca109 cells, cells were treated as
above and subjected to flow cytometry (Fig. 3). The percentage of G2/M
cells in group FR (29.18±9.26%) and group F (18.23±7.47%) was
greater than that in groups C (3.46±2.47%) and R (5.81±2.50%). The
difference was statistically significant (P<0.05). The data
suggested that a low dose of flavopiridol enhances the percentage
of Eca109 cells in phase G2/M when treated by
radiation.
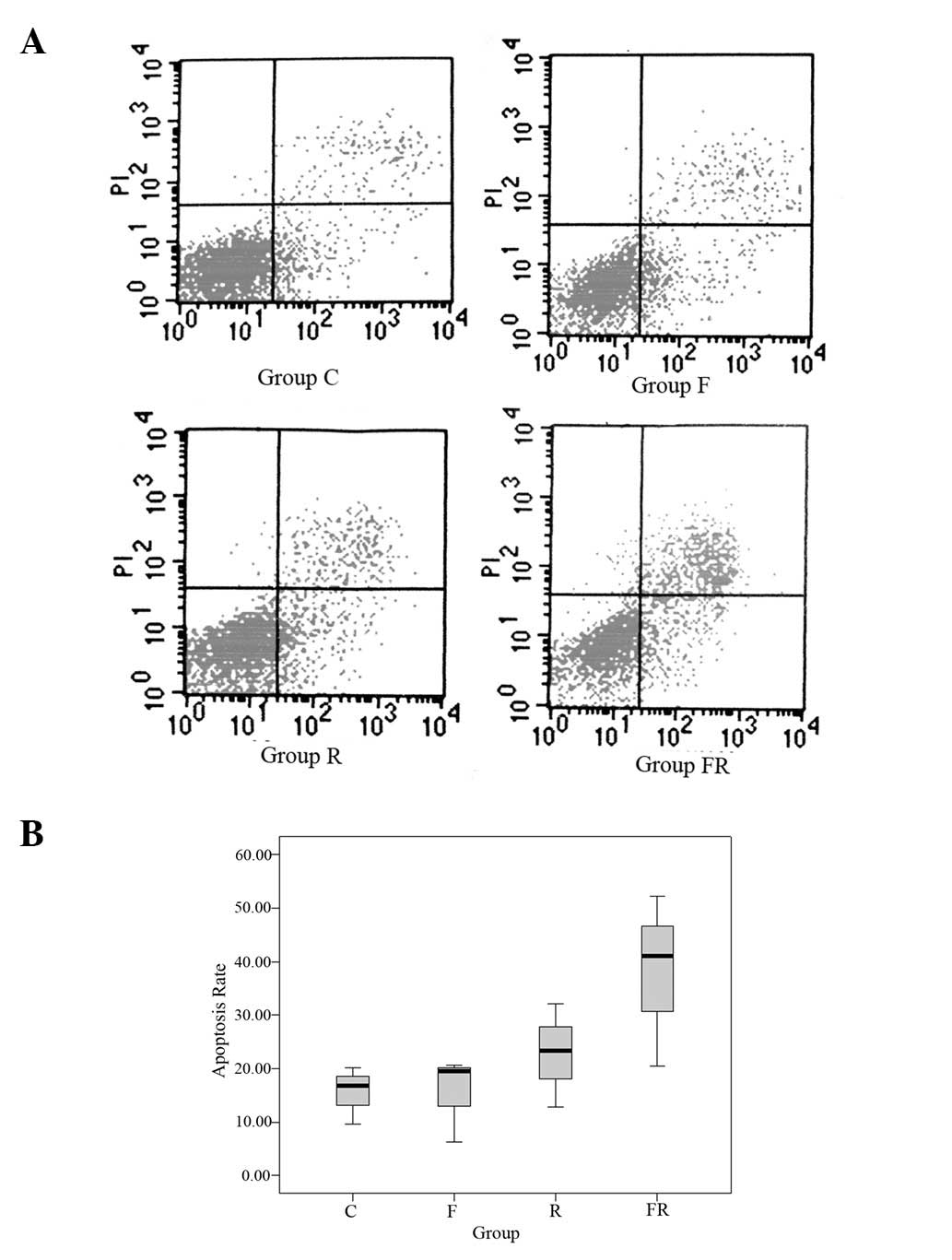
Annexin V-FITC/PI apoptosis detection was used to
determine whether flavopiridol enhanced apoptosis in Eca109 cells
induced by radiation (Fig. 4). The
control group showed 15.53±5.40% apoptosis, group F showed
15.50±7.95%, group R showed 22.76±9.71% and group FR showed
37.92±16.15%. The apoptosis rate in group FR was greater than that
of the other three groups. The difference was statistically
significant (P<0.05). The results revealed that flavopiridol
enhances apoptosis in Eca109 cells induced by radiation.
