Introduction
Soft tissue sarcomas (STS) are rare tumors of
mesenchymal origin primarily affecting adults, with liposarcoma
being the most frequent histopathological subtype (1,2). Cases
of intraperitoneal liposarcoma, particularly primary mesenteric
liposarcoma, are rare (3–5). Based on the WHO classification,
liposarcoma is classified as well-differentiated (WDL), myxoid,
including round/cell liposarcoma, pleomorphic or dedifferentiated
(DDL) (6). According to one study,
the presence of areas of dedifferentiation in the WDL type occurs
in only 7% of cases (7). DDLs are
more aggressive than WDLs without dedifferentiation and the rate of
recurrence has been reported to be 41% (8).
The present study describes a rare case of a primary
sclerosing liposarcoma originating from the root of the mesentery
with areas of dedifferentiation and features of a leiomyosarcoma.
The patient was treated with trabectedin (Yondelis®) and
a complete remission (CR) was noted following four cycles of this
regimen. Written informed consent was obtained from the
patient.
Case report
A 47-year-old female with a medical history of
hypertension, supraventricular arrhythmia and no clinically
relevant family history, presented with abdominal pain and fever.
The hematological and biochemical evaluations were within the
normal limits. The work-up revealed an abdominal mass. Therefore,
the patient underwent a laparotomy five months later. The mass was
identified as a liposarcoma originating from the root of the
mesentery. The liposarcoma measured ~11.5 cm and was present in the
left hypochondrium in contact with the pancreatic tail, but not
adhering to the adjacent organs. The pathology report described a
high-grade lobulated sclerosing liposarcoma, with focal loss of
differentiation and wide areas of necrosis (Fig. 1). The mass measured 15×11×10 cm and
was well circumscribed and encapsulated by a thin fibrous capsule.
The cut section revealed a fascicular fibrous non-tender tissue of
a grey-white color, with the morphological features of a
leiomyosarcoma. Immunohistochemistry (IHC) revealed S-100-positive
lipoblasts. The liposarcoma was also positive for desmin and smooth
muscle actin (SMA), which was consistent with the features of a
leiomyosarcoma. Ki-67 staining reactivity was 10% and the
liposarcoma was negative for c-Kit (also known as CD117),
platelet-derived growth factor receptor and epithelial membrane
antigen.
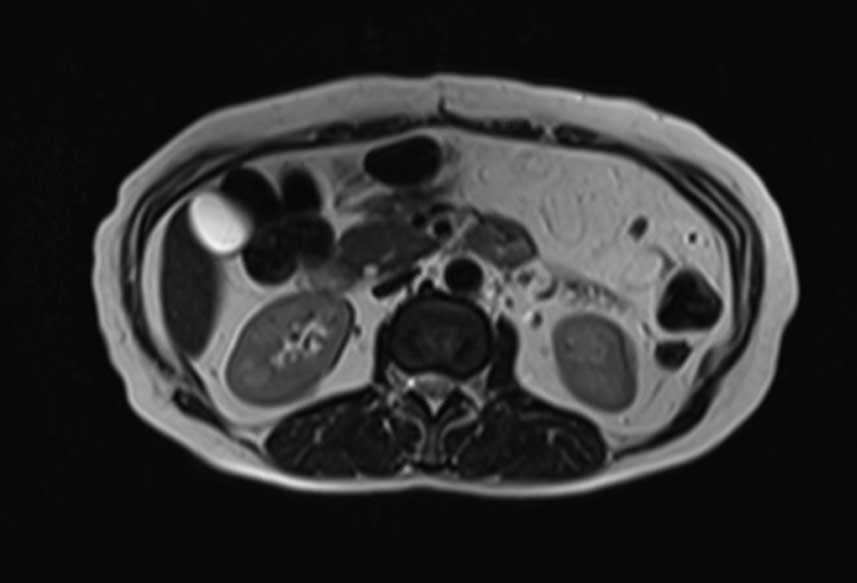
Three months later, the patient underwent an
additional laparotomy to remove a 12×10×3-cm mass of the same
histology as the first mass. Consequently, the patient was
administered iv chemotherapy with 50 mg/m2 doxorubicin
and 5 g/m2 ifosfamide. The re-evaluation with computed
tomography and magnetic resonance imaging (MRI) following the
completion of three cycles of chemotherapy revealed a local relapse
(Fig. 2).
