Introduction
Malignant melanoma occasionally shows a variety of
cytomorphological and architectural features, including balloon,
rhabdoid, small, myxoid, adenoid (pseudoglandular) and clear cell
types (1). Signet-ring cell
melanoma is one of the rarest histopathological variants of
malignant melanoma, characterized histopathologically by the
presence of tumor cells in which the nucleus is compressed to the
cellular periphery, appearing as signet-rings (2). This variant was initially described by
Sheibani and Battifora in 1988 (3)
and since then, 20 cases have been reported in English literature
(2–15). The current case report presents an
additional case of signet-ring cell melanoma with sentinel lymph
node metastasis, analyses of the immunohistochemical expression
profiles of the intermediate filaments and mammalian target of
rapamycin (mTOR) pathway proteins and review of the
clinicopathological features of this extremely rare variant of
malignant melanoma.
Case report
Patient presentation
A 68-year-old male without a past history of
malignant melanoma presented with a gradually enlarged black nodule
in the left thigh. Physical examination revealed a relatively
well-circumscribed nodule, measuring 25×20 mm in diameter, with
uneven pigmentation in the patients thigh. Systemic surveillance
failed to identify additional tumorous lesions other than the tumor
in the left thigh. Total resection of the nodule was performed and
subsequently, dissection of the sentinel lymph node. Written
informed consent was obtained from the patient.
Materials and methods
The formalin-fixed, paraffin-embedded tissue blocks
of the resected skin specimen and lymph nodes were cut into
3-μm-thick sections, deparaffinized and rehydrated. Each section
was stained with hematoxylin and eosin and then used for
immunostaining. Immunohistochemical analyses were performed using
an autostainer (BenchMark XT system; Ventana Medical System,
Tucson, AZ, USA) according to the manufacturer’s instructions. The
following primary antibodies were used: Mouse monoclonal antibodies
against α-internexin (2E3; Lab Vision Corp., Fremont, CA, USA),
cytokeratin (AE1/AE3 and CAM5.2; DakoCytomation, Glostrup, Denmark
and Becton-Dickinson, Franklin Lakes, NJ, USA, respectively), glial
