Introduction
Leukemia cutis (LC) is a rare condition that is
characterized by the presence of leukemic cell infiltration into
the dermis, subcutis, skin adnexa and blood vessels (1). The lesions usually present with
subcutaneous nodules, multiple papules or violaceous or brown
plaques (2). LC has various
cutaneous manifestations, which may make it difficult to
distinguish the disease from other dermatoses. The incidence of LC
was reported in 2–3% of patients with acute myeloid leukemia (AML)
(1).
Liver cancer is the fifth most common type of cancer
in Korea (3). Hepatocellular
carcinoma (HCC) accounts for 85–90% of all primary liver cancers,
with a median survival of less than one year (4). The most common risk factors for HCC
are chronic infection with hepatitis B virus or hepatitis C virus.
Heavy alcohol intake is a well-established HCC risk factor and
aflatoxin, obesity and diabetes are also possible risk factors
(5,6).
Multiple primary cancer is defined as a specific
malignant tumor, manifesting as more than one primary tumor that is
diagnosed in the same patient, either simultaneously or
sequentially (7,8). Synchronous multiple primary cancer is
defined as two or more tumors occurring within six months of each
other. The incidence of synchronous multiple primary cancer is
uncommon and it is even less common to observe a synchronous solid
tumor with a hematological malignancy. Recently, cases of solid
tumor synchronously presenting with hematological malignancy were
reported (7). However, the global
synchronous occurrence of two primary malignancies presenting as
AML and hepatocellular carcinoma is rare, particularly in cases of
AML presenting as LC with HCC. Although LC has been reported in
Korea, a case of LC associated with AML that was diagnosed
simultaneously with hepatocellular carcinoma and tonsillar
involvement has been identified in the present study. Therefore,
the present study describes this case with a review of the
literature. Written informed consent was obtained from the
patient’s family.
Case report
A 53-year-old male was referred to Konkuk University
Chungju Hospital (Chungju, Korea) for generalized cutaneous nodules
on the face, anterior chest wall and legs (Fig. 1), as well as a sore throat. The
patient had no history of medical systemic disease, with the
exception of hypertension. Furthermore, there was no history of
fever, trauma, weight loss, bleeding or any systemic illness.
However, the patient was a chronic alcoholic. At admission, the
hemoglobin level was 7.0 g/dl, the platelet count was
10.3×103/μl and the white blood cell (WBC) count was
20.8×103/μl. A differential count revealed the
following: Neutrophils, 10%; monocytes, 25%; blast cells, 24%;
carcinoembryonic antigen, 1.0 ng/ml; carbohydrate antigen 19-9,
12.2 U/ml; α-fetoprotein (AFP), 1,650 ng/ml; erythrocyte
sedimentation rate, 99 mm/h; and C-reactive protein, 14.1 mg/l. The
serology studies, which included hepatitis B surface antigen,
antibodies to hepatitis C virus and a serum antibody titer against
HIV, Mycobacterium and Cytomegalovirus, were all
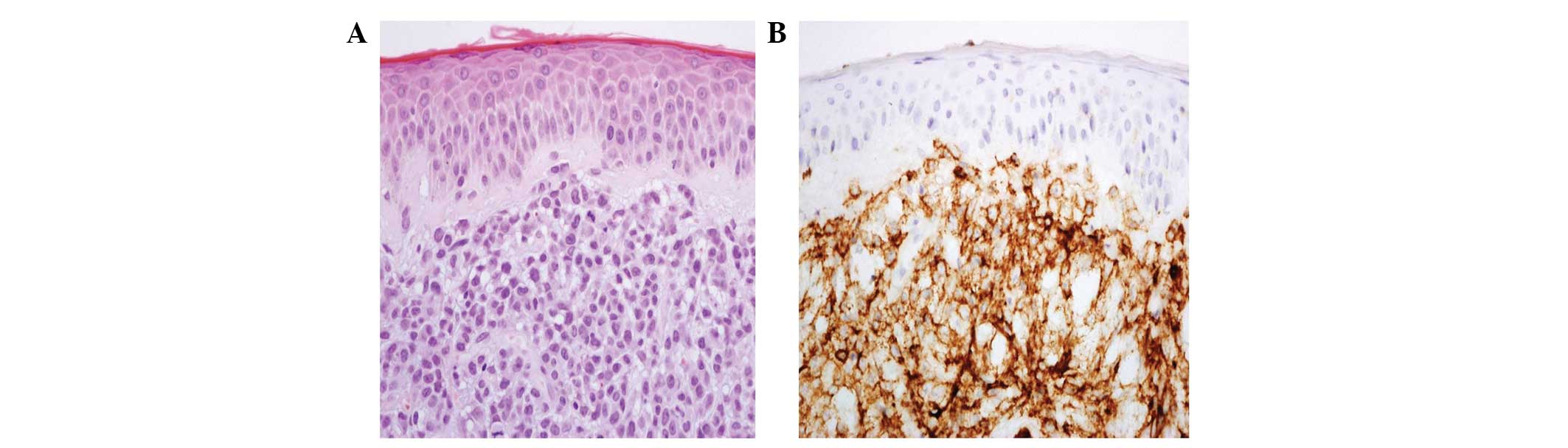
negative. Hematoxylin and eosin (H&E) staining of the upper and
deep dermis and a periappendiceal tissue skin biopsy specimen
demonstrated atypical monomorphic tumor cells with round to oval
vesicular nuclei and a moderate amount of cytoplasm infiltration
(Fig. 2). In addition, the
immunohistochemical staining revealed that the tumor cells were
positive for leukocyte common antigen and CD43, but negative for
CD34, CD68, C-kit, cytokeratin, myeloperoxidase and S-100 protein.
