Introduction
Primary cardiac sarcomas are extremely rare, with
angiosarcoma, fibrosarcoma, rhabdomyosarcoma and malignant fibrous
histiocytoma occurring in order of decreasing frequency (1). Synovial sarcomas arise from
mesenchymal tissue, which differentiates sufficiently to have the
histological appearance of synovium. The majority (80–95%) of
tumors are reported in the extremities of young adults, with
two-thirds being located in the lower limbs. Other sites of origin
include the head and neck, paravertebral region, chest and
abdominal wall (2). At present, few
cases of synovial sarcoma that occur in pericardium have been
published in the literature (1,3–8).
Pericardial synovial sarcoma is associated with an extremely poor
survival rate with a disease-free survival of 12 months after
surgery (1), and an overall
survival of 7 months (9). Due to
its rarity, there are no available treatment guidelines for this
disease. The present case report describes a case of pericardial
synovial sarcoma that was treated with surgery and adjuvant
chemotherapy. Informed consent was obtained from the patient.
Case report
Patient
A 45-year-old female presented in January 2010 with
progressive dyspnea upon exertion, a paroxysmal cough and night
sweats. The patient was diagnosed with tuberculous pericarditis and
received antituberculous drug therapy. A physical examination
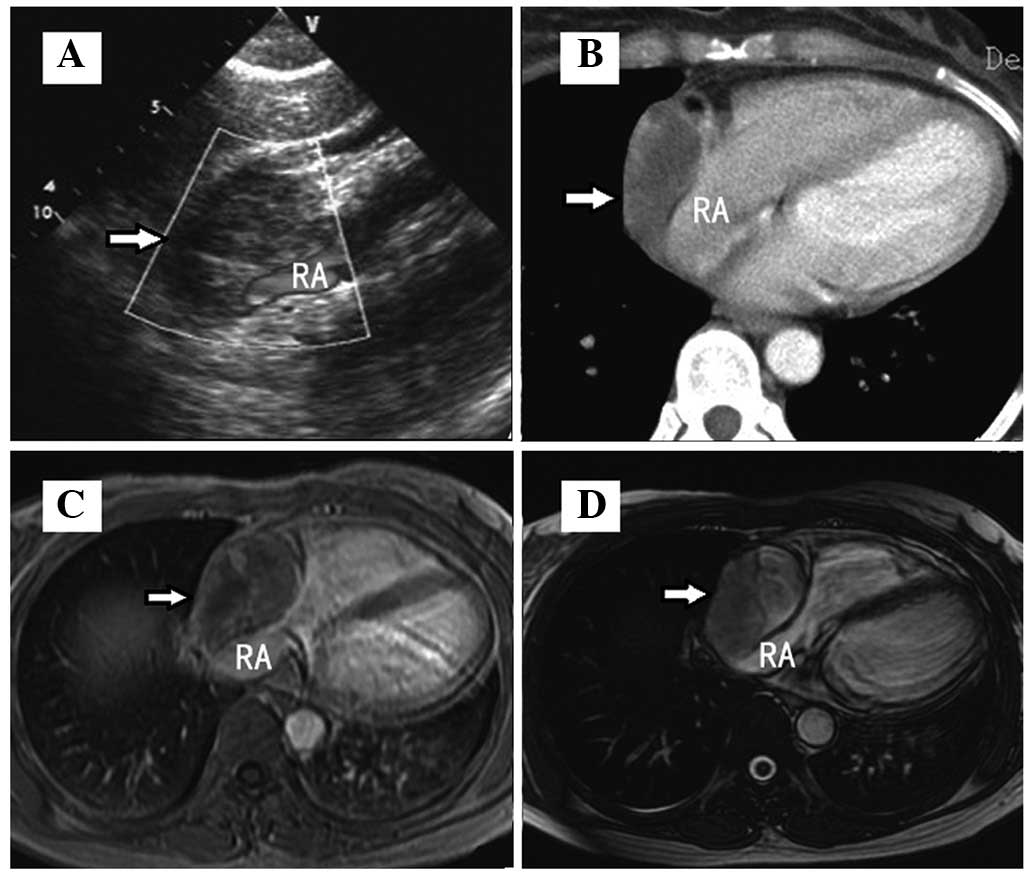
revealed quiet heart sounds. Echocardiography (iE33; Philips
Electronics, Amsterdam, The Netherlands) revealed a mass measuring
3.8×5.2 cm within the pericardial space and predominantly over the
right atrium, as well as a pericardial effusion. Contrast-enhanced
computed tomography (CT; Siemens AG, Erlangen, Germany)
demonstrated a low-attenuation lesion in the pericardium, with
inhomogeneous peripheral enhancement. Magnetic resonance imaging
(MRI; Signa Excite 1.5T, General Electric Company, Fairfield, CT,
USA) revealed a 3.4×5.2-cm, high-signal, heterogeneous,
multilocular mass on 2D fast imaging employing steady-state
acquisition (FIESTA) sequence images. The lesion was present in the
pericardium adjacent to the right atrium, which was significantly
extruded. T1-weighted post-gadolinium imaging identified mild
heterogeneous enhancement (Fig. 1).