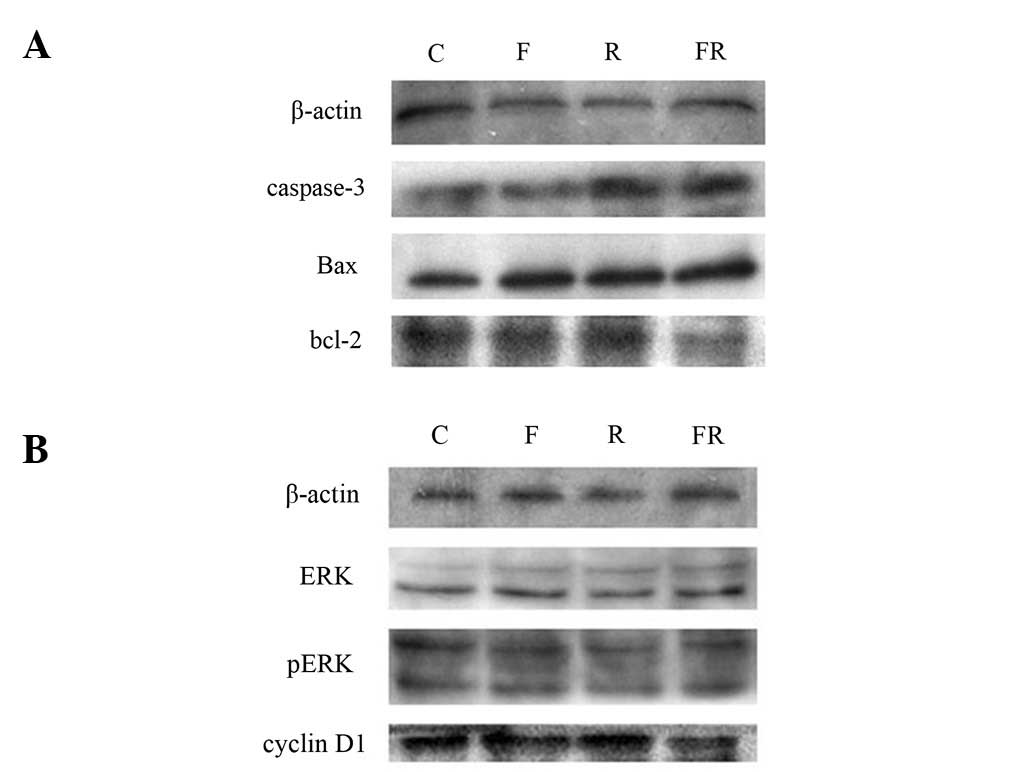
Western blot analysis
Caspase-3, Bax and Bcl-2 protein levels were
measured to determine whether flavopiridol influences apoptosis
proteins. As shown in Fig. 5A, the
level of caspase-3 and Bax in group FR increased significantly and
the level of Bcl-2 in group FR decreased significantly. It is
possible that cell death was caused by increasing levels of
apoptotic proteins caspase-3 and Bax. Cyclin D1 protein was also
detected to determine whether the increase in the percentage of
Eca109 cells in phase G2/M was due to the decreased
level of cyclin D1 protein. As shown in Fig. 5B, the level of cyclin D1 protein in
group FR was significantly decreased. It suggested that
flavopiridol enhanced phase G2/M in Eca109 by decreasing
the level of cyclin D1 protein. ERK/pERK protein was detected to
determine whether the Ras/Raf-1/Mek/ERK pathway was inhibited. The
result was negative. The difference was statistically significant
(P<0.05).