Subsequently, the patient was administered
trabectedin (1.5 mg/m2 in 24 h infusion every 21 days).
Following the completion of four cycles, the disease was
re-evaluated using MRI, and a complete remission (CR) was noted
according to the RECIST criteria (Fig.
3). With regard to the adverse effects and toxicity, the
patient complained of diffuse abdominal pain and exhibited grade II
vomiting and hepatic toxicity (421 U/l serum glutamic-pyruvic
transaminase and 241 U/l serum glutamic oxaloacetic transaminase).
Within a few days, the levels of transaminases had returned to
normal. The patient continued to experience a CR through 15 cycles
of trabectedin therapy.
Discussion
Cases of primary mesenteric liposarcoma are rare
(3–5). The present study describes a case of a
mixed-type mesenteric liposarcoma with areas of dedifferentiation
and the features of a leiomyosarcoma, including IHC positivity for
SMA and desmin. To the best of our knowledge, a case of a
mesenteric sclerosing liposarcoma with the morphological and IHC
features of a leiomyosarcoma has only been described once (9), while a review of 32 cases of
dedifferentiated retroperitoneal and mesenteric liposarcoma
included one case of a dedifferentiated mesenteric liposarcoma with
smooth muscle elements (5).
Surgical excision represents the cornerstone of
treatment for liposarcoma and has been reported to be successful in
mesenteric liposarcoma when a clear surgical margin can be achieved
(6). Surgery is often followed by
radiation and/or adjuvant chemotherapy. However, despite the best
locoregional control, disease relapse is common. The standard
chemotherapy for the treatment of liposarcoma is doxorubicin
combined with ifosfamide (6).
Trabectedin has emerged as a favorable option for patients with
advanced STSs (10). Trabectedin is
a second-line option and is approved for advanced
previously-treated STSs in the EU. The drug has shown to be
effective in leiomyosarcoma and liposarcoma. Notably, tissue
changes have been observed prior to tumor shrinkage (11).
Trabectedin is a marine-derived antineoplastic
compound isolated from the Caribbean tunicate Ecteinascidia
turbinata(12). A modification
in the DNA conformation leads to the inhibition of activated
transcription, while constitutive transcription appears unaffected
(13). Trabectedin binds the DNA
minor groove, which, in turn, induces DNA bending towards the major
groove. The modification of DNA conformation leads to the
inhibition of activated transcription (10). Trabectedin interferes with the
transcription factors, DNA binding proteins and DNA repair
pathways. Transcription-coupled nucleotide-excision repair appears
to be significant in the cytotoxicity of this agent (10). In addition, trabectedin has been
shown to modulate the production of cytokines and chemokines by
tumor and normal cells, thus altering the microenvironment of the
tumor (14).
The antitumor activity of trabectedin has been
mainly reported in cases of liposarcoma, particularly the
myxoid/round cell type, leiomyosarcomas and synovial sarcomas. The
efficacy and safety of trabectedin in a patient population
consisting of cases with advanced and/or metastatic liposarcomas or
leiomyosarcomas that have failed treatment with standard agents,
anthracyclines and ifosfamide, was assessed in a randomized,
multicenter, open-label, phase II trial. This randomized clinical
trial demonstrated a statistically significant increase in the time
to progression and a reduction in the relative risk of progression
for patients who were treated with trabectedin (15). Retrospective analyses of the
responses in patients with liposarcomas following the failure of
anthracyclines and ifosfamide identified the best responses to be
mainly partial responses, with CRs occurring most often in
myxoid-type liposarcoma cases (16,17).
Therefore, the achievement of a CR, as in the present case, appears
to occur rarely for liposarcomas of the histological type described
in the present study.
Neutropenia, thrombocytopenia, anemia, elevated
liver enzymes and increases in bilirubin levels comprise the usual
adverse effects of trabectedin. Low grade nausea and vomiting are
also frequently encountered. The patient in the present case
experienced grade II vomiting and elevated liver enzymes. As in
previous studies, these adverse effects were resolved quickly
(12,17,18).