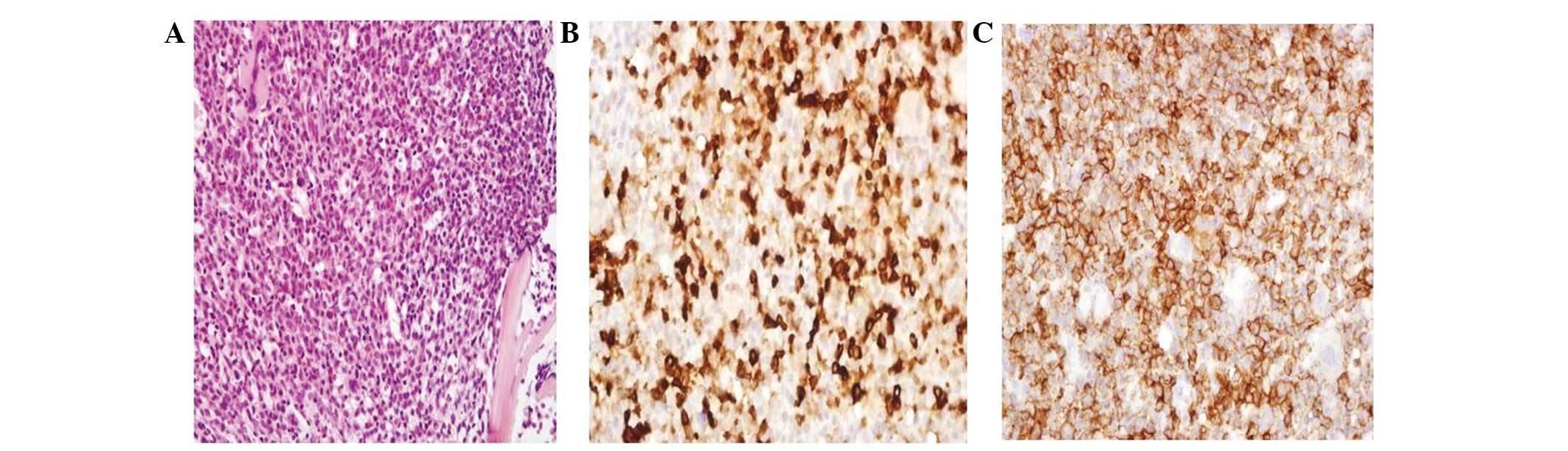
H&E staining of the bone marrow biopsy revealed packed marrow
with increased atypical cells, large nuclei with nuclear membranes,
dispersed chromatin and abundant cytoplasm. Furthermore, the cells
were positive for myeloperoxidase, CD43 and CD68 (Fig. 3). H&E staining of the right
tonsillar punch biopsy demonstrated several irregular fragments of
tonsils that revealed diffuse infiltration of the atypical cells.
The immunohistochemical staining revealed that the cells were
positive for myeloperoxidase, but negative for CD34, CD43 and CD20
(Fig. 4). Liver magnetic resonance
imaging (MRI) demonstrated a 6-cm ill-defined mass in hepatic
segments 4 and 8. H&E staining of the liver biopsy revealed
immature blasts infiltrating the hepatocellular carcinoma that were
positive for CD3 and CD68, but negative for myeloperoxidase and
CD34 (Fig. 5).