Discussion
From the present study, two conclusions may be
drawn. Firstly, flavopiridol enhances the radiosensitivity of
Eca109 cells. It increases the cell apoptosis induced by radiation.
Secondly, the radiosensitizing effect of flavopiridol may be
brought about by decreasing the level of cyclin D1 protein, thereby
increasing the percentage of cells in the G2/M
phase.
Cyclin D1 is overexpressed in several cancers
(14,15). It is also overexpressed in
esophageal cancer (16). Cyclin D1
drives cells into the S phase. The trigger is likely to be assembly
with its catalytic partners, CDK-4 and-6 (17). Flavopiridol decreases the level of
cyclin D1, and according to Camphausen et al it also
inhibits the activity of cdk-4 (10). Flavopiridol decreases the complex of
cyclin D1 with CDKs. This may explain why G2/M arrest
occurred. The transcriptional induction of cyclin D1 by growth
factors is dependent on the Ras/Raf-1/Mek/ERK pathway (18–20).
To elucidate whether flavopiridol reduced cyclin D1 by inhibiting
this pathway, another western blot analysis for ERK/pERK was
performed. There was no statistically significant difference. It
was hypothesized that flavorpiridol decreases the level of cyclin
D1 directly. The mechanism remains to be elucidated.
A cell’s relative radiosensitivity is determined by
the cell cycle phase. Cells are most radiosensitive in the
G2/M phase, less sensitive in the G1 phase
and least sensitive during the latter part of the S phase (5). Cell death was increased in the
flavopiridol with radiation group. Caspase-3, Bax and Bcl-2 protein
levels were investigated to elucidate the mechanism of cell death.
Caspase is a family of proteases that is the core component of an
intrinsic suicide machinery. Caspase-3 is a type of effector
caspase. It cleaves various cellular proteins leading to apoptotic
cell death (21). All pathways to
apoptosis converge on the activation of caspases. They may be
classified into two types depending on whether they require Bcl-2
family proteins. Bcl-2 family members have been grouped into three
classes. One class inhibits apoptosis (such as Bcl-2), the second
class promotes apoptosis (such as Bax) and the third binds and
regulates Bcl-2 proteins to promote apoptosis (22). Caspase-3 and Bax protein was
increased significantly in cells treated with flavopiridol and
radiation and Bcl-2 protein was significantly decreased. It may be
hypothesized that flavopiridol promotes Bax and inhibits Bcl-2,
thereby promoting caspase-3 to lead to apoptosis. This result was
consistent with the flow cytometry result.
These in vitro data suggest that flavopiridol
has radiosensitizing effects in Eca109 cells, but further
investigation is required for in vivo tumor models. The
mechanism also needs to be elucidated.
Acknowledgements
The authors would like to thank
Professor Lu Shen (The Central Laboratory of The Second Hospital of
Dalian Medical University, Dalian, China) for his helpful advice
and discussion. They also thank Chen Yinghai and Zheng Jin for
their excellent technical assistance.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008 v1.2, Cancer Incidence and
Mortality Worldwide: IARC CancerBase No. 10 (Internet).
International Agency for Research on Cancer; Lyon, France: pp.
2010http://globocan.iarc.fr.
Accessed March 15, 2012.
|
|
2
|
Brown LM and Devesa SS: Epidemiologic
trends in esophagealand gastric cancer in the United States. Surg
Oncol Clin N Am. 11:235–256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 4:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
MacLachlan TK, Sang N and Giordano A:
Cyclins, cyclin-dependent kinases and cdk inhibitors: implications
in cell cycle control and cancer. Crit Rev Eukaryot Gene Expr.