In conclusion, the current study presented a rare
case of a mixed-type mesenteric liposarcoma. The patient achieved a
CR following treatment with trabectedin. It thus appears that
trabectedin presents an option for cases of mesenteric liposarcoma
with areas of dedifferentiation that have recurred following
surgical excision and treatment with standard chemotherapeutic
agents, anthracyclines and ifosfamide.
References
|
1
|
Conyers R, Young S and Thomas DM:
Liposarcoma: Molecular genetics and therapeutics. Sarcoma.
2011:4831542011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dei Tos AP: Liposarcoma: new entities and
evolving concepts. Ann Diagn Pathol. 4:252–266. 2000.PubMed/NCBI
|
|
3
|
Jain SK, Mitra A, Kaza RCM and Malagi S:
Primary mesenteric liposarcoma: an unusual presentation of a rare
condition. J Gastrointest Oncol. 3:147–150. 2012.
|
|
4
|
Neuhaus SJ, Barry P, Clark MA, et al:
Surgical management of primary and recurrent retroperitoneal
liposarcoma. Br J Surg. 92:246–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hasegawa T, Seki K, Hasegawa F, et al:
Dedifferentiatted liposarcoma of the retroperitoneum and the
mesentery: varied growth patterns and histological grades - a
clinicopathological study of 32 cases. Hum Pathol. 31:717–727.
2000. View Article : Google Scholar
|
|
6
|
Skubitz KM and D’Adamo DR: Sarcoma. Mayo
ClinProc. 82:1409–1432. 2007. View Article : Google Scholar
|
|
7
|
Lucas DR, Nascimento AG, Sanjay BK and
Rock MG: Well-differentiated liposarcoma. The Mayo Clinic
experience with 58 cases. Am J Clin Pathol. 102:677–683. 1994.
|
|
8
|
Henricks WH, Chu YC, Goldblum JR and Weiss
SW: Dedifferentiated liposarcoma: a clinicopathological analysis of
155 cases with a proposal for an expanded definition of
dedifferentiation. Am J Surg Pathol. 21:271–281. 1997. View Article : Google Scholar
|
|
9
|
Russell MJ, Flynt FL, Harroff AL and
Fadere O: Dedifferentiated liposarcoma of the retroperitoneum with
extensive leiomyosarcomatous differentiation and human chorionic
gonadotropin production. Sarcoma. 2008:6580902008. View Article : Google Scholar
|
|
10
|
Cassier PA, Dufresne A, Blay JY and
Fayette J: Trabectedin and its potential in the treatment of soft
tissue sarcoma. Ther Clin Risk Manag. 4:109–116. 2008.
|
|
11
|
ESMO/European Sarcoma Network Working
Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
23(Suppl 7): vii92–vii99. 2012.
|
|
12
|
Carter NJ and Keam SJ: Trabectedin: A
review of its use in the management of soft tissue sarcoma and
ovarian cancer. Drugs. 67:2257–2276. 2007. View Article : Google Scholar
|
|
13
|
Friedman D, Hu Z, Kolb EA, et al:
Ecteinascidin-743 inhibits activated but not constitutive
transcription. Cancer Res. 62:3377–3381. 2002.
|
|
14
|
D’Incalci M and Galmarini CM: A review of
trabectedin (ET-743): a unique mechanism of action. Mol Cancer
Ther. 9:2157–2163. 2010.PubMed/NCBI
|
|
15
|
Demetri GD, Chawla SP, von Mehren M, et
al: Efficacy and safety of trabectedin in patients with advanced or
metastatic liposarcoma or leiomyosarcoma after failure of prior
anthracyclines and ifosfamide: Results of a randomized phase II
study of two different schedules. J Clin Oncol. 27:4188–4196. 2009.
View Article : Google Scholar
|
|
16
|
Grosso F, Jones RL, Demetri GD, et al:
Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated
myxoid liposarcomas: a retrospective study. Lancet Oncol.
8:595–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Christinat A and Leyvraz S: Role of
trabectedin in the treatment of soft tissue sarcoma. Onco Targets
Ther. 2:105–113. 2009.PubMed/NCBI
|
|
18
|
Schöffski P, Wolter P, Clement P, et al:
Trabectedin (ET-743): Evaluation of its use in advanced soft-tissue
sarcoma. Future Oncol. 3:381–392. 2007.PubMed/NCBI
|