fibrillary acid protein (GFAP; 6F2; DakoCytomation), HMB-45
(Novocastra Laboratories, Ltd., Newcastle upon Tyne, UK), Melan-A
(A103; Novocastra Laboratories, Ltd.), nestin (10C2; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA), peripherin (PJM50) and
vimentin (VIM3B4; both Novocastra Laboratories, Ltd.); and rabbit
polyclonal antibodies against S-100 protein (Nichirei Biosciences
Inc., Tokyo, Japan) and mTOR (7C10), 4E-BP1 (53H11) and
phosphorylated 4E-BP1 (p4E-BP1; Thr 37/46; 236B4) (all Cell
Signaling Technology Inc., Danvers, MA, USA).
Results
Histopathological examination of the resected thigh
nodule revealed proliferation of sheet-like or variable-sized nests
composed of medium-sized, round to oval neoplastic cells from the
entire dermis to the superficial subcutis. The majority of the
neoplastic cells exhibited slightly eosinophilic cytoplasm and
centrally located enlarged nuclei with conspicuous nucleoli and
specific cells exhibited melanin pigment within the cytoplasm. In
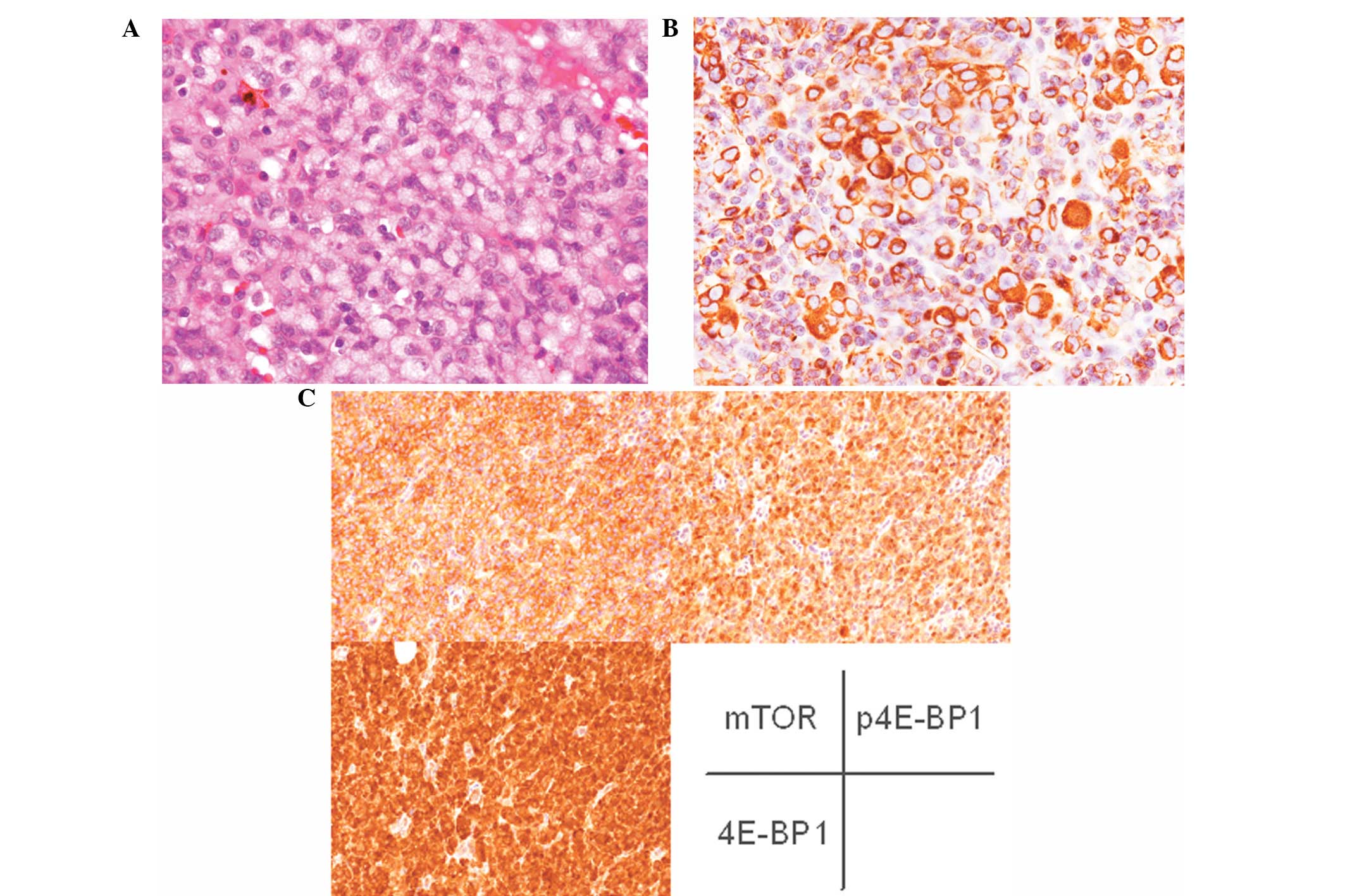
the lower section of the lesion, the neoplastic cells showing
signet-ring cell appearance were identified (Fig. 1A). These cells exhibited
eccentrically located enlarged nuclei with or without conspicuous
nucleoli and abundant pale cytoplasm (Fig. 1A). Melanin pigment was not notably
observed in the cytoplasm of the signet-ring cells. Mitotic figures
were occasionally identified in the entire lesion (6/10 high-power
fields). In addition, no atypical melanocytes were observed in the
overlying epidermis. Immunohistochemically, the tumor cells,
including signet-ring cells, were diffusely positive for S-100
protein, vimentin and Melan-A (Fig.
1B), but negative for HMB-45, cytokeratin (AE1/AE3 and CAM5.2),
nestin, peripherin, α-internexin and GFAP. Moreover, mTOR, 4E-BP1
and p4E-BP1 were diffusely expressed in the tumor cells (Fig. 1C).
The sentinel lymph node exhibited a metastatic
malignant melanoma with signet-ring cell component (Fig. 2). Immunohistochemical features of
the metastatic melanoma were identical to those of the skin.
According to these histopathological and
immunohistochemical features, an ultimate diagnosis of signet-ring
cell melanoma with sentinel lymph node metastasis was made.