A diagnosis of acute myelomonocytic leukemia
(AML-M4) was confirmed with skin, tonsil and hepatic involvement
and HCC. The patient was administered standard induction
chemotherapy with idarubicin (12 mg/m2/IV for 1–3 days)
and cytarabine (100 mg/m2/IV for 1–7 days). On the 36th
day, the bone marrow examination demonstrated complete remission
with 2.2% blast cells. With regard to the HCC, the patient was
treated by transcatheter hepatic arterial chemoembolization (TACE).
The patient was scheduled to undergo consolidation chemotherapy,
but the complete blood count revealed a WBC count of
21.7×103/μl (72% blasts). Therefore, relapse of AML was
suspected. The patient did not wish to undergo further treatment
and succumbed shortly thereafter, due to the progression of
AML.
Discussion
The prevalence of multiple synchronous primary
neoplasm is 2–10% in all the carcinomas that have been reported in
literature (8). The patients who
are diagnosed with cancer may have a 20% higher risk of a new
primary cancer compared with the general population (8). The synchronous occurrence of two
primary malignancies presenting as AML and hepatocellular carcinoma
is rare. LC refers to neoplastic leukemic cells that infiltrate
into the skin, most often in conjunction with systemic leukemia. In
the biopsy-comfirmed LC case studies, the prevalence of this
disease was 2–3%. Skin involvement in myeloid leukemia is
associated with monocytic differentiation, central nervous system
involvement and aneuploidy of chromosome 8 (9). Furthermore, AML accompanied by LC has
an aggressive and poor clinical course (10,11).
In the present study, liver MRI was performed due to the history of
chronic heavy alcoholism and an elevated AFP level of 1650 ng/ml
was observed. The MRI revealed a 6-cm ill-defined mass in hepatic
segments 4 and 8. The liver biopsy revealed that HCC cells and
immature blast cells coexisted. Following a diagnosis of acute
myelomonocystic leukemia, standard induction chemotherapy with
idarubicin and cytarabine was administered. Furthermore, the 6-cm
HCC lesion, the patient’s general condition and the laboratory
results made it impossible to perform a partial hepatectomy. The
6-cm mass was too large for radiofrequency ablation and
percutaneous ethanol injection. Therefore, TACE was performed.
However, prior to the consolidation chemotherapy, relapse of AML
was suspected, but the patient refused further treatment for AML.
As described previously, LC may be an indicator of acute leukemia
with poor prognosis. The patients who are diagnosed with a primary
cancer may develop a second primary cancer with variable clinical
manifestations. Due to the lack of guidelines for the standard
management of such a condition, the treatment modality should be
determined through therapeutic strategies that are specific to the
disease and performance status of the patient.
References
|
1
|
Cronin DM, George TI and Sundram UN: An
updated approach to the diagnosis of myeloid leukemia cutis. Am J
Clin Pathol. 132:101–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seymour JF, Pierce SA, Kantarjian HM,
Keating MJ and Estey EH: Investigation of karyotypic, morphologic
and clinical features in patients with acute myeloid leukemia blast
cells expressing the neural cell adhesion molecule (CD56).
Leukemia. 8:823–826. 1994.
|
|
3
|
Jung KW, Park S, Kong HJ, et al: Cancer
statistics in Korea: incidence, mortality, survival, and prevalence
in 2009. Cancer Res Treat. 44:11–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donato F, Tagger A, Gelatti U, et al:
Alcohol and hepatocellular carcinoma: the effect of lifetime intake
and hepatitis virus infections in men and women. Am J Epidemiol.
155:323–331. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gomaa AI, Khan SA, Toledano MB, Waked I
and Taylor-Robinson SD: Hepatocellular carcinoma: epidemiology,
risk factors and pathogenesis. World J Gastroenterol. 14:4300–4308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui Y, Liu T, Zhou Y, et al: Five cases
report of solid tumor synchronously with hematologic malignancy.
Cancer Res Treat. 44:63–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demandante CG, Troyer DA and Miles TP:
Multiple primary malignant neoplasms: case report and a
comprehensive review of the literature. Am J Clin Oncol. 26:79–83.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su WP: Clinical, histopathologic, and
immunohistochemical correlations in leukemia cutis. Semin Dermatol.
13:223–230. 1994.PubMed/NCBI
|
|
10
|
Paydaş S and Zorludemir S: Leukaemia cutis
and leukaemic vasculitis. Br J Dermatol. 143:773–779.
2000.PubMed/NCBI
|
|
11
|
Baer MR, Barcos M, Farrell H, Raza A and
Preisler HD: Acute myelogenous leukemia with leukemia cutis.
Eighteen cases seen between 1969 and 1986. Cancer. 63:2192–2200.
1989. View Article : Google Scholar : PubMed/NCBI
|