No positive mediastinal nodes were detected.
Surgery
A thoracotomy was performed and revealed marked soft
pericardial adhesions. The tumor was located between the
pericardial serous layer and the right atrium, arising from the
junction of the inferior vena cava and the pericardium. The tumor
was excised and a partial pericardiectomy was performed with
negative microscopic margins. The pericardial effusion cytology was
negative for malignant cells.
Tumor characteristics
The excised mass measured ∼5×6 cm and had a friable
texture consisting of blood clots and necrotic tissue.
Microscopically, the tumor exhibited a characteristic biphasic
appearance consisting of hypercellular spindled-cell sheets.
Hemorrhaging, necrosis, heteromorphism and Allotypic nuclear
division were also noted (Fig. 2).
Immunohistochemistry demonstrated positive staining for epithelial
membrane antigen (EMA), vimentin and Bcl-2, but negative staining
for CD99, CD34, CD68, S100, cytokeratin, desmin, calretinin,
HMBE-1, CK5/6 and smooth muscle actin, confirming a diagnosis of
biphasic synovial sarcoma. One and a half months later, the patient
was referred for adjuvant chemotherapy with a combination of
adriamycin (20 mg/m2, 3 times every 3 weeks) and
ifosfamide (3 g/m2/d on 3 subsequent days, 3-week
intervals).
Discussion
Primary sarcomas of the heart are rare (10) and include a number of
histopathological variants. In total, 8–10% of cardiac sarcomas are
synovial sarcomas, which are histologically classified into the
following 4 subtypes depending on the relative proportion of
epithelial and spindle cells: (i) Biphasic, (ii) monophasic fibrous
(spindle cell), (iii) monophasic epithelial and (iv) poorly
differentiated. Primary pericardial synovial sarcomas are the
rarest, with only 7 reports in the literature (1,3–8), and
are associated with the longest survival period of 14 years
(3).
In the early stages of pericardial synovial sarcoma,
symptoms are usually nonspecific or slight chest tightness is noted
(4). With the progression of
malignant tumors, symptoms may appear, including local invasion,
which may lead to arrhythmias and tamponade, and vascular invasion,
which may lead to dyspnea, chest pain or heart failure. On CT and
MRI, synovial sarcoma of the pericardium typically presents as a
solitary bulky mass that does not infiltrate the pericardium. CT is
useful for the identification of subtle soft tissue calcifications
and local bony changes. In a previous study by O’Sullivan et
al(2), MRI was considered to
represent the best modality for the detection and staging of soft
tissue tumors. Synovial sarcomas are usually multilocular with
internal septa and are well-defined in the majority of cases. In
the study by O’Sullivan et al Hemorrhaging was noted in
>40% of the lesions, which demonstrated high-intensity signals
on T1- and T2-weighted images. No difference was noted between MRI
characteristics for the monophasic and biphasic pathological
subtypes.