Discussion
In cutaneous neoplasms, the presence of signet-ring
cells have been reported in a variety of neoplasms, including
melanocytic nevi, malignant melanoma, squamous and basal cell
carcinoma, hidradenoma and malignant lymphoma (5). Ultrastructural examination revealed
that this characteristic morphology in signet-ring cell melanoma is
often imparted by the intracytoplasmic accumulation of intermediate
filaments, particularly vimentin (2). This observation corroborates the
immunohistochemical results of the present case report since
vimentin was found to be expressed in the cytoplasm of the
signet-ring cells, although, ultrastructural examination was not
performed. In addition, analyses of the expression profiles of
other intermediate filaments in the signet-ring cell melanoma were
not performed. The present case report clearly demonstrated that
intermediate filaments, including cytokeratin, peripherin,
α-internexin, GFAP and nestin, were not expressed in the
signet-ring cell melanoma, although, peripherin and nestin are
commonly expressed in malignant melanoma (16). These results indicate that
intracytoplasmic accumulation of vimentin, but not other types of
intermediate filaments, contributes to the development of the
characteristic morphology of signet-ring cell melanoma.
Table I summarizes
the clinicopathological features of the 20 previously reported
cases of signet-ring cell melanoma as well as the present case.
This disease commonly affects middle-aged males (average age of
57.8-years and male/female ratio of 14:7), however, young
individuals may also be affected (range, 18–85-years-old).
Metastatic signet-ring cell melanoma in a patient with an unknown
primary tumor has been documented (2) and six cases, including the present
case, exhibited no primary sites (Table
I). The present case exhibited no in situ component in
the overlying epidermis and the patient had no past history of
malignant melanoma and tumorous lesions with the exception of the
tumor in the thigh, therefore, the cutaneous lesion may not be
confirmed as the primary lesion.
 | Table IClinicopathological features of
signet-ring cell melanoma. |
Table I
Clinicopathological features of
signet-ring cell melanoma.
| Case no. | Age, years | Gender | Site | Primary | S-100protein | HMB-45 | Vimentin | Author |
|---|
| 1 | 35 | Male | Right axillary lymph
node | Unknown | + | + | + | Sheibani and
Battifora |
| 2 | 63 | Female | Inguinal lymph
node | Skin | + | + | + | Sheibani and
Battifora |
| 3 | 57 | Female | Lung | NA | − | + | + | Bonetti et
al |
| 4 | 55 | Male | Arm (recurrence) | Arm | + | + | + | Nakhleh et
al |
| 5 | 40 | Male | Thigh
(recurrence) | Thigh | + | + | + | Nakhleh et
al |
| 6 | 80 | Male | Left leg | NA | + | + | + | Al-Talib et
al |
| 7 | 27 | Male | Multiple
metastases | Unknown | + | + | + | Eckert et
al |
| 8 | 84 | Male | Right forearm | Unknown | + | + | NA | LiVolsi et
al |
| 9 | 33 | Female | Axillary lymph
node | Arm | + | − | NA | LiVolsi et
al |
| 10 | 85 | Female | Skin and inguinal
lymph node | Left foot | + | + | NA | LiVolsi et
al |
| 11 | 56 | Male | Left ear | NA | + | + | NA | LiVolsi et
al |
| 12 | 84 | Male | Inguinal lymph
node | Righ foot | + | + | + | Tsang et
al |
| 13 | 55 | Male | Abdomen | Unknown | + | + | + | Won et al |
| 14 | 55 | Male | Peritoneal
effusion | Abdomen | + | + | + | Niemann et
al |
| 15 | 72 | Female | Left arm | NA | + | + | + | Breier et
al |
| 16 | 18 | Female | Inguinal lymph
node | NA | NA | + | + | Bastian et
al |
| 17 | 61 | Female | Right shoulder | Right shoulder | + | − | + | Rutten et
al |
| 18 | 76 | Male | Anterior chest | Anterior chest | + | + | + | Rutten et
al |
| 19 | 69 | Male | Left shoulder | Left shoulder | + | − | + | Kacerovska et
al |
| 20 | 41 | Male | Supraclavicular | Unknown | + | + | NA | Russo et
al |
| Present | 68 | Male | Left thigh | Unknown | + | − | + | Ishida et
al |
Metastatic signet-ring cell melanoma may be a
diagnostic issue (4) as it has been
reported that signet-ring cells are occasionally present only in
metastatic sites and the signet-ring cell melanoma component
occasionally lacks melanin pigments in the cytoplasm.
Immunohistochemical analyses are useful for generating a correct
diagnosis. This type of tumor usually shows positive
immunoreactivity for melanocytic markers, including S-100 protein,
Melan-A and HMB-45. However, it is important to recognize that
exceptions to this phenotype exist as S-100 protein-negative (1/20
cases) or HMB-45-negative cases (4/21 cases) have been documented
(Table I) (2,4).
Therefore, a combination of these markers is useful for
establishing a diagnosis.