5:127–156. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zafonte BT, Hulit J, Amanatullah DF,
Albanese C, Wang C, Rosen E, Reutens A, Sparano JA, Lisanti MP and
Pestell RG: Cell-cycle dysregulation in breast cancer therapies
targeting the cell cycle. Front Biosci. 5:938–961. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sedlacek HH: Mechanisms of action of
flavopiridol. Crit Rev Oncol Hematol. 38:139–170. 2001. View Article : Google Scholar
|
|
7
|
Monga M and Sausville EA: Developmental
therapeutics program at the NCI: molecular target and drug
discovery process. Leukemia. 16:520–526. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Senderowicz AM and Sausville: Preclinical
and clinical development of cyclin-dependent kinase modulators. J
Natl Cancer Inst. 92:376–387. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaur G, Stetler-Stevenson M, Sebers S,
Worland P, Sedlacek H, Myers C, Czech J, Naik R and Sausville E:
Growth inhibition with reversible cell cycle arrest of carcinoma
cells by flavone L86-8275. JNCI J Natl Cancer Inst. 84:1736–1740.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Camphausen K, Brady K, Burgan W, Carra M,
Russell J, Bull E and Tofilon P: Flavopiridol enhances human tumor
cell radio-sensitivity and prolongs expression of gammaH2AX foci.
Mol Cancer Ther. 3:409–416. 2004.PubMed/NCBI
|
|
11
|
Byrd JC, Shinn C, Waselenko JK, Fuchs EJ,
Lehman TA, Nguyen PL, Flinn IW, Diehl LF, Sausville E and Grever
MR: Flavopiridol induces apoptosis in chronic lymphocytic leukemia
cells via activation of caspase-3 without evidence of bcl-2
modulation or dependence on functional p53. Blood. 92:3804–3816.
2008.PubMed/NCBI
|
|
12
|
Patel V, Senderowicz AM, Pinto D, Igishi
T, Raffeld M, Quintanilla-Martinez L, Ensley JF, Sausville EA and
Gutkind JS: Flavopiridol, a novel cyclin-dependent kinase
inhibitor, suppresses the growth of head and neck squamous cell
carcinomas by inducing apoptosis. J Clin Invest. 102:1674–1681.
1998. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Bhuiyan M and Sarkar FH: Induction
of apoptosis and inhibition of c-erbB-2 in MDA-MB-435 cells by
genistein. Int J Oncol. 15:525–533. 1999.PubMed/NCBI
|
|
14
|
Fredersdorf S, Burns J, Milne AM, Packham
G, Fallis L, Gillett CE, Royds JA, Peston D, Hall PA, Hanby AM,
Barnes DM, Shousha S, O’Hare MJ and Lu X: High level expression of
p27kip1 and cyclin D1 in some human breast cancer cells: Inverse
correlation between the expression of p27kip1 and degree of
malignancy in human breast and colorectal cancers. Proc Natl Acad
Sci USA. 94:6380–6385. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gansauge S, Gansauge F, Ramadani M, Stobbe
H, Rau B, Harada N and Beger HG: Overexpression of cyclin D1 in
human pancreatic carcinoma is associated with poor prognosis.
Cancer Res. 57:1634–1637. 1997.PubMed/NCBI
|
|
16
|
Adélaide J, Monges G, Dérdérian C, Seitz
JF and Birnbaum D: Esophageal cancer and amplification of the human
cyclin D gene CCND1/PRAD1. Br J Cancer. 71:64–68. 1995.PubMed/NCBI
|
|
17
|
Blagosklonny MV and Pardee AB: The
restriction point of the cell cycle. Cell Cycle. 1:103–110. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Filmus J, Robles AI, Shi W, et al:
Induction of cyclin D1 overexpression by activated ras. Oncogene.
9:3627–3633. 1994.PubMed/NCBI
|
|
19
|
Aktas H, Cai H and Cooper GM: Ras links
growth factor signaling to the cell cycle machinery via regulation
of cyclin D1 and the cdk inhibitor p27KIP1. Mol Cell Biol.
17:3850–3857. 1997.PubMed/NCBI
|
|
20
|
Winston JT, Coats SR, Wang YZ and Pledger
WJ: Regulation of the cell cycle machinery by oncogenic ras.
Oncogene. 12:127–134. 1996.PubMed/NCBI
|
|
21
|
Chang HY and Yang X: Proteases for cell
suicide: functions and regulation of caspases. Microbiol Mol Biol
Rev. 64:821–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|