Due to the rarity of primary pericardial synovial
sarcoma, there are no standard treatment guidelines currently
available (3,5). However, surgical resection is widely
accepted as a primary treatment. Depending on the location of the
tumor, various surgical approaches may be selected. Radiotherapy is
recommended for positive resection margins to reduce local
recurrence rates. However, cardiac irradiation may lead to
long-term cardiac damage. In the case of a strong family history of
cardiovascular problems, the total dose of radiation must be
restricted to avoid long-term toxicity. Notably, no standardized
chemotherapy protocol is currently followed. According to a
previous study, adriamycin or a combination of adriamycin and
ifosfamide represent the most effective chemotherapeutic agents
(3). In the present case, the
combination of adriamycin and ifofamide was used with a
satisfactory curative effect.
Immunohistochemical markers, including vimentin, EMA
and cytokeratin, are used to aid the pathological diagnosis of
synovial sarcoma (4). However,
further cytogenetic analysis may also be necessary. From all the
patients with synovial sarcoma, >90% have a t(X;18)
translocation mutation, which has not been found to be associated
with other sarcomas. This translocation involves the SSX1 or SSX2
gene on the X chromosome (Xp11) and the SYT gene on chromosome 18
(18q11). Subtypes of these translocations have been revealed to
correlate with the various histological subtypes of synovial
sarcoma (11).
Although primary cardiac synovial sarcoma is
associated with an extremely poor survival rate, for tumors of
pericardial origin, the overall survival remains unknown. In the
present case, following surgical excision and post-operative
adjuvant chemotherapy, the patient has remained clinically free of
disease for 32 months.
References
|
1
|
Al-Rajhi N, Husain S, Coupland R, McNamee
C and Jha N: Primary pericardial synovial sarcoma: a case report
and literature review. J Surg Oncol. 70:194–198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O’Sullivan PJ, Harris AC and Munk PL:
Radiological features of synovial cell sarcoma. Br J Radiol.
81:346–356. 2008.
|
|
3
|
Van der Mieren G, Willems S, Sciot R, et
al: Pericardial synovial sarcoma: 14-year survival with
multimodality therapy. Ann Thorac Surg. 78:e41–e42. 2004.PubMed/NCBI
|
|
4
|
Moorjani N, Peebles C, Gallagher P and
Tsang G: Pericardial synovial sarcoma. J Card Surg. 24:349–351.
2009. View Article : Google Scholar
|
|
5
|
de Zwaan C, Bekkers SC, van Garsse LA,
Jansen RL and van Suylen RJ: Primary monophasic mediastinal,
cardiac and pericardial synovial sarcoma: a young man in distress.
Neth Heart J. 15:226–228. 2007.PubMed/NCBI
|
|
6
|
Schumann C, Kunze M, Kochs M, Hombach V
and Rasche V: Pericardial synovial sarcoma mimicking pericarditis
in findings of cardiac magnetic resonance imaging. Int J Cardiol.
118:e83–e84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anand AK, Khanna A, Sinha SK, Mukherjee U,
Walia JS and Singh AN: Pericardial synovial sarcoma. Clin Oncol (R
Coll Radiol). 15:186–188. 2003. View Article : Google Scholar
|
|
8
|
Akerström F, Santos B, Alguacil AM,
Orradre JL, Lima P and Zapardiel S: Pericardial synovial sarcoma.
Thorac Cardiovasc Surg. 59:175–177. 2011.
|
|
9
|
Oizumi S, Igarashi K, Takenaka T, et al:
Primary pericardial synovial sarcoma with detection of the chimeric
transcript SYT-SSX. Jpn Circ J. 63:330–332. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burke AP, Cowan D and Virmani R: Primary
sarcomas of the heart. Cancer. 69:387–395. 1992. View Article : Google Scholar
|
|
11
|
Clark J, Rocques PJ, Crew AJ, et al:
Identification of novel genes, SYT and SSX, involved in the
t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma.
Nat Genet. 7:502–508. 1994. View Article : Google Scholar : PubMed/NCBI
|