In addition, the current case report is the first to
analyze the expression profiles of mTOR pathway proteins in
signet-ring cell melanoma. mTOR is a central protein involved in
carcinogenesis, since it phosphorylates 4E-BP1 which leads to cell
proliferation, cell cycle progression and angiogenesis. It has been
previously reported that the mTOR pathway is activated in malignant
melanomas in contrast to benign melanocytic nevi. Therefore, the
mTOR inhibitor is hypothesized to represent a promising therapeutic
agent for various types of carcinomas. Specific clinical studies
with regard to the mTOR inhibitor have been performed in malignant
melanoma (17). The present case
study demonstrates that mTOR pathway proteins are activated in
signet-ring cell melanoma. Therefore, the mTOR inhibitor may be a
potential candidate for the treatment of this type of tumor.
References
|
1
|
Banerjee SS and Harris M: Morphological
and immunophenotypic variations in malignant melanoma.
Histopathology. 36:387–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rütten A, Huschka U, Requena C,
Rodríguez-Peralto JL and Requena L: Primary cutaneous signet-ring
cell melanoma: a clinico-pathologic and immunohistochemical study
of two cases. Am J Dermatopathol. 25:418–422. 2003.PubMed/NCBI
|
|
3
|
Sheibani K and Battifora H: Signet-ring
cell melanoma. A rare morphologic variant of malignant melanoma. Am
J Surg Pathol. 12:28–34. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kacerovska D, Sokol L, Michal M and
Kazakov DV: Primary cutaneous signet-ring cell melanoma with
pseudoglandular features, spindle cells and oncocytoid changes. Am
J Dermatopathol. 31:81–83. 2009. View Article : Google Scholar
|
|
5
|
Bastian BC, Kutzner H, Yen Ts and LeBoit
PE: Signet-ring cell formation in cutaneous neoplasms. J Am Acad
Dermatol. 41:606–613. 1999.PubMed/NCBI
|
|
6
|
Bonetti F, Colombari R, Zamboni G and
Chilosi M: Signet ring melanoma, S-100 negative. Am J Surg Pathol.
13:522–523. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakhleh RE, Wick MR, Rocamora A, Swanson
PE and Dehner LP: Morphologic diversity in malignant melanomas. Am
J Clin Pathol. 93:731–740. 1990.PubMed/NCBI
|
|
8
|
al-Talib RK and Theaker JM: Signet-ring
cell melanoma: light microscopic, immunohistochemical and
ultrastructural features. Histopathology. 18:572–575. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eckert F, Baricevic B, Landthaler M and
Schmid U: Metastatic signet-ring cell melanoma in a patient with an
unknown primary tumor. Histologic, immunohistochemical, and
ultrastructural findings. J Am Acad Dermatol. 26:870–875. 1992.
View Article : Google Scholar
|
|
10
|
LiVolsi VA, Brooks JJ, Soslow R, Johnson
BL and Elder DE: Signet cell melanocytic lesions. Mod Pathol.
5:515–520. 1992.PubMed/NCBI
|
|
11
|
Tsang WY, Chan JK and Chow LT: Signet-ring
cell melanoma mimicking adenocarcinoma. A case report. Acta Cytol.
37:559–562. 1993.PubMed/NCBI
|
|
12
|
Won JH, Ahn SK, Lee SH, Lee WS and Kim SC:
Signet-ring cell melanoma: poor prognostic factor? Br J Dermatol.
131:135–137. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niemann TH and Thomas PA: Melanoma with
signet-ring cells in a peritoneal effusion. Diagn Cytopathol.
12:241–244. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Breier F, Feldmann R, Fellenz C, Neuhold N
and Gschnait F: Primary invasive signet-ring cell melanoma. J Cutan
Pathol. 26:533–536. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Russo JJ, Barr KL, Scanlan LZ,
Chapman-Fredricks J, Herrera L, Dinges MM and Vincek V: Signet ring
cell melanoma, Brenner sign, and elevated vascular endothelial
growth factor. J Am Acad Dermatol. 65:444–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brychtova S, Fiuraskova M, Hlobilková A,
Brychta T and Hirnak J: Nestin expression in cutaneous melanomas
and melanocytic nevi. J Cutan Pathol. 34:370–375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dronca RS, Allred JB, Perez DG, et al:
Phase II Study of Temozolomide (TMZ) and Everolimus (RAD001)
Therapy for Metastatic Melanoma: A North Central Cancer Treatment
Group Study, N0675. Am J Clin Oncol. Jan 24–2013.(Epub ahead of
print).
|